Advancements in the preparation of high-performance liquid chromatographic organic polymer monoliths for the separation of small-molecule drugs
2018-04-17XiliDingJingYngYumingDong
Xili Ding,Jing Yng,Yuming Dong,b,*
aInstitute of Pharmaceutical Analysis,School of Pharmacy,Lanzhou University,Lanzhou,Gansu 730000,PR China
bLanzhou Universty-Techcomp(China)Ltd.Joint Laboratory of Pharmaceutical Analysis,Lanzhou,Gansu 730000,PR China
1.Introduction
As a new fourth generation of chromatographic separation media,monolithic columns with good permeability and high mass transfer speed play an increasingly important role in analytical investigations in the fields of environmental science,pharmaceutical analysis,food and chemistry[1,2].Depending on the type of substrate,monoliths can be divided into three categories:organic polymer monoliths,inorganic monoliths(mainly silica monoliths)and hybrid monoliths[3–5].Inorganic silica monoliths use the alkoxysilane as the main material,with columns prepared by direct sintering or the use of a sol-gel.The preparation of organic polymer monolithic columns usually involves light-or heat-induced polymerization using crosslinking agents,porogens and initiators[6,7]as raw materials.Compared with inorganic silica monoliths,the preparation process for polymeric monoliths is relatively simple,with an abundant choice of monomer species,a wide pH range(2–12)and easy surface modification[8].Therefore,polymeric monoliths have wide range of applications,and are extensively used for the separation and enrichment of complex samples.The overall structure of micropores is an important factor that determines the separation performance of organic polymer monoliths.Therefore,a suitable pore structure is essential to obtain good resolution.Although organic polymer monoliths prepared by traditional methods have many advantages,there have few mesopores and almost no micropores,and thus their ability to separate small molecules is poor[9,10].According to the International Union of Pure and Applied Chemistry(IUPAC),micropores have pore sizes of≤ 2 nm,mesopores have pore sizes of 2–50 nm,and macropores have pore sizes of>50 nm.Thus,in recent years,studies on high-performance liquid chromatographic monoliths have concentrated on polymeric materials,which are suitable for separating small molecules.To improve the performance of organic polymer monoliths for separating small molecules,researchers have mainly examined optimization of the preparation conditions and surface modifications with metal-organic frameworks or nanomaterials[11,12].
2.Factors affecting the polymerization reaction of monolithic column
The structure of a polymer monolithic column must provide a large surface area,similar to those achieved using silica monoliths.Although macropores are necessary to achieve monolithic columns with good permeability,they have little effect on the overall surface area of a polymer monolithic column[13].Therefore,the current literature has focused mostly on finding preparation processes that both retain the advantages of high velocity and increase the overall surface area of the column.From the viewpoint of chemical polymerization,several factors can influence the surface area and permeability of the column,including the selection of monomer and crosslinker;the proportion of monomer,crosslinker,and porogen;and the polymerization temperature and reaction time[14].
2.1.Selection of monomer and crosslinker
The selection of monomer and crosslinker not only affects the formation of the pore structure in the polymer monolith,but also determines the chemical composition of the polymer monolith,which is the main factor affecting the performance of the monolith.Note that the density and rigidity of the prepared column depend,to a large extent,on the nature and the initial concentration of the monomer[15].Therefore,to obtain high column efficiency and strong mechanical stability,the selection of the monomer is a very important step.
At present,because various monomers have been used to prepare different monoliths for the separation of different small molecules,investigations into the influence of monomer type on the structure of the monolith are ongoing.
Based on the type of monomer,organic polymer-based monolithic columns can be divided into three categories:styrenes[16],acrylates[17],and acrylamides[18].Different monomers have different advantages and disadvantages,as summarized in Table 1.
In 2014,Bai[19]prepared a monolithic column with a uniform framework,good permeability,and high column efficiency.The monolith was applied as a high-performance liquid chromatography(HPLC)stationary phase to separate alkaline,acidic and neutral small molecules.The results showed that alkaline,acidic small molecular compounds were separated quickly and efficiently on the monolithic column(Fig.1).The good performance of the column was related to the uniform pore structure,originating from the use of trimethylolpropane triacrylate(TMPTA),which contains three terminal double bonds(Fig.2).
Liu et al.[20]used a 1-dodecene polymeric monolith to separate benzotriazole,benzene,biphenyl,anthracene,and other small molecules successfully.The combination of 1-dodecene,which is highly hydrophobic,with an acrylate results in a monolith with the desired pore structure.They also optimized the preparation conditions and found that the amount of crosslinking agent directly affected the column pressure.Excessive amounts of crosslinking agent caused a high column pressure,which decreased the permeability of the column.On the contrary,when the amount of crosslinking agent is too low,the monolith structure will be loose.They also found that too much porogen led to low mechanical strength,as well as a loose monolith structure.
It has been reported that higher crosslinker concentrations can provide higher mechanical stability and a higher surface area[21].Liu et al.[22]has suggested that significant advantages are realizedwhen asingle-monomer/crosslinkerisused,including straightforward optimization of the polymerization solution,improved column-to-column reproducibility,better mechanical stability,and higher surface area owing to a highly crosslinked network.The effect of monomer content on the overall column efficiency,porosity,and surface area was investigated.It was foundthat the surface area,porosity,and column efficiency increased when the monomer content was reduced from 20%to 17.5%[23].Highly crosslinked networks resulting from crosslinking a single monomer were found to enhance the concentrations of mesopores in and the surface areas of polymeric monoliths.Li et al.[24]synthesized monolithic columns with four different monomers and optimized the preparation processes.The optimized monoliths synthesized from each of the crosslinked monomers showed high permeability,with little swelling or shrinkage observed in solvents with different polarities.Some common monomers used in the preparation of monoliths[17,19,22,23,25]are shown in Table 2.
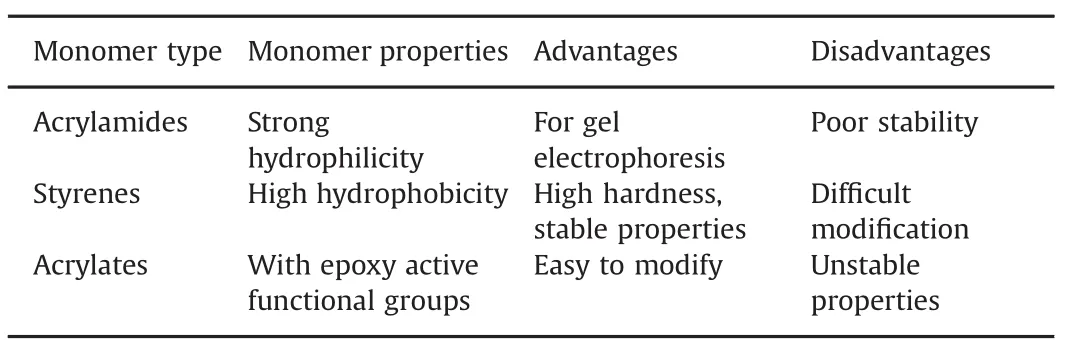
Table 1 Properties of different monomer types.
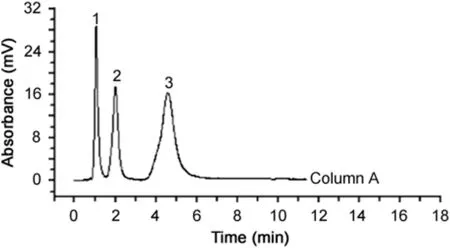
Fig.1.Chromatogram showing the separation of small molecules on the poly(TMPTA-co-EDMA)column.Conditions:mobile phase:methanol:water(75:25,v/v);f l ow rate:1.0mL/min.Analytes:(1)1H-benzotriazole,(2)p-xylene,(3)biphenyl[19].
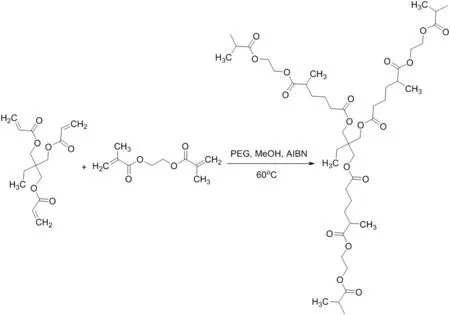
Fig.2.Synthetic route for the poly(TMPTA-co-EDMA)monolith[19].
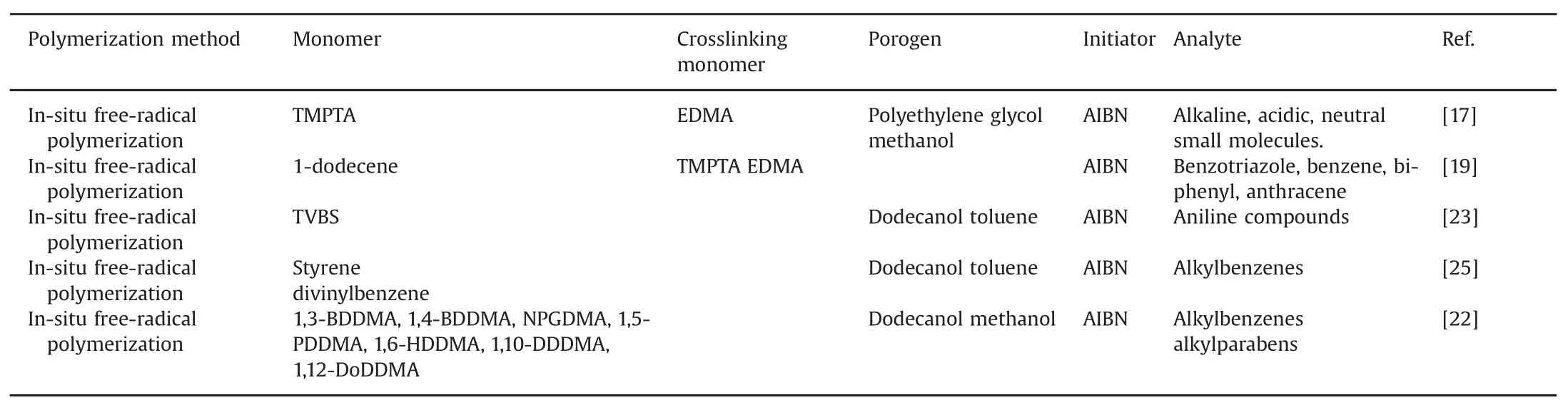
Table 2 Common monomers in the preparation of monoliths.
2.2.Proportion of monomer,crosslinker,and porogen
The proportion of monomer,crosslinker,and porogen is very important for the preparation of polymer monoliths.In particular,the proportion of monomer and crosslinking agent has a very signif i cant impact on the pore structure and chemical properties of a monolith.If the ratio of monomer to crosslinking agent is too large,the pore size will be too small and reduce the permeability;in contrast,if the pore size is too large,efficient separation cannot be achieved.
Poly(vinyl ester resin-co-ethylene glycol dimethacrylate)(poly(VER-co-EDMA))was prepared to investigate the influence on the proportion of crosslinking agent and monomer[26].The results showed that the retention time of toluene increased as the monomer concentration increased,with the best efficiency obtained when the monomer concentration was 30%.When the monomer concentration was increased further,the column exhibited poor permeability(Fig.3).
In 2014,Wei[27]employed pentaerythritol triacrylate(PETA)and triallyl isocyanurate(TAIC)as monomers,azodiisobutyronitrile(AIBN)as an initiator,cetyl alcohol as a porogen,and methanol as a solvent to prepare a monolith through a free radical polymerization process.As the content of TAIC or PETA increased,the pore structure of the monolith became too dense,causing an increase of the column pressure.In contrast,when the content of TAIC or PETA decreased,the pore structure was loose.Therefore,the proportion of monomers and crosslinker was important for the performance of this monolithic column.
Hao[28]used 1-dodecene as a monomer,VER as a crosslinking agent,dodecanol as a porogen,and a redox initiator to prepare a poly(C12-co-VER)monolithic column.Benzoic acid,p-xylene,p-amino azobenzene,benzene,terephthalic acid,naphthol,anthrone,and other small molecules were separated successfully using this column(Fig.4).
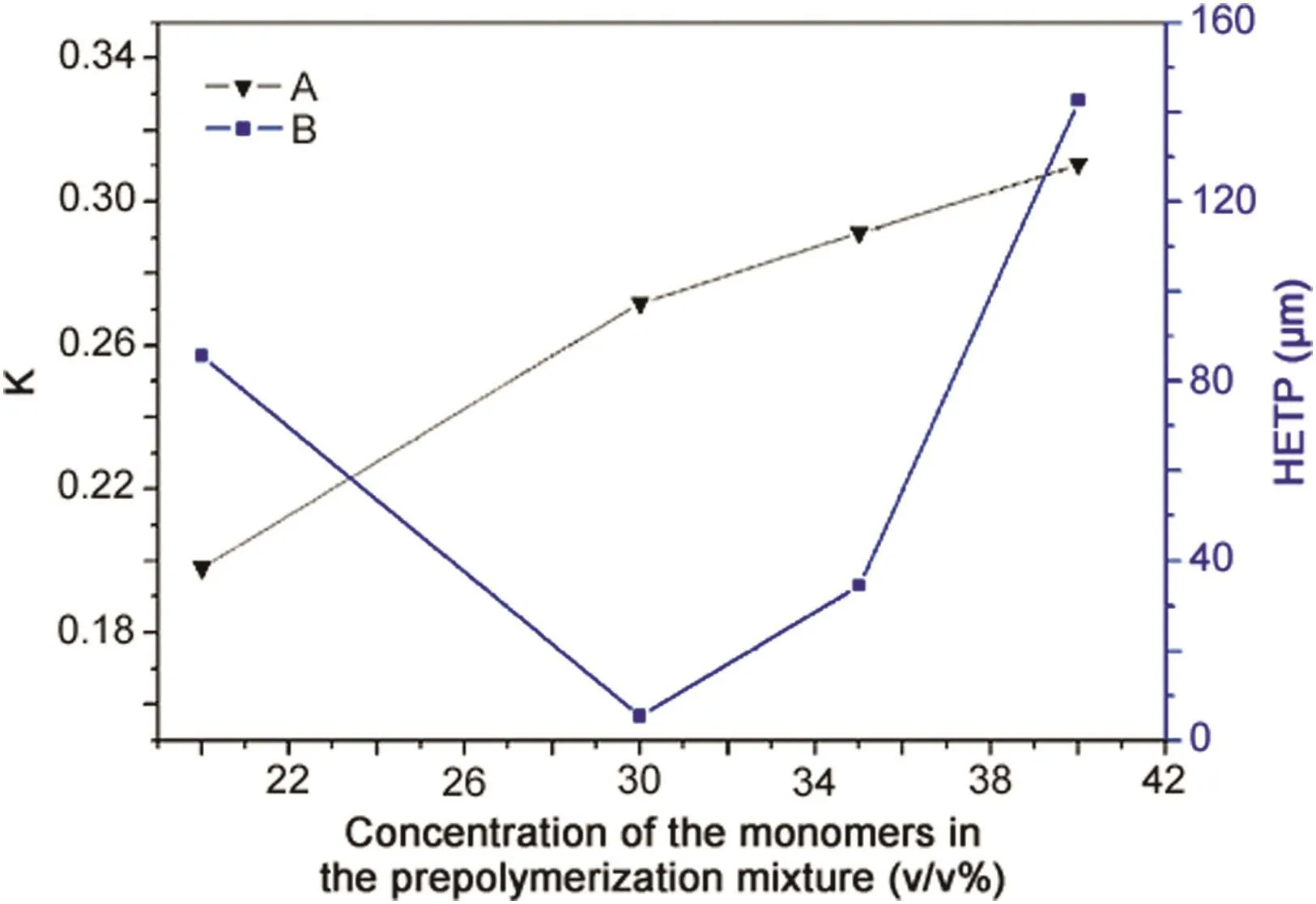
Fig.3.(A)Effects of monomer content on the retention abilities of poly.(VER-co-EDMA)monolithic columns.(B)Effects of monomer content on the efficiency of poly(VER-co-EDMA)monolithic columns.Conditions:effective length:100 mm× 150 μm i.d.;test compound:toluene;mobile phase:methanol:water(80:20,v/v);f l ow rate:3 μL/min;UV detection at 214 nm[26].
Several monoliths with different proportions of 1-dodecene,VER,and dodecanol were used to examine how the extent of polymerization impacts performance.The results indicated that increasing the amount of monomer or crosslinking agent increased the column pressure.Meanwhile,the initiation method also influences the speed of the polymerization reaction and the pore size obtained,thereby affecting the performance of monolith(Fig.5).As shown in Fig.5,the redox method is better than the thermal method for preparing poly(C12-co-VER)monolith columns.
Dou et al.[29]used[2-(methacryloyloxyethyl)ethyl]dimethyl(3-sulfopropyl)ammoniumhydroxide(SPE)asamonomer,N-ethyl-N,N-dimethyl-1-dodecanaminium hydroxide(EDMA)as a crosslinking agent,AIBN as an initiator and n-propanol,1,4-butanediol,and water as porogens to prepare a SPE-co-EDMA monolithic column.The authors investigated the effect of the amount of initiator on the speed of the polymer reaction and the size of the pores.When the amount of initiator was too low,polymerization speed was very slow,and the reaction did not proceed to a significant extent.In contrast,when the amount of initiator was too large,the rate of monomer polymerization was fast,the polymer pore size was smaller,and the permeability decreased.
In summary,increasing the amount of crosslinking agent produces monoliths with smaller pore sizes and dense structures,whereas reducing the amount of crosslinking agent produces monoliths with larger pores sizes,leading to loose structures.The monomer polymerization speed is slow when less initiator is used,whereas using excessive initiator decreases pore size.
2.3.Porogen choice
An appropriate porogen is very important when preparing polymer monoliths,because the porogen can control the pore properties.The choice of porogen can affect the performance of the monolith significantly.Monoliths prepared with a single porogen have loose pore structures,poor stability,and low separation performance.Thus,two kinds of porogens have been commonly used.The first type of porogen,in which the monomer is soluble,results in polymer monoliths with relatively large pore sizes.The other type porogen,in which the monomer is insoluble,results in a smaller pore structure.In recent years,binary and ternary porogens have often been used in the preparation of monolithic organic polymer columns[30–41](Table 3).
Liu et al.[22]investigated how the choice of porogen impacted column performance,and the results showed that dodecanol and methanol were good and poor solvents,respectively,when 1,12-dodecanediol dimethacrylate(1,12-DoDDMA)was used to prepare the monolith.They also examined the impacts of others porogens,including methanol,isobutanol,toluene,tetrahydrofuran(THF),and acetonitrile(ACN),on the performance of the monolithic column.Toluene,THF,and ACN were found to be"good"solvents,whereas methanol and isobutanol were “poor”solvents for 1,12-DoDDMA.Methanol,isobutanol,and decanol or dodecanol formed a compact porous structure.However,a gel structure was formed when toluene,THF,or ACN was combined with decanol or dodecanol.Although decanol could also be used to prepare monolithic column,the performance of the prepared monolithic column was poor.When isobutanol and dodecanol were combined to prepare a monolithic column,the column pressure was high(more than 3000 psi at a f l ow rate of 100 nL/min).Therefore,methanol and dodecanol were the preferred porogen combination.
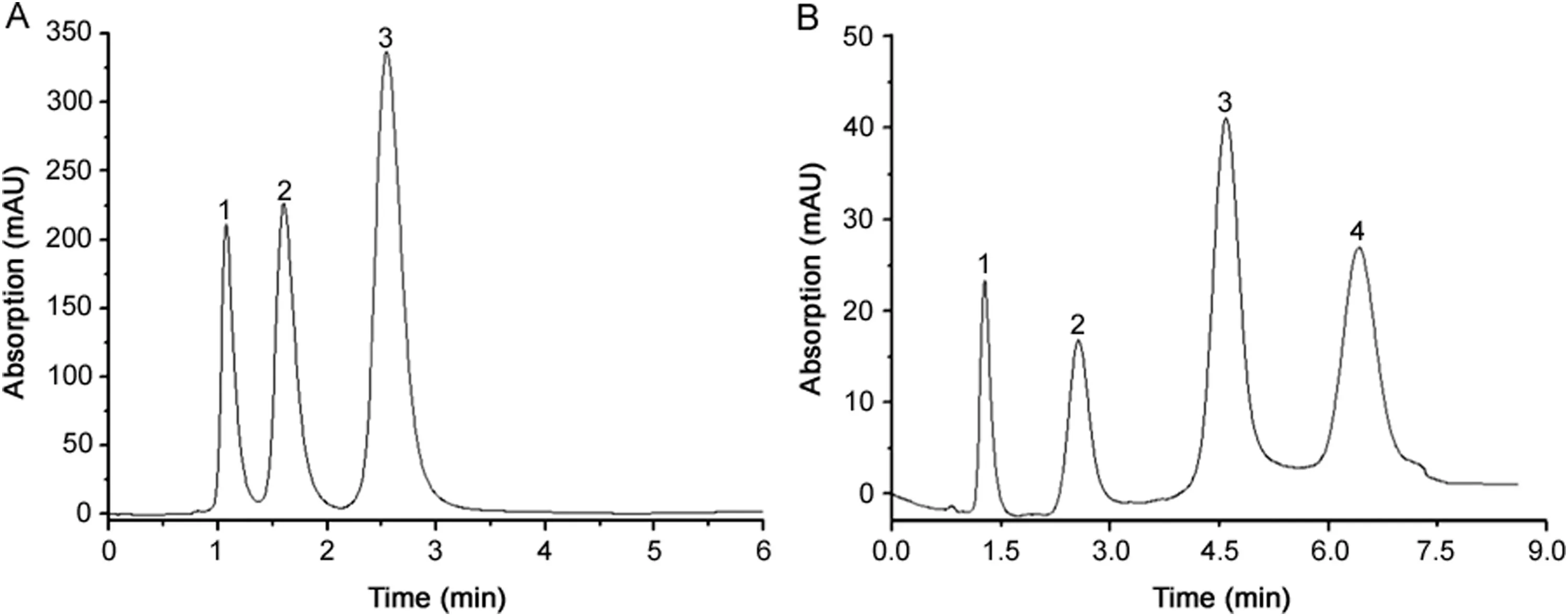
Fig.4.Elution pro fi les of small molecules on a poly(C12-co-VER)column.Conditions:effective length:50 mm×4.6 mm i.d.:mobile phase:methanol:water(75:25,v/v);fl ow rate:1.0 mL/min,UV detection at 254 nm.Samples A:(1)benzoic acid,(2)xylene,(3)p-aminoazobenzene;Samples B:(1)benzene,(2)terephthalic acid,(3)1-naphthol,(4)anthrone[28].
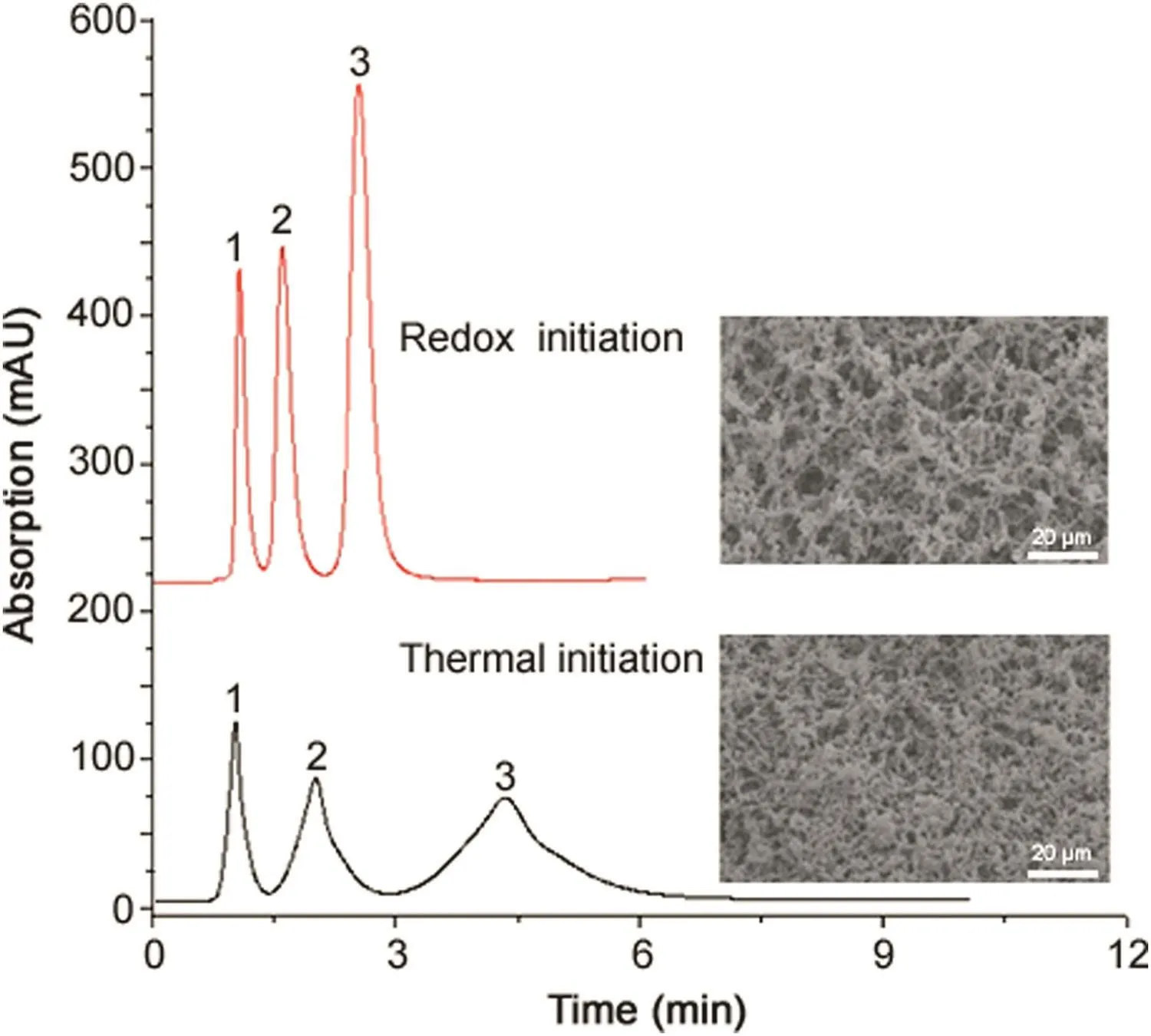
Fig.5.Comparison of monolithic columns obtained using different initiation methods.Analytes:(1)benzoic acid,(2)xylene,(3)p-aminoazobenzene;mobile phase:methanol:water(75:25,v/v)[28].
Gao et al.[37]used[3-(methacryloylamino)propyl]dimethyl(3-sulfopropyl)ammonium hydroxide inner salt(SPP)as a zwitterionic monomer,PETA as a crosslinking agent,and ethanol-ethylene glycol as a porogen to synthesize a hydrophilic SPP-co-PETA monolith,on which phenolic compounds were successfully separated.Then they compared the hydrophilic properties of monolithic columns prepared using various porogens when nuclear glycosides and phenolic compounds were used as model compounds.The results indicated that the monoliths obtained when ethanol-ethylene glycol was used as a porogen had high column efficiency and better separation performance,whereas the monolith obtained when methanol-1,4-butanediol glycol was used as a porogen had good permeability.
In 2014,Li et al.[38]used hexanediol dimethacrylate(HDDA)and butylmethacrylate(BMA)as monomers,EDMA as a crosslinking agent,and dodecyl alcohol as a porogen to prepare a polymer monolith with good permeability,high mechanical stability and a large surface area.Benzene,amines,and phenols were successfully separated on the prepared monolithic column.Subsequently,the effects of different porogens,including dodecanol,isopropyl alcohol,polyethylene glycol,cyclohexanol,and 1,4-butanediol,on the monolithic structure were examined.The results showed that using isopropyl alcohol,polyethylene glycol,cyclohexanol,or 1,4-butanediol as a porogen to prepare the monolith resulted in poor permeability.Dodecanol was the best choice of porogen because the obtained structure had uniform pores and the column had appropriate hardness,good permeability,and low back pressure.Zhong et al.[39]investigated the impact of different porogens on monolithic column preparation.The monolith was too soft to be used as a stationary phase when polyethylene glycol(PEG)200 was used.Further,connections between pores were lacking when a single porogen(1,2-propanediol or 1-hexadecanol)was used.The monoliths obtained using PEG-200 and 1,2-propanediol as co-porogens had a robust granulous structure and low back pressure.The binary porogen realized both full solubility of the monomers and a robust stationary phase for HPLC(Fig.6).
2.4.Polymerization time and temperature
The polymerization temperature controls the pore distribution in a monolith and is thus a very important factor,affecting the formation of the pore structure and the surface area.High temperatures reduce permeability and lead to high back pressures.In contrast,when the polymerization temperature is too low,the reaction speed is slow and the reaction may not proceed.The polymerization time significantly affects the internal structure of a polymer monolith.Longer polymerization times result in further reactions of the monomer,which reduces the polymer pore size and lowers the porosity.
Tong[42]compared the pore size distributions of polymer monoliths prepared using the same polymerization mixture at different temperatures.The results indicated that when the temperature was 70°C,the pore size was about 100nm,whereas when the temperature was 130°C,the pore size was about 1000 nm.If temperature is too low,the polymerization reaction cannot proceed.As the temperature increases,the molecular weight of the polymer will increase and the number of macropores will also increase,which reduces the surface area of the column.However,when the temperature is too high,the reaction speed is too fast.
Niu[26]prepared a poly(VER-co-EDMA)monolith with a three-dimensional network structure when the polymerization temperature was 70 °C or 80 °C.However,the reaction rate was too fast,resulting in a microsphere-packed structure that caused poor permeability.When the reaction temperature was 40 °C or 50 °C,polymerization did not occur.AIBN was used as a radical initiator in this reaction,and as its decomposition temperature was 60–85°C,it could not initiate the polymerization reaction when the reaction temperature was below 60°C.However,when thetemperature was too high,the reaction was not controlled,leading to an uneven column structure.
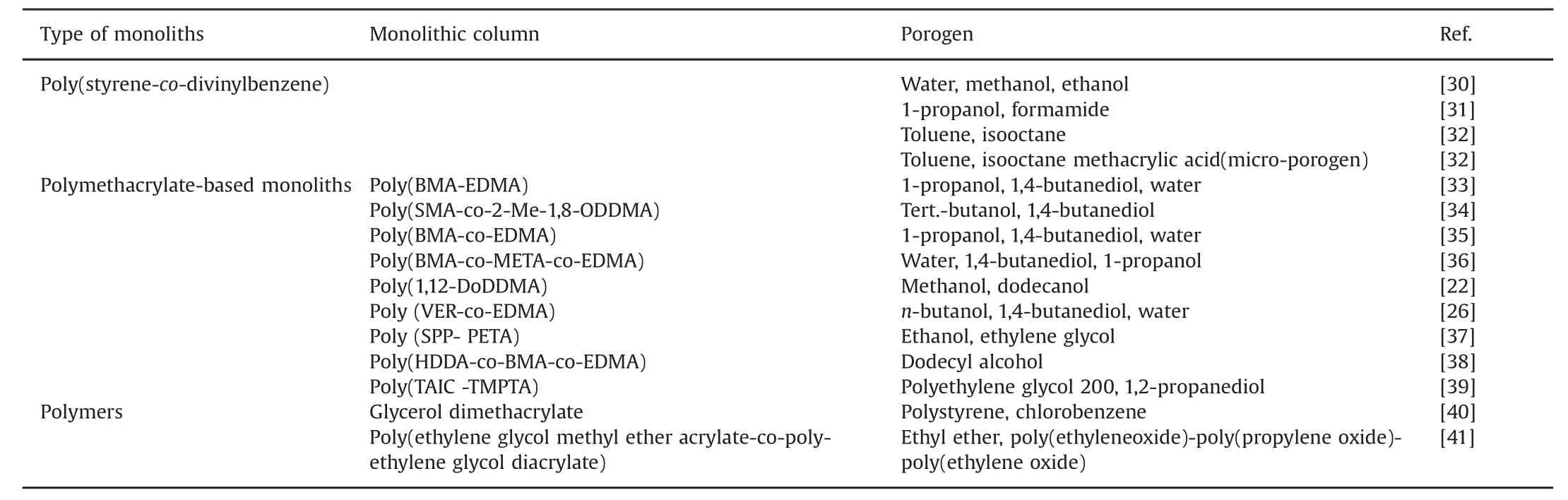
Table 3 Common porogens used in the preparation of monoliths.

Fig.6.SEM photographs of the porous structures obtained with different porogen compositions.(A)PEG-200,(B)1,2-propanediol,(C)1-hexadecanol,and(D)PEG-200/1,2-propanediol(4:9,v/v)[39].
In 2014,Liu et al.[43]used N-isopropylacrylamide(NIPAAm)and TMPTA as monomers and EDMA as a crosslinking agent to prepare polymer monoliths.The column temperature was adjusted to improve the column efficiency for separating small molecules.Increasing the column temperature from 25 °C to 70 °C was found to reduce the retention time of small compounds.Increasing the temperature improves diffusion of the solutes,which can help to achieve rapid separation.However,elution is only affected by the hydrophobicity of the compounds and the character of the stationary phase.As the hydrophobility of nisoldipine is greater than that of nifedipine,nisoldipine has a faster elution rate.Considering the effect of temperature on poly(HDDA-co-BMA-co-EDMA)monolith,polymerization temperatures of 50 °C,60 °C,70 °C,and 80 °C were investigated.When the temperature was 50°C,the pore structure was relatively loose and the stability was poor.When the temperature was 70 °C or 80 °C,the pore structure was too compact and the monolith had a high back pressure.Thus,the ideal temperature for a good pore structure and permeability was 60°C[38].
Nischang and Brüggemann[44]examined the effect of polymerization time on the performance of monolithic poly(BMA-co-EDMA)columns.The authors demonstrated that a monolithic column prepared under incomplete conversion conditions exhibited good performance for the separation of alkylbenzenes,whereas a similar column polymerized for 48h failed completely.They also observed a signif i cant decrease in permeability with increasing polymerization time,as supported by SEM micrographs(Fig.7).
3.Modif i ed polymer monoliths
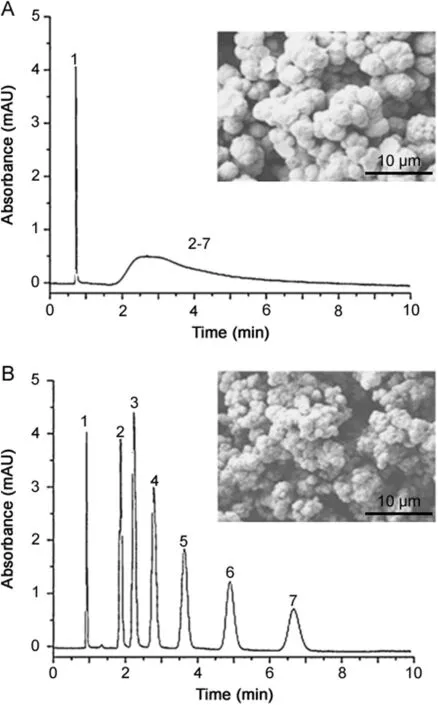
Fig.7.Isocratic separation of alkylbenzenes using monolithic poly(BMA-co-EDMA)capillary columns polymerized for(A)48 h and(B)0.5 h in a 100 μm i.d.capillary.Conditions:column:200 mm × 100 μm i.d.;mobile phase:50%aqueous acetonitrile;flow rate:1.6 μL/min;linear f l ow velocity:4.6mm/s(A)and 3.6 mm/s(B);back pressure:3.92MPa(A)and 1.14 MPa(B).Analytes:(1)uracil,(2)benzene,(3)toluene,(4)ethylbenzene,(5)propylbenzene,(6)butylbenzene,(7)pentylbenzene.The inset SEM micrographs show the morphologies of the monoliths[44].
Although organic polymer monoliths have many advantages,their weak mechanical stabilities and low surface areas are not suitable for the separation of small molecules,which limits their application to pharmaceutical analysis.Hence,many studies have focused on overcoming these disadvantages in recent years.Modification of polymer realizes significant improvements in performance.For example,click chemistry reactions can be used in increasing the surface areas.Monoliths modified using ultra-high crosslinking technologies have large surface areas,good stabilities,and high column efficiencies.Monoliths modif i ed with metal-organic frameworks have a network structure skeletons,adjustable pore sizes,and good thermal and chemical stabilities.Adding nanomaterials into monoliths provides relatively good mechanical stability.There are two main ways to functionalize polymer monoliths.The first method uses functional monomers to prepare polymer monoliths.The second one is the modification after polymerization.Current methods for modifying polymer monoliths include whole column functionalization,hypercrosslinking,incorporation of metal-organicframeworks,and addition of nanomaterials.
3.1.Functionalization of polymer monoliths
3.1.1.Click chemistry and grafting
Click chemistry was proposed by Kolb et al.in 2001[45].Subsequently,thiol-ene and thiol-alkyne click chemistry have also been suggested.The thiol-ene click reaction is a rapid and simple process that has a very important role in the preparation of functional polymers and surface modification[46–48].In particular,over the past two years,the thiol-ene click reaction has been applied in the field of monolith preparation,allowing expansion of the scope of available monomers.Therefore,monolith surface functional groups have become more diverse[49,50].Functional groups can be grafted onto the surface of a monolith by click chemistry,and reports on the use of this method to prepare monolithic columns with high efficiency and good permeability have increased recently.When functional monomers are used to prepare a monolith,the monolith has active surface.Two-step grafting methods have been explored to functionalize monoliths.In the first step,an initiating group is formed under UV irradiation.A hydrogen atom on the surface of the polymer is removed by benzophenone as a grafted initiator,releasing a free radical.In the second step,a reaction occurs with a single monomer.A monolithic column prepared by the two-step grafting method has the function of blocking protein adsorption[51–54].
Tijunelyte et al.[55]prepared a polymeric material using N-acryloxysuccininimide and ethylene dimethacrylate,and then grafted ethylene glycol and mercaptoethanol on the surface using a two-step thiol-ene click reaction.The reaction was carried out at a high temperature.The prepared monolithic column could be used for hydrophilic interaction liquid chromatography(HILIC)and reversed-phase chromatography.Replacement of the allyl amine with propargylamine allowed preparation of a hydrophilic monolith containing amine moieties that could be used for the separation of phenols by HILIC.
Lv et al.[56]used thiol-ene click chemistry to modify the surface of a poly(glycidyl methacrylate/ethylene dimethacrylate)(GMA-EDMA)monolith.The monolith was an excellent substrate for functionalization via the thiol-ene click reaction and suitable for reversed-phase and HILIC separations.
Sun et al.[57]developed a simple one-step in situ “click”modification strategy for the preparation of hydrophobic organic monolithic columns.Compared with the blank column,the stationary phases with higher hydrophobicities obtained by"click"modification had longer retention times with better resolution for five proteins.
3.1.2.Zwitterionic monomers
To obtain high efficiencies for high throughput separation,it is important to consider monomers of different natures.Many types of monomers have been reported,including hydrophilic,ionic,chiral,and zwitterionic monomers.Recently,an increasing number of zwitterionic monomers have been reported that can be used to adjust the surface chemistry of a polymer monolith.Owing to their good chemical and thermal stabilities and pH tolerance,zwitterionic monomers have attracted increasing interest.
In 2012,Gao et al.[37]used SPP as a zwitterionic monomer,PETA as a crosslinking agent,AMPS as an electroosmotic flow donator,AIBN as an initiator,and ethanol-ethylene glycol as a porogen to synthesize a hydrophilic(SPP-co-PETA)monolith,on which phenolic compounds were successfully separated.The use of ethanol-ethylene glycol as the porogen provided the prepared monolithic column with high column efficiency and better separation performance.
In 2014,sulfobetaine-based zwitterionic hydrophilic monoliths were synthesized by using PEG/methanol as a novel binary porogen.The obtained monolithic column exhibited good performance for the separation of nucleosides,phenols,amines,and other polar compounds[58].
In recent years,ionic liquids(ILs)have been used as comonomers to synthesize monolithic columns.IL-based monolithic columns have structural homogeneity of the structure and good column efficiency as a result of the properties of ILs,including high thermal stability,low volatility,good adjustability,high electrolytic conductivity,and miscibility[59].Compared with a column prepared without an IL,the IL-based column has a uniform and porous skeleton structure,with good permeability and performance(Fig.8)[60].
3.2.Hypercrosslinking polymerization technique
The hypercrosslinking polymerization technique was first reported by Davankov et al.[61–63]in the late 1960s.The technique can increase surface areas and has mostly been used to prepare poly(styrene-divinylbenzene).Increasing the surface area of a column will help to improve the performance for separating small molecules[64].
Škeříková and Urban[65]functionalized the surface of monoliths with 4,4´-azobis(4-cyanovaleric acid)(ACVA),followed by surface grafting polymerization of[2-(methacryloyloxy)ethyl]dimethyl(3-sulfopropyl)ammonium hydroxide(MEDSA)as a functional monomer,to prepare hypercrosslinked stationary phases applicable for HILIC.Interestingly,the prepared monolithic columns provided a dual retention mechanism,combining reversedphase and hydrophilic interaction chromatography,controlled by the composition of the mobile phase.The column has been used in 1D and 2D chromatography of polar phenolic compounds.
MayaandSvec[66]hypercrosslinkedpoly(styrene-divinylbenzene)monolithsusing a Fe3+catalyzed Friedel–Crafts reaction involving 4,4′-bis(chloromethyl)-1,1′-biphenyl as an external crosslinker,and the column exhibited good chromatographic performance.The hypercrosslinked column was tested for the isocratic reversed-phase liquid chromatography separation of a mixture comprising acetone and six alkylbenzenes,and the column eff i ciencies for the retained analytes exceeded 70,000 plates/m.
In 2015,Simona[67]used 1,8-diaminooctane to hypercrosslink a poly (styrene-co-vinylbenzyl chloride-co-divinylbenzene)monolithiccolumn viaanucleophilicsubstitution reaction.The concentration of 1,8-diaminoctane,together with the hypercrosslinking time and temperature,was optimized.To improve the permeability of the prepared columns,the hypercrosslinking modification process was combined with early termination of the polymerization reaction and a decreased polymerization temperature.Further,modification of the residual chloromethyl groups with 2-aminoethanesulfonic acid (taurine)provided monolithic columns suitable for separating small polar molecules by HILIC.The prepared column provided a dual-retention mechanism,including hydrophilic interactions and reversed-phase liquid chromatography,that can be controlled by the composition of the mobile phase.The prepared column was successfully used for the isocratic separation of low-molecular-weight phenolic acids.
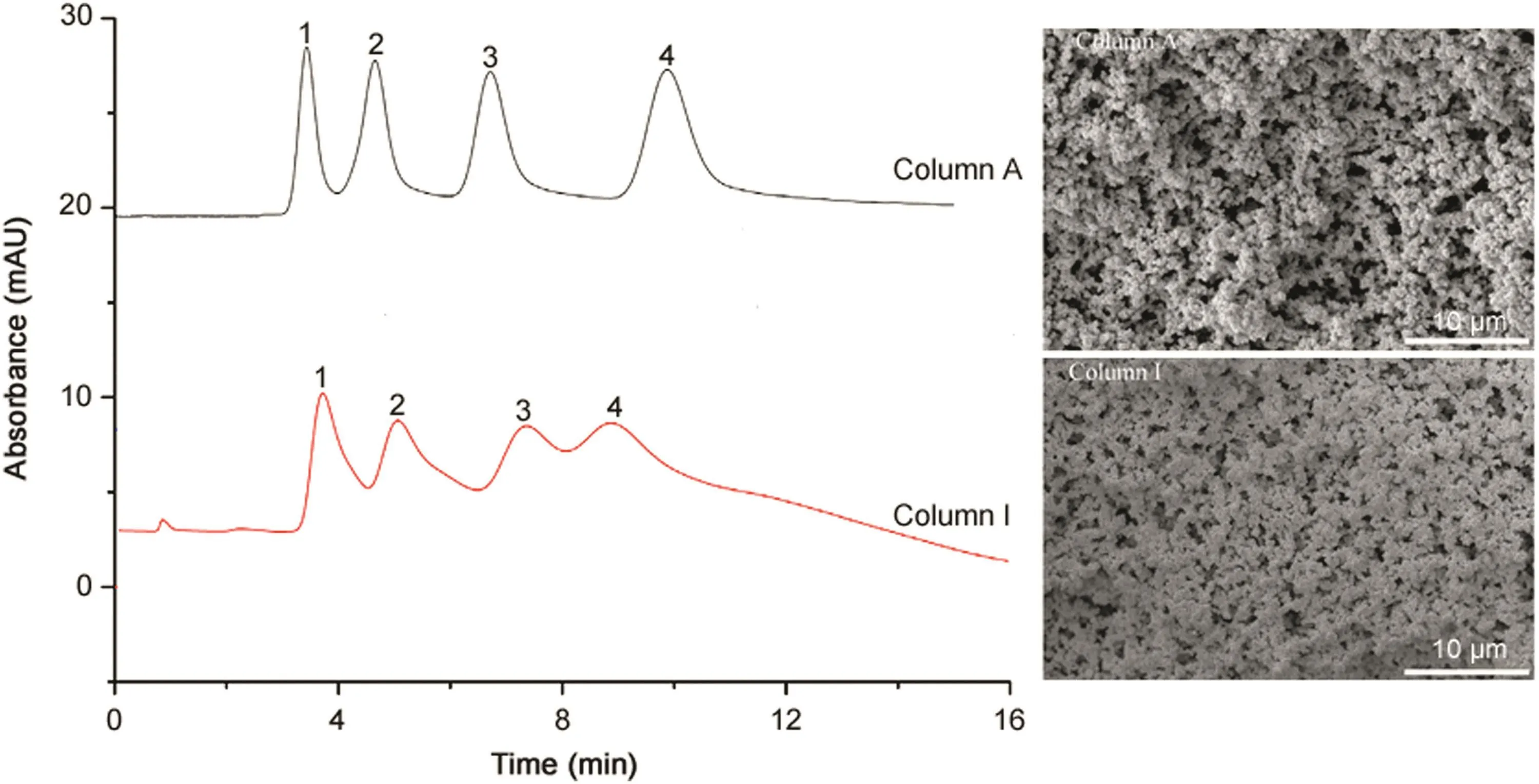
Fig.8.Separation of toluene and its homologues on different monoliths.Conditions:monolith size:50 mm×4.6 mm i.d.;mobile phase:ACN:water(42:58,v/v);f l ow rate:1.0 mL/min;concentration:0.01 mol/L;injection volume:2.0 μL;detection wavelength:254 nm.Analytes:(1)toluene,(2)ethylbenzene,(3)propylbenzeneand,(4)butylbenzene.Column A:IL-based monolithic column;column I:without IL.The inset SEM micrographs show the morphologies of the monoliths[60].
Until now,hypercrosslinking has only been demonstrated with styrene-based monoliths and it has been proven useful for separating small molecules in the reversed-phase and HILIC modes.Although several other polymers,such as polyaniline,polypyrrole,polyarylate,polyxylylene,polyamide,and polypyridine,have already been hypercrosslinked[68],they have not yet been prepared as monolithic columns.Inaddition,thedevelopmentofhypercrosslinking polymerization methods is required for the preparation of polyacrylate-and polymethacrylate-based monolithic columns.
3.3.Metal-organic frameworks
Metal-organic frameworks(MOFs),also called porous coordination polymers,are a new class of hybrid inorganic-organic microporous crystalline materials self-assembled from metal ions and organic linkers via coordination bonds[69].Owing to their fascinating structures and unusual properties,such as large surface areas,structural diversity,good thermal and chemical stabilities,uniform and regular pore sizes,and the availability of framework functionality,MOFs have great potential for separation applications[70].
In 2013,Fu et al.[71]investigated the incorporation of MOF UiO-66 into a porous poly(MAA-co-EDMA)monolith to enhance the HPLC separation of small molecules with high column efficiency and good reproducibility.The introduction of UiO-66 increased the strength of hydrophobic interactions between the analyte and the monolithic column,resulting in longer retention times.Huang et al.[72]prepared the MOF MIL-101(Cr),which has a large surface area and good stability.Then they prepared a MOF monolith by proportionate mixing of MIL-101(Cr),BMA,EDMA,AMPS,and AIBN.Compared with the column without MOF modification,the MOF monolith column had a larger surface area,improved permeability,and a nanoporous structure,which allowed the efficient separation of xylene isomers.
MOF HKUST-1 nanoparticles have been incorporated into poly(glycidylmethacrylate-co-ethylene dimethacrylate)(HKUST-1-poly(GMA-co-EDMA))monoliths to afford stationary phases with enhanced chromatographic performance for small molecule separation in the reversed-phase capillary liquid chromatography.While the bare poly(GMA-co-EDMA)monolith exhibited poor resolution(Rs < 1.0)and low efficiency(800–16,300plates/m),the addition of a small amount of HKUST-1 nanoparticles to the polymerization mixture provided increased resolution(Rs≥1.3)and high efficiency,ranging from 16,300 to 44,300 plates/m[73].
3.4.Nanomaterials
Nanotechnology had a major impact and promoted advances in medicine,biology,environmental science,energy,electronics,and other fields[74,75].In recent years,gold nanoparticles,carbon nanotubes,and other nanomaterials have been used in functionalized organic polymer monoliths.The introduction of nanomaterials,such as carbon nanotubes and graphene,has revealed the potential for such materials in the enrichment and separation of complex samples[76,77].Since the discovery of zero-dimensional fullerenes,researchers have been particularly concerned with the use of carbon nanomaterials to overcome the poor mechanical stability of polymer monoliths[78–80].
3.4.1.Metal nanoparticles
Nanoparticles (NPs) are ultra fine particles with sizes of 1–100 nm.The particle size decreases rapidly as the ratio of surface atoms in a NP and the total number of atoms increases,resulting in a strong volume effect(small size effect),the quantum size effect,and surface and macroscopic quantum tunneling effects.
A novel approach has been developed for porous polymer monoliths hypercrosslinked to obtain large surface areas and modified with zwitterionic functionalities through the attachment of Au NPs in a layered architecture.The combination of hypercrosslinking hydrophobic poly(4-methylstyrene-co-vinylbenzene chloride-co-divinylbenzene)monoliths with a hydrophilic layered structure including Au NPs embedded in a polyethyleneimine layer and functionalized with cysteine enabled the preparation of a very efficient monolithic stationary phase for the separation of small molecules by HILIC.A column efficiency of 51,000 plates/m was achieved for cytosine[81].
A porous polymethacrylate ester-based monolith was prepared using BMA and EDMA in a binary porogenic solvent of 1,4-butanediol and 1-propanol[82].Injecting a Au NP colloid into the monolith resulted in a homogeneous coverage of Au NPs,which were physically adsorbed on the monolith surface.The Au NP-modified poly(BMA-EDMA)monolith was found to show obvious SERS enhancement,with the SERS activity dependent on the size of the Au NPs.
3.4.2.Carbon nanotubes
Carbon nanotubes can be used to prepare monoliths for the efficient separation of various compounds.The surfaces of such monoliths are strongly hydrophobic.Hence,monoliths containing carbon nanotubes are very useful as stationary phases for the reversed-phase separation of small molecules.Owing to the unique characteristics of nanoparticles,such as their large surface-to-volume ratios and their properties that differ from those of the corresponding bulk materials,the application of nanomaterials in separation science is growing[83–85].
Li et al.[86]prepared a poly(vinylbenzyl chloride-ethylene dimethacrylate)(VBC-EDMA)column that incorporated singlewall carbon nanotubes(SWNT).The retention behavior of neutral compounds on the poly(VBC-EDMA-SWNT)monolith was examined by separating a mixture of small organic molecules using micro-HPLC.The results indicated that the incorporation of SWNT enhanced the chromatographic retention of small neutral mole-cules in reversed-phase HPLC presumably because of their strongly hydrophobic characteristics.
Multiwalled carbon nanotubes have been entrapped in monolithic poly(glycidyl methacrylate-co-ethylene dimethacrylate)columns to afford a stationary phase with enhanced performance for the separation of small molecules by reversed-phase chromatography[87].While the column with no nanotubes exhibited an efficiency of only 1800plates/m,the addition of a small amount of nanotubes to the polymerization mixture increased the efficiency to over 35,000plates/m.The addition of THF to the typical aqueous ACN eluent improved the peak shape and increased the column efficiency to 44,000 plates/m,as calculated for the benzene peak.
In 2015,Qi[88]prepared two polymer monolithic columns.One was modified by In2O3NPs and the other was modified by 3-trimethoxysilyl propyl methacrylate(γ-MAPS)and sodium titanate nanotubes(NaTiNTs).The prepared monolithic columns were used to determine organic residues in food by HPLC or HPLCMS/MS.
In 2012,Li et al.[89]used GMA as a monomer and EDMA as a crosslinking agent to prepare a polymer monolith containing carbon nanotubes.The column efficiency was considerably improved compared with that of the polymer monolith without adding carbon nanotubes.Benzenes,alkyl ketones,and other small molecules were successfully separated on the prepared monolithic column.
3.4.3.Graphene
Graphene(GN)is a two-dimensional sp2-hybridized nanocarbon material.Similar to metal NPs,carbon nanotubes,and other nanocarbon materials,GN can be incorporated into polymer monoliths to modify the surface of the polymer monolith and improve the efficiency for the separation of small molecules.However,polymer monoliths modified with GN and used for the separation of small molecules have rarely been reported.Therefore,further investigations of GN-modified monoliths are required to determine the possible potential applications and advantages of such materials.
In 2013,Li et al.[90]used graphene oxide as a crosslinking agent to prepare a methyl acrylate polymer monolith from GMA and EDMA.The prepared monolith was used as a HPLC stationary phase for the isocratic separation of model compounds,such as hydrophobic steroids and polar aniline.Compared with the polymer monolith that prepared by a traditional method,the cofunctionalized graphene oxide polymer monolith showed greatly improved ability for the separation of model small molecules.This study reported a new preparation method for polymer monoliths containing nanomaterials.
In 2015,Zheng[91]prepared a poly(BMA-co-EDMA-ALA-β-CD-Cu2O)monolith by using graphene and beta-cyclodextrin(β-CD).Owing to the addition of inorganic nanomaterials and GN,the polymer monolith exhibited considerable improvement in separation effects.The polymer monolith had a homogeneous structure with good penetrability.Under certain conditions,the prepared monolith showed enrichment efficiency for the trace analysis of polychlorinated biphenyls(PCBs)in wine samples.
4.Conclusions and outlook
Over the past few years,the technology for preparing polymer monoliths has been developed.Many achievements have been made in methods for preparing polymer monoliths that are suitable for the separation of small molecules.Many polymer monoliths have been successfully prepared with high column efficiency,good permeability and high efficiency for the separation of small molecules.In this review,we have summarized the methods reported in recent studies,such as carefully selecting the monomer and crosslinker;adjusting the ratio of monomer,crosslinking agent,and porogen;controlling polymerization temperatureand polymerization time;modifying thepolymer monolithic column using click chemistry and boron affinity technology;using zwitterionic monomers and super-high crosslinking technology;and adding nanomaterials,which can be applied to achieve uniform columns with compact pore structures and sufficiently large surface areas.
Although some defects that made monoliths unsuitable for the separation of small molecules have been improved using these methods,there are still some problems.We can know from the reported literatures that it is easy for organic polymer monolithic column to form accumulation structure in the process of reaction,which lead to unexpected pores structure of monolithic columns.This is still a problem to be solved in the preparation of a polymer monolithic column.Therefore,research efforts are still being directed toward obtaining uniform,compact network structures when preparing polymer monoliths.
The preparation of polymer monoliths containing nanomaterials is a promising research area.Although some examples of such polymer monolithic columns have been reported in recent years,most of them are monolithics silica columns containing nanomaterials.Relatively few polymer monoliths containing nanomaterials have been reported.However,the addition of nanomaterials improves the separation performance of polymer monoliths,realizing columns with good reproducibility and selectivity.Although many optimized methods for preparing polymer monoliths have been reported,they are not always simple and efficient.Thus,there is considerably scope for improving the preparation methods for polymer monoliths.With rapid developments in science and technology,we will gain a deeper understanding of important problems in monolith polymerization processes,such as the process of forming the pore structure and the factors that affect pore formation and swelling.In this way,we will not only be able to improve reported methods,but also discover new,more convenient,and efficient ways to prepare polymer monoliths that can be used to separate small molecules.
The requirements for separating complex matrices are becoming more stringent,for example,environmental issues received considerable worldwide attention owing to their direct influence on human health.Therefore,it is very important to find methods for the effective and selective enrichment of pollutants.Modified polymer monoliths may be applicable to this important task.For example,polymer monoliths modified with large ring polyamine compounds have enhanced abilities for ion exchange and hydrophobic interactions,which can largely improve the selective enrichment performance and the enrichment efficiency.As polymer monoliths have increasingly played an important role in the fields of medicine and environmental science,the preparation of these materials has become an important research subject.Further theoretical and practical insight for the development of new application areas and the separation of actual samples will allow new methods to be established for the preparation of polymer HPLC monolithic column for the separation of small molecules drugs.
Conflicts of interest
The authors declare that there are no conflicts of interest.
[1]F.Svec,Organic polymer monoliths as stationary phases for capillary HPLC,J.Sep.Sci.27(2004)1419–1430.
[2]R.D.Arrua,M.Talebi,T.J.Causon,et al.,Review of recent advances in the preparation of organic polymer monoliths for liquid chromatography of large molecules,Anal.Chim.Acta 738(2012)1–12.
[3]K.Liu,P.Aggarwal,J.S.Lawson,et al.,Organic monoliths for high-performance reversed-phase liquid chromatography,J.Sep.Sci.36(2013)2767–2781.
[4]Z.Liu,J.Ou,Z.Liu,J.Liu,et al.,Separation of intact proteins by using polyhedral oligomeric silsesquioxane based hybrid monolithic capillary columns,J.Chromatogr.A 1317(2013)138–147.
[5]K.Cabrera,D.Lubda,H.M.Eggenweiler,et al.,R&D biochemistry and separation,Merck KgaA:A new monolithic-type HPLC column for fast separations,J.High Resolut.Chromatogr.23(2000)93–99.
[6]N.Ishizuka,H.Minakuchi,K.Nakanishi,et al.,Chromatographic properties of miniaturized silica rod columns,J.High Resolut.Chromatogr.21(1998)477–479.
[7]Z.Lin,X.Tan,R.Yu,et al.,One-pot preparation of glutathione–silica hybrid monolith for mixed-mode capillary liquid chromatography based on"thiolene"click chemistry,J.Chromatogr.A 1355(2014)228–237.
[8]P.Jandera,Advances in the development of organic polymer monolithic columns and their applications in food analysis―a review,J.Chromatogr.A 1313(2013)37–53.
[9]J.D.Hayes,A.Malik,Sol–gel monolithic columns with reversed electroosmotic flow for capillary electrochromatography,Anal.Chem.72(2000)4090–4099.
[10]Z.Liu,J.Ou,H.Lin,et al.,Preparation of polyhedral oligomeric silsesquioxanebased hybrid monolith by ring-opening polymerization and post-functionalization via thiol-ene click reaction,J.Chromatogr.A 1342(2014)70–77.
[11]H.Wang,J.Ou,H.Lin,et al.,Chromatographic assessment of two hybrid monoliths prepared via epoxy-amine ring-opening polymerization and methacrylate-based free radical polymerization using methacrylate epoxy cyclosiloxane as functional monomer,J.Chromatogr.A 1367(2014)131–140.
[12]Z.Zhang,F.Wang,B.Xu,et al.,Preparation of capillary hybrid monolithic column with sulfonate strong cation exchanger for proteome analysis,J.Chromatogr.A 1256(2012)136–143.
[13]F.Svec,Quest for organic polymer-based monolithic columns affording enhanced efficiency in high performance liquid chromatography separations of small molecules in isocratic mode,J.Chromatogr.A 1228(2012)250–262.
[14]J.Seidl,J.Malinský,K.Dušek,et al.,Makroporöse Styrol-Divinylbenzol-Copolymere und ihre Verwendung in der Chromatographie und zur Darstellung von Ionenaustauschern,Adv.Polym.Sci.5(1967)113–213.
[15]G.Guiochon,Monolithic columns in high-performance liquid chromatography,J.Chromatogr.A 1168(2007)101–168.
[16]M.J.Gray,P.J.Slonecker,G.Dennis,A column capacity study of single,serial,and parallel linked rod monolithic high performance liquid chromatography columns,J.Chromatogr.A 1096(2005)92–100.
[17]Z.Xu,L.Yang,Q.Wang,Different alkyl dimethacrylate mediated stearyl methacrylate monoliths for improving separation efficiency of typical alkylbenzenes and proteins,J.Chromatogr.A 1216(2009)3098–3106.
[18]A.Maruška,A.Rocco,O.Komyšova,et al.,Synthesis and evaluation of polymeric continuous bed(monolithic)reversed-phase gradient stationary phases for capillary liquid chromatography and capillary electrochromatography,J.Biochem.Biophys.Methods 70(2007)47–55.
[19]X.M.Bai,Study on the Preparation and Application of TMPTA Monolithic Columns,Heibei University,Baoding,China,2014.
[20]H.Y.Liu,Y.M.Ma,X.Qin,et al.,Preparation of poly dodecone monolithic column for HPLC separations of small molecules,J.Hebei Univ.:Nat.Sci.Ed.35(2015)147–152.
[21]A.Greiderer,L.Trojer,C.W.Huck,et al.,Influence of the polymerisation time on the porous and chromatographic properties of monolithic poly(1,2-bis(p-vinylphenyl))ethane capillary columns,J.Chromatogr.A 1216(2009)7747–7754.
[22]K.Liu,H.D.Tolley,M.L.Lee,Highly crosslinked polymeric monoliths for reversed-phase capillary liquid chromatography of small molecules,J.Chromatogr.A 1227(2012)96–104.
[23]S.H.Lubbad,M.R.Buchmeiser,Fast separation of low molecular weight analytes on structurally optimized polymeric capillary monoliths,J.Chromatogr.A 1217(2010)3223–3230.
[24]Y.Li,H.D.Tolley,M.L.Lee,Preparation of monoliths from single crosslinking monomers for reversed-phase capillary chromatography of small molecules,J.Chromatogr.A 1218(2011)1399–1408.
[25]I.Nischang,I.Teasdale,O.Brüggemann,Towards porous polymer monoliths for the efficient,retention-independent performance in the isocratic separation of small molecules by means of nano-liquid chromatography,J.Chromatogr.A 1217(2010)7514–7522.
[26]W.J.Niu,Preparation and Evaluation of Capillary Columns of Poly(vinyl ester resin),Heibei University,Baoding,China,2013.
[27]D.Wei,The Preparation and Application of Poly(PETA-co-TAIC)and poly(modified epoxy acrylate resin)Monolithic Columns,Heibei University,Baoding,China,2014.
[28]M.B.Hao,Preparation of Poly C12 Monolith and its Application in the Separation of Benzene Series,Heibei University,Baoding,2014.
[29]A.Dou,C.Zou,Y.Shan,et al.,Preparation and application of mixed-mode capillary polymer monolithic column,Chin.J.Chromatogr.32(2014)447–451.
[30]I.Gusev,X.Huang,C.Horváth,Capillary columns with in situ formed porous monolithic packing for micro high-performance liquid chromatography and capillary electrochromatography,J.Chromatogr.A 855(1999)273–290.
[31]Z.Kucerová,M.Szumski,B.Buszewski,et al.,Alkylated poly(styrene-divinylbenzene)monolithic columns for mu-HPLC and CEC separation of phenolic acids,J.Sep.Sci.30(2007)3018–3026.
[32]A.Svobodová,T.Křížek,J.Sirc,et al.,Monolithic columns based on a poly(styrene-divinylbenzene-methacrylic acid)copolymer for capillary liquid chromatography of small organic molecules,J.Chromatogr.A 1218(2011)1544–1547.
[33]P.Coufal,M.Cihák,J.Suchánková,et al.,Methacrylate monolithic columns of 320μm I.D.for capillary liquid chromatography,J.Chromatogr.A 946(2002)99–106.
[34]Z.Xu,L.Yang,Q.Wang,Different alkyl dimethacrylate mediated stearyl methacrylate monoliths for improving separation efficiency of typical alkylbenzenes and proteins,J.Chromatogr.A 1216(2009)3098–3106.
[35]D.Moravcová,P.Jandera,J.Urban,et al.,Characterization of polymermonolithic stationary phases for capillary HPLC,J.Sep.Sci.26(2003)1005–1016.
[36]Y.Huo,P.J.Schoenmakers,W.T.Kok,Efficiency of methacrylate monolithic columns in reversed-phase liquid chromatographic separations,J.Chromatogr.A 1175(2007)81–88.
[37]Y.Gao,Y.Wang,C.Wang,et al.,Preparation of new hydrophilic monolithic columns and their applications in capillary liquid chromatography and pressurized capillary electrochromatography,Chin.J.Chromatogr.30(2012)487–494.
[38]R.Li,J.Zhong,M.Hao,et al.,Preparation of a porous functional polymer and its application in the separation of small molecules in conjunction with HPLC,Anal.Methods 6(2014)589–595.
[39]J.Zhong,M.Hao,R.Li,et al.,Preparation and characterization of poly(triallyl isocyanurate-co-trimethylolpropane triacrylate)monolith and its applications in the separation of small molecules by liquid chromatography,J.Chromatogr.A 1333(2014)79–86.
[40]H.Aoki,T.Kubo,T.Ikegami,et al.,Preparation of glycerol dimethacrylatebased polymer monolith with unusual porous properties achieved via viscoelastic phase separation induced by monodisperse ultra high molecular weight poly(styrene)as a porogen,J.Chromatogr.A 1119(2006)66–79.
[41]Y.Li,H.D.Tolley,M.L.Lee,Preparation of polymer monoliths that exhibit size exclusion properties for proteins and peptides,Anal.Chem.81(2009)4406–4413.
[42]S.S.Tong,Development of Novel Separation and Enrichment Approaches Based on Carbon Nanomaterials,Jilin University,Changchun,China,2013.
[43]H.Liu,X.Bai,D.Wei,et al.,High-performance liquid chromatography separation of small molecules on a porous poly(trimethylol propane triacrylateco–N-isopropylacrylamide-co-ethylene dimethacrylate)monolithic column,J.Chromatogr.A 1324(2014)128–134.
[44]I.Nischang,O.Brüggemann,On the separation of small molecules by means of nano-liquid chromatography with methacrylate-based macroporous polymer monoliths,J.Chromatogr.A 1217(2010)5389–5397.
[45]H.C.Kolb,M.G.Finn,K.B.Sharpless,Click chemistry:diverse chemical function from a few good reactions,Angew.Chem.Int.Ed.Engl.40(2001)2004–2021.
[46]G.Jian,Y.Liu,X.He,et al.,Click chemistry:a new facile and efficient strategy for the preparation of Fe3O4nanoparticles covalently functionalized with IDACu and their application in the depletion of abundant protein in blood samples,Nanoscale 4(2012)6336–6342.
[47]J.E.Moses,A.D.Moorhouse,The growing applications of click chemistry,Chem.Soc.Rev.36(2007)1249–1262.
[48]M.Slater,M.Snauko,F.Svec,et al.,“Click Chemistry”in the preparation of porous polymer-based particulate stationary phases for μ-HPLC separation of peptides and proteins,Anal.Chem.78(2006)4969–4975.
[49]H.Huang,Y.Jin,M.Xue,,et al.,A novel click chitooligosaccharide for hydrophilic interaction liquid chromatography,Chem.Commun.45(2009)6973–6975.
[50]L.Moni,A.Ciogli,I.D'Acquarica,et al.,Synthesis of sugar-based silica gels by copper-catalysed azide–alkyne cycloaddition via a single-step azido-activated silica intermediate and the use of the gels in hydrophilic interaction chromatography,Chemistry 16(2010)5712–5722.
[51]T.Suksrichavalit,K.Yoshimatsu,V.Prachayasittikul,et al.,“Clickable”aff i nity ligands for effective separation of glycoproteins,J.Chromatogr.A 1217(2010)3635–3641.
[52]Z.Liu,J.Ou,H.Lin,et al.,Preparation of monolithic polymer columns with homogeneous structure via photoinitiated thiol-yne click polymerization and their application in separation of small molecules,Anal.Chem.86(2014)12334–12340.
[53]H.Lin,J.Ou,Z.Liu,et al.,Thiol-epoxy click polymerization for preparation of polymeric monoliths with well-def i ned 3D framework for capillary liquid chromatography,Anal.Chem.87(2015)3476–3483.
[54]L.Chen,J.Ou,Z.Liu,et al.,Fast preparation of a highly efficient organic monolith via photo-initiated thiol-ene click polymerization for capillary liquid chromatography,J.Chromatogr.A 1394(2015)103–110.
[55]I.Tijunelyte,J.Babinot,M.Guerrouache,et al.,Hydrophilic monolith with ethylene glycol-based grafts prepared via surface confined thiol-ene click photoaddition,Polymer 1(2012)29–36.
[56]Y.Lv,Z.Lin,F.Svec,“Thiol–ene”click chemistry:a facile and versatile route for the functionalization of porous polymer monoliths,Analyst 137(2012)4114–4118.
[57]X.Sun,D.Lin,X.He,et al.,A facile and efficient strategy for one-step in situ preparation of hydrophobic organic monolithic stationary phases by click chemistry and its application on protein separation,Talanta 82(2010)404–408.
[58]Y.Kuang,Synthesis and chromatographic evaluation of sulfobetaine-based capillary zwitterionic hydrophilic monolithic column using a binary porogenic agent of polyethylene glycol/methanol,Se Pu 32(2014)388–394.
[59]M.Armand,F.Endres,D.R.MacFarlane,et al.,Ionic-liquid materials for the electrochemical challenges of the future,Nat.Mater.8(2009)621–629.
[60]H.Zhang,L.Bai,Z.Wei,et al.,Fabrication of an ionic liquid-based macroporous polymer monolithic column via atom transfer radical polymerization for the separation of small molecules,Talanta 149(2016)62–68.
[61]V.A.Davankov,S.V.Rogozhin,M.P.Tsyurupa,Influence of polymeric matrix structure on performance of ion-exchange resins,Solvent Extr.Ion.Exc.,1977:29–81.
[62]V.A.Davankov,M.P.Tsyurupa,Structure and properties of hypercrosslinked polystyrene—the f i rst representative of a new class of polymer networks,React.Polym.13(1990)27–42.
[63]V.A.Davankov,M.Tsyurupa,M.Ilyin,et al.,Hypercross-linked polystyrene and its potentials for liquid chromatography:a mini-review,J.Chromatogr.A 965(2002)65–73.
[64]A.V.Pastukhov,M.P.Tsyurupa,V.A.Davankov,Hypercrosslinked polystyrene:a polymer in a non-classical physical state,J.Polym.Sci.Polym.Phys.37(1999)2324–2333.
[65]V.Škeříková,J.Urban,Highly stable surface modif i cation of hypercrosslinked monolithic spillary columns and their application in hydrophilic interaction chromatography,J.Sep.Sci.36(2013)2806–2812.
[66]F.Maya,F.Svec,A new approach to the preparation of large surface area poly(styrene-co-divinylbenzene)monoliths via knitting of loose chains using external crosslinkers and application of these monolithic columns for separation of small molecules,Polymer 55(2014)340–346.
[67]S.Janků,V.Škeříková,J.Urban,Nucleophilic substitution in preparation and surface modif i cation of hipercrosslinked stationary phases,J.Chromatogr.A 1388(2015)151–157.
[68]V.Davankov,M.Tsyurupa,Hypercrosslinked Polymeric Networks and Adsorbing Materials,Elsevier 2011:315–358.
[69]Y.Y.Fu,Metal-organic Frameworks and its Composites for Liquid Chromatographic Separation,Nankai University,Tianjin,China,2013.
[70]S.Yang,F.Ye,C.Zhang,et al.,In situ synthesis of metal–organic frameworks in a porous polymer monolith as the stationary phase for capillary liquid chromatography,Analyst 140(2015)2755–2761.
[71]Y.Y.Fu,C.X.Yang,X.P.Yan,Incorporation of metal–organic framework UiO-66 into porous polymer monoliths to enhance the liquid chromatographic separation of small molecules,Chem.Commun.49(2013)7162–7164.
[72]H.Y.Huang,C.L.Lin,C.Y.Wu,et al.,Metal organic framework–organic polymer monolith stationary phases for capillary electrochromatography and nano-liquid chromatography,Anal.Chim.Acta 779(2013)96–103.
[73]S.Yang,F.Ye,Q.Lv,et al.,Incorporation of metal-organic framework HKUST-1 into porous polymer monolithic capillary columns to enhance the chromatographic separation of small molecules,J.Chromatogr.A 1360(2014)143–149.
[74]A.Z.Zhang,G.F.Ye,J.Y.Lu,et al.,Preparation and characterization of polymer solid phase microextraction monolith modif i ed with gold nanoparticle,Chin.J.Anal.Chem.39(2011)1247–1250.
[75]Z.Zhang,Z.Wang,Y.Liao,et al.,Applications of nanomaterials in liquid chromatography:opportunities for separation with high eff i ciency and selectivity,J.Sep.Sci.29(2006)1872–1878.
[76]C.Nilsson,S.Birnbaum,S.Nilsson,Use of nanoparticles in capillary and microchip electrochromatography,J.Chromatogr.A 1168(2007)212–224.
[77]C.Wu,Y.Liang,Q.Zhao,et al.,Boronate affinity monolith with a gold nanoparticle modified hydrophilic polymer as a matrix for the highly specif i c capture of glycoproteins,Chemistry 20(2014)8737–8743.
[78]S.Wang,C.Liu,H.Wang,et al.,A surface-enhanced Raman scattering optrode prepared by in situ photoinduced reactions and its application for highly sensitive on-chip detection,ACS Appl.Mater.Interfaces 6(2014)11706–11713.
[79]Y.C.Pan,X.Wang,H.Zhang,et al.,Gold-nanoparticle functionalized–poro uspolymer monolith enclosed in capillary for on-column SERS detection,Anal.Methods 7(2015)1349–1357.
[80]O.Sedlacek,J.Kucka,F.Svec,et al.,Silver-coated monolithic columns for separation in radiopharmaceutical applications,J.Sep.Sci.37(2014)798–802.
[81]Y.Lv,Z.Lin,F.Svec,Hypercrosslinked large surface area porous polymer monoliths for hydrophilic interaction liquid chromatography of small molecules featuring zwitterionic functionalities attached to gold nanoparticles held in layered structure,Anal.Chem.84(2012)8457–8460.
[82]H.Ren,L.Liu,H.Liu,et al.,Preparation of porous polymer monolith modified with gold nanoparticles and on-column surface-enhanced Raman spectroscopy detection,J.Hubei Univ.36(2014)238–247.
[83]Z.Zhang,B.Yan,Y.Liao,et al.,Nanoparticle:is it promising in capillary electrophoresis?Anal.Bioanal.Chem.391(2008)925–927.
[84]C.Nilsson,S.Nilsson,Nanoparticle-based pseudostationary phases in capillary electrochrom atography,Electrophoresis 27(2006)76–83.
[85]C.D.Tran,S.Challa,Fullerene-impregnated ionic liquid stationary phases for gas chromatography,Analyst 133(2008)455–464.
[86]Y.Li,Y.Chen,R.Xiang,et al.,Incorporation of single-wall carbon nanotubes into an organic polymer monolithic stationary phase for μ-HPLC and capillary electrochromatography,Anal.Chem.77(2005)1398–1406.
[87]S.D.Chambers,F.Svec,J.M.Fréchet,Incorporation of carbon nanotubes in porous polymer monolithic capillary columns to enhance the chromatographic separation of small molecules,J.Chromatogr.A 1218(2011)2546–2552.
[88]R.F.Qi,Preparation of Monolithic Polymeric Column Modif i ed by Nanomaterials and its Application in Analysis of Organic Residues in Food,Jilin University,Changchun,China,2015.
[89]Q.Li,Y.Du,H.Tang,et al.,Ultra sensitive surface-enhanced Raman scattering detection based on monolithic column as a new type substrate,J.Raman Spectrosc.43(2012)1392–1396.
[90]Y.Li,L.Qi,H.Ma,et al.,Preparation of porous polymer monolithic column using functionalized graphene oxide as a functional crosslinker for high performance liquid chromatography separation of small molecules,Analyst 138(2013)5470–5478.
[91]H.J.Zheng,Preparation of Macrocyclic Compound Modif i ed Polymer Monolithic Column and its Application in Separation and Analysis,Jilin University,Changchun,China,2015.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Novel degradation products of argatroban:Isolation,synthesis and extensive characterization using NMR and LC-PDA-MS/Q-TOF
- Physicochemical characterization,the Hirshfeld surface,and biological evaluation of two meloxicam compounding pharmacy samples
- Structural confirmation of sulconazole sulfoxide as the primary degradation product of sulconazole nitrate
- Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L.fruit:In silico and in vivo approaches
- Quantitative determination of erlotinib in human serum using competitive enzyme-linked immunosorbent assay
- Ultrasensitive electrochemical determination of metronidazole based on polydopamine/carboxylic multi-walled carbon nanotubes nanocomposites modified GCE
