Snail在 前列腺癌中的研究进展
2018-04-26刘妍徐勇
刘妍,徐勇
Snail是近年发现的锌指转录因子,在果蝇中胚层形成中起重要作用,并且与许多肿瘤的发生密切相关。上皮-间质转化(epithelial-mesenchymal transition,EMT)是肿瘤侵袭和转移的关键步骤。转录因子能够诱导EMT的发生,调控EMT相关基因的表达。近年来研究发现,snail在前列腺癌(prostate cancer,PCa)中高表达,并在 PCa发生发展和侵袭转移中发挥重要作用。本文就snail调控PCa一系列信号通路以及对PCa的预后评估及治疗作一综述。
1 Snail的结构
Snail基因定位于人类第20号染色体20q12.3,全长5 882 bp,含有3个外显子。Snail为snail超家族成员之一,snail超家族成员中包括snail1(snail)、snail2(slug)和 snail3(smuc)[1],由高度保守的锌指结构羧基末端DNA结合区和多变的氨基酸末端调控区组成。锌指是C2H2型,由2个β-折叠紧密连接1个α-螺旋组成,其氨基末端包含SNAG反式激活区域,与DNA大沟接触,2个保守的cys和his(C2H2)与Zn离子相连,并与以6个碱基CAGGTG为核心的位置相连,发挥转录功能[2-3]。Snail基因上具有糖原合成激酶3β(glycogen synthase kinase 3β,GSK-3β)结合位点,GSK-3β 与 snail结合后发生磷酸化[4],并且可以与特异核心碱基序列基因上的E-box盒连接调节其表达,发挥转录抑制因子的作用[5]。
DNA甲基化是表观遗传学基因表达调节的重要途径之一。DNA甲基化可以调节snail基因的表达。Snail在EMT和间质-上皮转化(mesenchymalepithelial transition,MET)过程中具有重要的作用,并且维持间充质细胞的形态。据文献报道,snail的转录水平与其DNA甲基化水平呈负相关[6]。Snail基因DNA甲基化后,snail启动子区域的活性显著降低,组蛋白去乙酰酶促使组蛋白去乙酰化,抑制snail基因的表达。在小鼠的癌症研究中,snail转录与其最接近的启动子DNA甲基化密切相关,但是在人类snail基因的DNA甲基化作用尚鲜见文献报道。
2 Snail的功能
2.1 Snail在胚胎发育和伤口愈合中的作用 Ma等[7]研究发现,snail在妊娠小鼠子宫上皮细胞基质部位过表达,但在胚胎未形成的小鼠体内没有检测到。胚胎发育过程中snail可以抑制紧密连接蛋白表达[8],促进胚胎穿过内膜与周围血管建立联系,促进胚胎发育。Aomatsu等[9]通过对成年雄性BALB/c小鼠的研究表明,snail和slug可以促进人角膜上皮细胞基底层的生长,在角膜损伤的愈合方面具有重要的作用。
2.2 Snail对细胞周期和细胞生长的调控作用 在胚胎发育过程中,snail通过抑制细胞周期蛋白D2(cyclin D2)基因的表达和增加p21 Waf1/Cip1的表达,调控G1期和G1/S控制点,抑制细胞凋亡[10]。另外,在培养经转化生长因子β(transforming growth factor β,TGF-β)处理的大鼠肝细胞中,伴随 snail的高表达,只有少数经EMT过程的细胞能够存活[11]。因此,snail在胚胎发育过程中发挥重要作用。
2.3 Snail在肿瘤干细胞EMT的发生和机体免疫调节中的作用 高表达的snail促进肿瘤干细胞在基质微环境中发生EMT,使肿瘤干细胞向远处转移。Snail通过调控免疫细胞因子和调节性T细胞,导致树突状细胞损伤,发生免疫抑制,促进肿瘤的进展。Mani等[12]发 现 ,人 类 乳 腺 上 皮 细 胞(human mammary epithelial cells,HMLE)中异常表达的 snail可以引起HMLE聚集成团,促进EMT的发生。这些HMLE具有间充质表型,大量存在于肿瘤形成初期,类似于乳腺肿瘤干细胞(cancer stem cell,CSC)。Kurrey等[13]在卵巢癌的研究中也有相似的发现,snail能有效调节卵巢癌细胞的生长并且参与CSC的自我更新和无限增殖作用,间接地提升肿瘤细胞的自我更新能力,增加富含CD44+/CD24-标志物的卵巢CSC的数量。
2.4 Snail在肿瘤细胞侵袭和迁移中的作用 Snail可以抑制上皮细胞的黏附,促使上皮细胞与间质细胞转换,增强细胞侵袭能力,导致EMT的发生。在EMT过程中,EMT标志性蛋白——上皮细胞钙黏蛋白(E-cadherin)下调[14],snail和神经钙黏素(N-cadherin)上调。在多数肿瘤和胚胎发育过程中,转换的上皮细胞分离出来,极性发生改变,迁移到机体其他部位,逐渐转换成为具有侵袭能力的间质细胞。Snail可以通过多条信号通路启动EMT的发生,发挥重要作用[15-17]。
2.5 Snail通过多条信号通路在EMT发生中的作用 信号通路主要包括TGF-β/表皮生长因子(epidermal growth factor,EGF)、肿瘤坏死因子-β(tumor necrosis factor β,TNF-β)、受体酪氨酸激酶(receptor tyrosine kinases,RTK)、Notch、骨成型蛋白质(bone morphogenetic protein,BMP)以及 Wnt/β-连环蛋白(β-catenin)等。Snail通过多条信号通路能够降低E-cadherin和上皮细胞标志蛋白表达,并且可以促进间质细胞标志蛋白表达;而小干扰RNA沉默表达 snail时,低表达的 snail能够促进 E-cadherin过表达,并且降低间质细胞标志蛋白表达[18]。在snail蛋白的中央区域有许多磷酸化位点,snail可以通过这些磷酸化位点来调节其活性[19]。
3 Snail与PCa的关系
3.1 Snail在PCa中的作用 研究发现,snail在PCa组织中高表达,并与PCa的Gleason评分密切相关,由于snail在PCa发展进程中起着重要的作用,snail的表达水平可以用于PCa患者病情的预测,并可为 PCa患者 Gleason评分提供参考[20]。Huang等[21]利用构建荧光素酶报告基因及染色质免疫沉淀技术检测到PCa组织中转录因子snail高表达,通过抑制糖基转移酶LARGE2的表达,导致α-肌营养不良蛋白聚糖(α-dystroglycan,α-DG)亚糖基化,从而影响PCa的进程。有报道显示,snail在PCa细胞系PC-3中高表达[22]。PCa细胞系DU145中snail也可以异常高表达[23]。此外,PCa细胞系ARCaP中snail的高表达还可以通过调节氧化应激酶提升活性氧(reactive oxygen species,ROS)的表达水平,促进肿瘤的进展[24]。Emadi等[25]发现 PCa 的8个细胞系中,LNCaP和PC-3细胞中的snail基因表达水平是最高的,沉默snail表达后LNCaP和PC-3细胞大量减少,细胞凋亡显著增加,低表达的snail可以促进E-cadherin蛋白的表达,降低波形蛋白(vimentin)和N-cadherin蛋白的表达并抑制细胞浸润,诱导一个完整的MET发生。因此,snail是PCa细胞重要的生存因子和细胞衰老的抑制因子,影响PCa发展的进程。
3.2 Snail通过多条信号通路在PCa中的激活机制 Chen等[26]发现,伴随 snail的上调,TGF-β 和EGF表达也升高,导致人白细胞抗原Ⅰ类分子(human leucocyte antigenⅠ,HLA-Ⅰ)在 PCa中低表达。Snail和HLA-Ⅰ呈负相关表达,这有利于PCa细胞逃离免疫监视。Smith等[20]得出了相似的研究结论,认为snail在EMT过程中作为一个关键的转录因子,能促进PCa的发生发展。Liu等[27]发现,PCa细胞能通过RTK-磷脂酰肌醇-3-激酶(PI3K)-蛋白激酶 B(protein kinase B,PKB)/GSK-3β信号通路表达较高水平的snail和成纤维细胞生长因子(basic fibroblast growth factor,bFGF),而 E-cadherin呈低表达。bFGF通过稳定snail蛋白并且增强其转录活性,促进PC-3的EMT和肿瘤转移,提示PCa细胞的EMT表型转化。而用RNA干扰技术沉默snail基因表达后,PCa中snail基因表达降低,E-cadherin表达升高,提示PCa细胞的EMT表型逆转。沉默的snail表达也使PCa细胞大量减少,证明snail可以介导PCa细胞发生EMT。Ju等[28]发现,TGF-β可以通过BMP-SMAD4信号通路上调PCa细胞中snail的表达水平,促进PCa细胞增殖。Wang等[29]也报道了snail通过Notch信号通路促进PCa细胞发生EMT,提高癌细胞的侵袭能力。Tao等[30]发现,snail通过 miR-128调控的核糖体 S6蛋白激酶抗体(RPS6KB1)/缺氧诱导因子-1α(hypoxia inducible factor 1α,HIF-1α)/丙 酮 酸 激 酶 M2(PKM2)信号通路调节PCa细胞的生长和能量代谢。此外,snail在去势抵抗性前列腺癌(castrationresistant prostate cancer,CRPC)中的表达明显低于激素依赖性PCa[31]。当PCa细胞中snail水平下降时,miR-128水平会增高,减少糖代谢和乳酸生成,抑制RPS6KB1、HIF-1α 和 PKM2 的表达。Santiago等[32]报道称,淋巴样增强子结合因子 1(lymphoid enhancer binding factor 1,LEF1)作为 T 细胞因子(T-cell factor,TCF)家族成员之一,通过 Wnt/β-catenin信号通路激活EMT相关基因以及转录因子snail,促进 PCa细胞增殖、侵袭、迁移。Hao等[33]对 YKL-40基因的研究也获得了相似的结论,见图1。
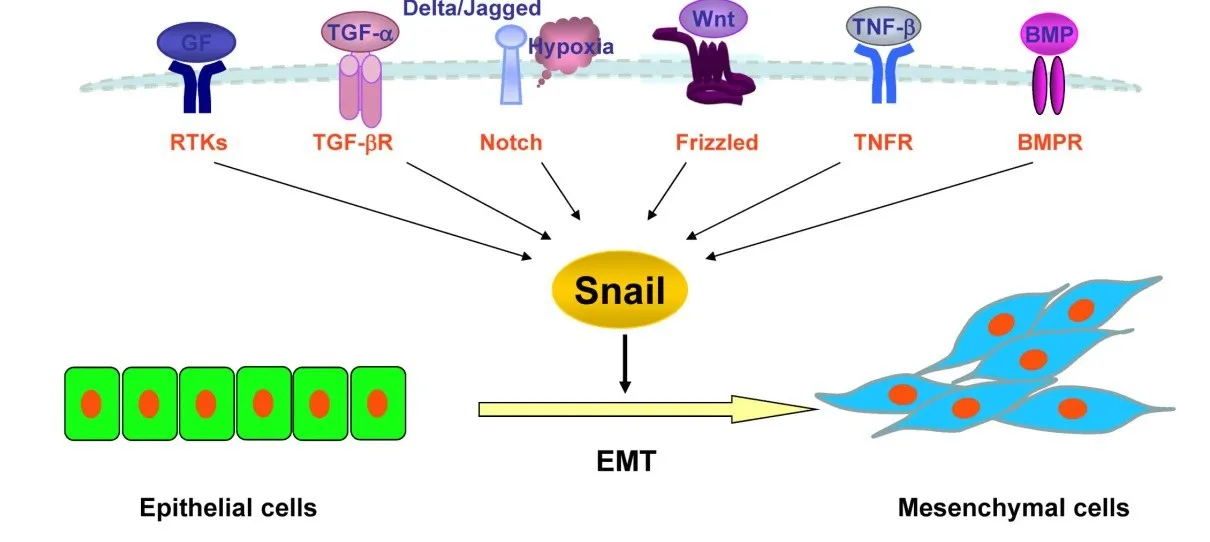
Fig.1 Schematic diagram of the signaling pathways associated with snail-induced EMT in prostate cancer图1 Snail调控PCa发生EMT的信号通路示意图
3.3 Snail在PCa细胞侵袭和转移中的作用 Snail能够下调细胞与细胞之间的黏附蛋白E-cadherin,上调间质细胞蛋白vimentin,增强癌细胞的侵袭和转移能力。Snail还可以通过MAPK信号通路对整合素蛋白进行调控,减少PCa细胞与纤连蛋白和Ⅰ型胶原蛋白等细胞外基质(extracellular matrix,ECM)的黏附能力,促进癌细胞与之分离并转移,增强PCa细胞的侵袭能力,在细胞解离和代谢方面发挥重要的作用[34]。Lv 等[35]也报道称,低氧环境能提高PCa细胞的侵袭能力,高表达的snail通过调节侵袭相关基因,在PCa细胞侵袭方面发挥作用;同时,低氧环境还可以上调HIF-1α和肿瘤坏死因子α(tumor necrosis factor α,TNF-α);更重要的是,TNF-α与HIF-1α共同增强snail的稳定性。Snail在PCa中高度表达,抑制E-cadherin的表达并且促进侵袭相关基因基质金属蛋白酶9(matrix metalloproteinase 9,MMP-9)、纤连蛋白以及波形蛋白表达增高,最终影响PCa细胞侵袭和转移。Osorio等[36]在snail增加PCa细胞侵袭力方面也得到了相同的结论。
3.4 Snail在PCa预后评价和治疗中的作用 Wen等[37]研究证实,snail高表达的PCa患者生化复发可能会较早出现,患者需要早期进行辅助性治疗。Poblete等[38]发现,Gleason 评分高的 PCa患者体内snail基因的表达水平更高,可以通过snail表达水平对PCa的进展进行评估。这一发现为PCa患者的早期诊断及个体化治疗等方面提供了广阔的应用前景。
Neal等[39]研究显示,snail可以通过抑制乳腺丝抑蛋白(maspin)启动子区域的活性而抑制其蛋白的表达,导致PCa细胞间黏附降低,促进PCa细胞的转移以及EMT的发生。因此,snail可以作为PCa治疗的靶向基因,通过促进乳腺丝抑蛋白在PCa细胞中重新表达,阻止PCa的进程。Barnett等[24]研究证实,snail调控氧化应激酶并激活细胞外信号调节激酶(extracellular signal-regulated kinase,ERK),增加ROS,调节EMT的发生。因此,snail将可能成为阻止PCa进程的靶向基因。
值得注意的是,Tang等[40]研究发现,牛磺酸对EMT相关基因以及snail具有抑制作用,在PCa细胞中作为EMT的抑制剂发挥作用,从而应用于PCa的靶向治疗。此外,藤黄酸也可以起到相同的作用,降低 PCa细胞的侵袭能力[41]。
恩杂鲁胺作为雄激素受体抑制剂,能够竞争性地抑制雄激素(androgen)与雄激素受体(androgen receptor,AR)的结合,抑制PCa细胞的增殖并导致其死亡。在恩杂鲁胺作用下,PCa细胞中的snail基因表达降低,临床广泛地将恩杂鲁胺用于PCa的雄激素阻断治疗。但是近年来,Miao等[31]通过对C42细胞和CRPC组织的研究表明,恩杂鲁胺能够增强PCa细胞上皮细胞-间质转化可塑性(epithelial-tomesenchymal plasticity,EMP),同时伴随 AR 的降低、snail的激活以及间质细胞标志物的表达,促进PCa细胞的转移,认为采用恩杂鲁胺治疗PCa可能存在治疗抵抗。因此snail对于长期使用恩杂鲁胺的CRPC患者的疗效仍有待于进一步持续监测[42]。
综上所述,目前研究表明转录因子snail在PCa中高度表达,在PCa的发生发展中起重要的作用。因此,snail有望成为PCa新的临床诊断指标,为PCa的早期诊断、预后判断提供帮助,为建立PCa个性化基因治疗提供新的基因靶点。
[1]Kaufhold S,Bonavida B.Central role of snail1 in the regulation of EMT and resistance in cancer:a target for therapeutic intervention[J].J Exp Clin Cancer Res,2014,33:62.doi:10.1186/s13046-014-0062-0.
[2]Villarejo A,Cortés-Cabrera A,Molina-Ortíz P,et al.Differential role of snail1 and snail2 zinc fingers in E-cadherin repression and epithelial to mesenchymal transition[J].J Biol Chem,2014,289(2):930-941.doi:10.1074/jbc.M113.528026.
[3]Prokop JW,Liu Y,Milsted A,et al.A method for in silico identification of SNAIL/SLUG DNA binding potentials to the E-box sequence using molecular dynamics and evolutionary conserved amino acids[J].J Mol Model,2013,19(9):3463-3469.doi:10.1007/s00894-013-1876-y.
[4]Zhao J,Ou B,Han D,et al.Tumor-derived CXCL5 promotes human colorectal cancer metastasis through activation of the ERK/Elk-1/Snail and AKT/GSK3β/β-catenin pathways[J].Mol Cancer,2017,16(1):70.doi:10.1186/s12943-017-0629-4.
[5]Izawa G,Kobayashi W,Haraguchi M,et al.The ectopic expression of Snail in MDBK cells does not induce epithelial-mesenchymal transition[J].Int J Mol Med,2015,36(1):166-172.doi:10.3892/ijmm.2015.2215.
[6]Chen Y,Wang K,Qian CN,et al.DNA methylation is associated with transcription of Snail and Slug genes[J].Biochem Biophys Res Commun,2013,430(3):1083-1090.doi:10.1016/j.bbrc.2012.12.034.
[7]MaXH,Hu SJ,Yu H,etal.Differentialexpression of transcriptional repressor snail gene at implantation site in mouse uterus[J].Mol Reprod Dev,2006,73(2):133-141.
[8]De Chiara L,Andrews D,Watson A,et al.miR302 regulates SNAI1 expression to control mesangial cell plasticity[J].Sci Rep,2017,7:42407.doi:10.1038/srep42407.
[9]Aomatsu K,Arao T,Abe K,et al.Slug is upregulated during wound healing and regulates cellular phenotypes in corneal epithelial cells[J].Invest Ophthalmol Vis Sci,2012,53(2):751-756.doi:10.1167/iovs.11-8222.
[10]Sun G,Guzman E,Balasanyan V,et al.A molecular signature for anastasis,recovery from the brink of apoptotic cell death[J].J Cell Biol,2017,216(10):3355-3368.doi:10.1083/jcb.201706134.
[11]ValdesF,AlvarezAM,LocascioA,etal.Theepithelial mesenchymal transition confers resistance to the apoptotic effects of transforming growth factor Beta in fetal rat hepatocytes[J].Mol Cancer Res,2002,1(1):68-78.
[12]Mani SA,Guo W,Liao MJ,et al.The epithelialmesenchymal transition generates cells with properties of stem cells[J].Cell,2008,133(4):704-715.doi:10.1016/j.cell.2008.03.027.
[13]Kurrey NK,Jalgaonkar SP,Joglekar AV,et al.Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells[J].Stem Cells,2009,27(9):2059-2068.doi:10.1002/stem.154.
[14]Wallesch M,Pachow D,Blücher C,et al.Altered expression of E-cadherin-related transcription factors indicates partial epithelialmesenchymal transition in aggressive meningiomas[J].J Neurol Sci,2017,380:112-121.doi:10.1016/j.jns.2017.07.009.
[15]Zheng H,Li W,Wang Y,et al.Glycogen synthase kinase-3 beta regulates snail and beta-catenin expression during Fas-induced epithelial-mesenchymal transition in gastrointestinal cancer[J].Eur J Cancer,2013,49(12):2734-2746.doi:10.1016/j.ejca.2013.03.014.
[16]Wang H,Fang R,Wang XF,et al.Stabilization of snail through AKT/GSK-3 beta signaling pathway is required for TNF-alphainduced epithelial-mesenchymal transition in prostate cancer PC3 cells[J].Eur J Pharmacol,2013,714(1/2/3):48-55.doi:10.1016/j.ejphar.2013.05.046.
[17]Nagarajan D,Melo T,Deng Z,et al.ERK/GSK3beta/snail signaling mediates radiation-induced alveolar epithelial-to-mesenchymal transition[J].Free Radic Biol Med,2012,52(6):983-992.doi:10.1016/j.freeradbiomed.2011.11.024.
[18]Yonemori K,Kurahara H,Maemura K,et al.Impact of Snail and E-cadherin expression in pancreatic neuroendocrine tumors [J].Oncol Lett,2017,14(2):1697-1702.doi:10.3892/ol.2017.6306.
[19]Tarasewicz E,Rivas L,Hamdan R,et al.Inhibition of CDK-mediated phosphorylation of Smad3 results in decreased oncogenesis in triple negative breast cancer cells[J].Cell Cycle,2014,13(20):3191-3201.doi:10.4161/15384101.2014.950126.
[20]Smith BN,Odero-Marah VA.The role of snail in prostate cancer[J].Cell Adh Migr,2012,6(5):433-441.doi:10.4161/cam.21687.
[21]Huang Q,Miller MR,Schappet J,et al.The glycosyltransferase LARGE2 is repressed by Snail and ZEB1 in prostate cancer[J].Cancer Biol Ther,2015,16(1):125-136.doi:10.4161/15384047.2014.987078.
[22]Furuya S,Endo K,Takahashi A,et al.Snail suppresses cellular senescence and promotes fibroblast-led cancer cell invasion[J].FEBS Open Bio,2017,7(10):1586-1597.doi:10.1002/2211-5463.12300.
[23]Luo W,Tan P,Rodriquez M,et al.Leucine-rich repeat-containing G protein-coupled receptor 4(Lgr4)is necessary for prostate cancer metastasis via epithelial-mesenchymal transition[J].J Biol Chem,2017,292(37):15525-15537.doi:10.1074/jbc.M116.771931.
[24]Barnett P,Arnold RS,Mezencev R,et al.Snail-mediated regulation of reactive oxygen species in ARCaP human prostate cancer cells[J].Biochem Biophys Res Commun,2011,404(1):34-39.doi:10.1016/j.bbrc.2010.11.044.
[25]Emadi Baygi M,Soheili ZS,Schmitz I,et al.Snail regulates cell survival and inhibits cellular senescence in human metastatic prostate cancer cell lines[J].Cell Biol Toxicol,2010,26(6):553-567.doi:10.1007/s10565-010-9163-5.
[26]Chen XH,Liu ZC,Zhang G,et al.TGF-β and EGF induced HLAI downregulation is associated with epithelial-mesenchymal transition(EMT)through upregulation of snail in prostate cancer cells[J].Mol Immunol,2015,65(1):34-42.doi:10.1016/j.molimm.2014.12.017.
[27]Liu ZC,Wang HS,Zhang G,et al.AKT/GSK-3β regulates stability and transcription of snail which is crucial for bFGF-induced epithelial– mesenchymal transition of prostate cancer cells[J].Biochim Biophys Acta,2014,1840(10):3096-3105.doi:10.1016/j.bbagen.2014.07.018.
[28]Ju X,Casimiro MC,Gormley M,et al.Identification of a cyclin D1 network in prostate cancer that antagonizes epithelial-mesenchymal restraint[J].Cancer Res,2014,74(2):508-519.doi:10.1158/0008-5472.CAN-13-1313.
[29]Wang W,Wang L,Mizokami A,et al.Down-regulation of E-cadherin enhances prostate cancer chemoresistance via Notch signaling[J].Chin J Cancer,2017,36(1):35.doi:10.1186/s40880-017-0203-x.
[30]Tao T,Li G,Dong Q,et al.Loss of snail inhibits cellular growth and metabolism through the miR-128-mediated RPS6KB1/HIF-1α/PKM2 signaling pathway in prostate cancer cells[J].Tumour Biol,2014,35(9):8543-8550.doi:10.1007/s13277-014-2057-z.
[31]Miao L,Yang L,Li R,et al.Disrupting androgen receptor signaling induces snail-mediated epithelial-mesenchymalplasticity in prostate cancer[J].Cancer Res,2017,77(11):3101-3112.doi:10.1158/0008-5472.CAN-16-2169.
[32]Santiago L,Daniels G,Wang D,et al.Wnt signaling pathway protein LEF1 in cancer,as a biomarker for prognosis and a target for treatment[J].Am J Cancer Res,2017,7(6):1389-1406.
[33]Hao H,Wang L,Chen H,et al.YKL-40 promotes the migration and invasion of prostate cancer cells by regulating epithelial mesenchymal transition[J].Am J Transl Res,2017,9(8):3749-3757.
[34]Neal CL,Mckeithen D,Odero-Marah VA.Snail negatively regulatescelladhesion to extracellularmatrix and integrin expression via the MAPK pathway in prostate cancer cells[J].Cell Adh Migr,2011,5(3):249-257.
[35]Lv L,Yuan J,Huang T,et al.Stabilization of Snail by HIF-1α and TNF-α is required for hypoxia-induced invasion in prostate cancer PC3 cells[J].Mol Biol Rep,2014,41(7):4573-4582.doi:10.1007/s11033-014-3328-x.
[36]Osorio LA,Farfán NM,Castellón EA,et al.Snail transcription factor increases the motility and invasive capacity of prostate cancer cells[J].Mol Med Rep,2016,13(1):778-786.doi:10.3892/mmr.2015.4585.
[37]Wen YC,Chen WY,Lee WJ,et al.Snail as a potential marker for predicting the recurrence of prostate cancer in patients at stage T2 after radical prostatectomy[J].Clin Chim Acta,2014,431:169-173.doi:10.1016/j.cca.2014.01.036.
[38]Poblete CE,Fulla J,Gallardo M,et al.Increased snail expression and low syndecan levels are associated with high Gleason grade in prostate cancer[J].Int J Oncol,2014,44(3):647-654.doi:10.3892/ijo.2014.2254.
[39]Neal CL,Henderson V,Smith BN,et al.Snail transcription factor negatively regulates maspin tumor suppressor in human prostate cancer cells[J].BMC Cancer,2012,12:336.doi:10.1186/1471-2407-12-336.
[40]Tang Y,Kim YS,Choi EJ,et al.Taurine attenuates epithelialmesenchymal transition-related genes in human prostate cancer cells[J].Adv Exp Med Biol,2017,975:1203-1212.doi:10.1007/978-94-024-1079-2_96.
[41]Lu L,Tang D,Wang L,et al.Gambogic acid inhibits TNF-αinduced invasion of human prostate cancer PC3 cells in vitro through PI3K/Akt and NF-kB signaling pathways[J].Acta Pharmacol Sin,2012,33(4):531-541.doi:10.1038/aps.2011.180.
[42]Sidaway P.Enzalutamide promotes mesenchymal plasticity via snail activation[J].Nat Rev Urol,2017,14(6):325.doi:10.1038/nrurol.2017.55.
