Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L.fruit:In silico and in vivo approaches
2018-04-17PrhuSrinivsnVijykumrSwminthnKothndrmnMnogrPlni
Prhu Srinivsn,S.Vijykumr,*,Swminthn Kothndrmn,Mnogr Plni
aComputational Phytochemistry Lab,PG and Research Department of Botany and Microbiology,A.V.V.M Sri Pushpam College(Autonomous),Poondi 613 503,Tamil Nadu,India
bDepartment of Physics,RKM Vivekananda College(Autonomous),Mylapore,Chennai 600 004,India
1.Introduction
Diabetes mellitus(DM)is a metabolic disorder characterized by hyperglycemia resulting from defects in insulin action,insulin secretion from pancreatic beta cells,or both[1].In addition,DM is a group of diseases characterized by chronic hyperglycemia due to deficiency of insulin action,a common basis of diabetes that leads to characteristic abnormalities in the metabolism of carbohydrate,lipid,and protein[2].The total number of diabetic patients worldwide was estimated to increase from 171 million in the year 2000 to 366 million in 2030[3],although the latter level was already attained in 2011 according to the International Diabetes Federation.A major part of this increase occurred in Asia,mainly China and India[4].A popular theory on meal-induced insulin secretion(the“incretin effect”)states that glucose or any other drug is more effective on the pancreatic cells when administered orally than when given through intravenous or subcutaneous injections[5].The World Health Organization(WHO)recommends plant-based drugs as an alternative medicine[6].Renewed attention to alternative medicines and natural therapies has raised researchers'interest in traditional herbal medicine.Because of their perceived effectiveness,with minimal side effects and relatively low costs,herbal drugs are prescribed widely,even when the contents of their biologically active constituents are unknown[7].Hence,people are seeking traditional medicines for the management of DM.
Flavonoids are naturally occurring compounds widely distributed as secondary metabolites in the plant kingdom.They are recognized for having beneficial clinical properties,such as anti inflammatory[8],cardioprotective[9],antiviral,antibacterial,and anticancer activities[10].Quercetin is one of the flavonoids found in the fruits of medicinal plants,such as Phyllanthus emblica and Vaccinium oxycoccos.We have observed that the tribal people of Pachamalai hills use Amla fruits(P.emblica)as a traditional medicine for diabetes.P.emblica is referenced in “Rasayana,”a branch of the 5000-year-old Indian medical system “Ayurveda,”which focuses on enhancing good health,preventing diseases by boosting the immune system,as well as rejuvenating and revitalizing the body and mind[11].It is still used extensively not only in India,but also in Iran,Iraq,Thailand,China,Italy,Germany,and other countries as a laxative,diuretic,astringent,and antiemetic.It is also used to treat other ailments,including anemia,jaundice,and tumors[12].
Computational biology and bioinformatics play an important role in drug design and screening.Molecular docking of a drug molecule with its target molecule can provide vital information about the drug-receptor binding and affinity.Bioinformatics and molecular docking studies helped us determine the efficacy of quercetin,found in P.emblica,in the treatment of DM.These computational results motivated us to further the study by purifying quercetin from the extract of P.emblica fruit.We then conducted in vivo studies in an animal model of diabetes with the purified quercetin.
2.Materials and methods
2.1.Computational tools and database source(ligand and protein)
This study was carried out using the Schrödinger software suite.Maestro(version 10.2)of the Schrödinger suite contains programs such as ligprep,sitemap,grid generation,and glide XP dock[13].Suitable ligands were retrieved from the ChemSpider database[14].These ligand molecules were converted to 3D structures in the Schrödinger suite.Protein molecules of glycogen phosphorylase(PDB:1NOI)and peroxisome proliferator-activated receptor gamma(PPAR-γ,PDB:3G9E)were retrieved from the protein data bank[15].
2.2.Protein preparation
The protein for docking was prepared using the protein preparation wizard of the Schrödinger suite.The missing side chains,back chains,and residues were updated.Occasional water molecules present in the crystal structure,owing to the prevailing conditions at the time of crystallization,may prove to be problematic;any such water molecules were removed in the protein preparation process.The removal of water molecules increases the entropy,but it is offset by an accompanying loss in enthalpy.
2.2.1.Validation of binding site
Binding site validation plays a vital role in molecular docking.Site validation analysis displays ligand-binding locations,amino acid residues,and site scores.The site map of the entire protein molecule is generated by validating the binding sites using the site map generation tool in Maestro version 10.2 of the Schrödinger suite.Once the site map was generated,we chose only one binding site based on the site score.The binding site of the largest volume was taken for grid generation.
2.2.2.Grid generation
Grid generation is very important for fixing the target site of protein molecules.The phytocompounds or commercial drugs were docked with the target in order to determine the docking parameters with the help of Grid-based ligand docking.A grid box was generated for docking the ligand at the centroid of the target site[16].
2.3.Ligand preparation
The ligand preparation was performed using the LigPrep(2.2)module[17].The drawn ligand was geometry optimized via the Optimized Potentials for Liquid Simulations 2005(OPLS2005)force field.LigPrep is a utility in the Schrödinger suite for the purpose of combining tools to generate 3D structures from 1D(Smiles)and 2D(SDF)representations,which probes tautomers and stereoisomers for geometric minimization of ligands.
2.4.Molecular docking
Molecular docking was performed in the form of flexible docking for the prediction of binding affinity,ligand efficiency,and inhibitory constant.All the ligands were docked with the target active site using Glide Xtra precision(XP).Active compounds will only have positioning that avoids penalties and receives favorable scores for accurate hydrophobic contacts between the protein and ligand.
2.5.Plant material
The fruits of P.emblica were collected from the Pachamalai hills during January 2016.The plant was authenticated by taxonomists in the Rapinat herbarium,St.Josephs College,Trichy,India.A voucher specimen(PHC1125)of the plant material was deposited at the Pushpam Herbarium Cabinet,Department of Botany,A.V.V.M Sri Pushpam College(Autonomous),Poondi,Thanjavur,India.
2.6.Extract preparation
Fresh fruits were washed under running water,cleaned,and air-dried at 35°C.After drying,the fruit was ground to powder and kept dry in an air tight container until extraction.This powder,dissolved in methanol,served as the extract tested in this study.
2.7.Phytocompound isolation
Methanolic fruit extract of P.emblica was chromatographed on a silica gel column.Solvent mixtures of increasing polarity composed of methanol and chloroform(methanol up to 90%)were used for elution,and the fractions were collected.The purity of all the fractions collected was determined by thin layer chromatography on silica gel with methanol and petroleum ether(90:10,v/v)as the mobile phase.Spots were visualized by spraying the plates with 20%antimony chloride in chloroform.The column fractions that showed a single spot of the same Rf value were pooled.
2.8.Experimental animals
Albino Wister male rats(7–8 weeks old,weighing 150–200 g)were purchased from the GSA Animal Farm,Mayiladuthurai,Tamil Nadu,India.The animals were fed with standard pellet diet(Chakan Oil Mills,Sangli)and water ad libitum.Ethical clearance was obtained from the Institutional Animal Ethics Committee and the experiments were conducted according to the Indian National Science Academy guidelines for the use and care of experimental animals(CPCSEA/265).
2.9.Induction of diabetes and treatments
Streptozotocin(STZ)was dissolved freshly in 100 mM citrate buffer(pH 4.5)and the calculated amount(60 mg/kg)of the solution was injected intraperitoneally to overnight-fasted rats.Blood glucose was checked 48 h later and animals showing blood glucose values greater than 250 mg/dL were deemed diabetic and included in the experiments.Experimental rats were divided into six groups(one normal control group and five diabetic groups)with six animals per group.Treatments were given orally,once a day,in the following manner:
Group I:Normal control(without any drug treatment);
Group II:Diabetes(STZ-injected rats);
Group III:Diabetes(STZ-injected rats)treated with 25 mg/kg
body weight quercetin;
Group IV:Diabetes(STZ-injected rats)treated with 50 mg/kg
body weight quercetin;
Group V:Diabetes(STZ-injected rats)treated with 75 mg/kg
body weight quercetin;
Group VI:Diabetes(STZ-injected rats)treated with 10 mg/kg
body weight metformin;
Quercetin and metformin were orally administered daily for 28 days.Body weights and blood glucose levels of overnight-fasted rats were measured weekly.At the end of the experimental period,the animals were fasted overnight and blood samples were collected for various biochemical estimations.
2.10.Biochemical determinations
The serum was separated by centrifugation of the blood samples at 3000g for 10 min in a microcentrifuge.Then,biochemical parameters,such as blood glucose,triglycerides(TG),high-density lipoprotein(HDL),low-density lipoprotein(LDL),total cholesterol(TC),and very-low-density lipoprotein(VLDL),were determined.The hepatic glycogen levels were measured as per the method described by Kemp and Kits Van Hejnijen[18].
2.11.Histopathological study
After termination of the experiment and euthanasia of the rats,the livers were subjected to histopathological studies.They were cut into small species(1 mm×1 mm×1 mm),preserved7 in 10%formaldehyde in saline for 48 h,dehydrated by treating successively with increasing concentrations of ethyl alcohol(50%,80%,95%,and 100%),cleared in xylene,and embedded in paraffin.Samples were cut into ultrathin sections with an ultramicrotome,stained with hematoxylin and eosin dye,and mounted in neutral deparaffinated xylene(DPX)medium for microscopic observation of cells.The sections were examined for necrosis,fatty changes,hyaline degeneration,and ballooning degeneration.
2.12.Statistical analysis
All the data were statistically evaluated and the significance of various treatments was calculated.Results are expressed as means±standard error(S.E)and comparisons among the groups were made by analysis of variance(ANOVA),followed by Student's ttests.A value of P<0.01 was considered significant.
3.Results and discussion
3.1.Validation of binding sites
Ligand binding site validation was performed for all binding sites in the target molecule and the binding cavity was chosen based on the site score(Table 1).The 3D structure of the target molecule is shown in Fig.1A and the active site is shown in Fig.1B.
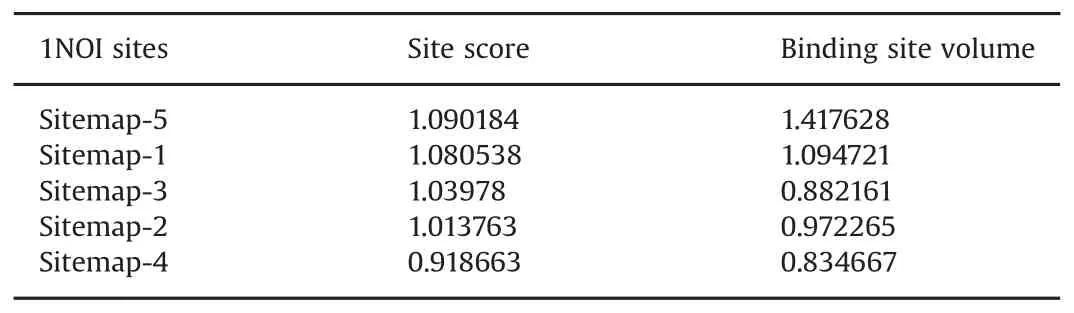
Table 1 Target site score and ligand contact volume of binding cavity in glycogen phosphorylase(GP).
3.2.Molecular docking
3.2.1.Glycogen phosphorylase(GP)protein
Molecular docking studies clearly revealed that quercetin has a higher docking score with GP than does any other ligand.Inhibition of glycogenolysis has been proposed as a therapeutic strategy for the treatment of type 2 diabetes.GP catalyzes the first step in glycogen degradation as the rate limiting enzyme.It is expected that inhibition of GP will inhibit glycogenolysis,reduce hepatic glucose production,and lower blood glucose,thereby providing a potential new treatment for type 2 diabetes[19–21].
The fruit of P.emblica,contains quercetin and gallic acid also.The computational approach used involved docking of the ligands(quercetin and gallic acid)to form a complex with GP and PPAR-γ.Computational studies showed that quercetin interacts with GP residues GLY177,TRP173,THR85,and THR219(Fig.2A).The backbones of GLY177 and TRP173 formed hydrogen bonds with the ligand OH groups,while the side chains of THR85 and THR219 formed hydrogen bonds.These interactions are represented as two types of arrows from protein to ligand;the first is a solid blue line(H-bond backbone)and the other is a dotted blue line(H-bond side chain)(Fig.2B).In addition,another ligand molecule,gallic acid,has the second docking score of-6.48347(Table 2).The docked molecule interacts with GP residues ILE166,ARG40,ASN178,and TRP173(Fig.3A).The backbones of ILE166 and TRP173 residues form hydrogen bonds with the ligand OH groups,while the side chains of ARG40 and ASN173 form hydrogen bonds with the ligand OH groups(Fig.3B).According to the report by Paramaguru et al.[22],quercetin interacts more favorably with GP than gallic acid affinity.They also suggest that quercetin has potent antihyperglycemic drug properties[22].
3.2.2.PPAR-γprotein
The other protein molecule,PPAR-γ,was docked with the two phytocompounds(quercetin and gallic acid)and one synthetic ligand,metformin.The 3D structure of the PPAR-γbinding cavity is represented in Fig.4,and the site scores are displayed in Table 3.In this docking analysis,the interactions between protein and ligands were examined.The results show that quercetin is the best ligand and a potentially potent inhibitor for PPAR-γ.The docking scores are listed in Table 4.Quercetin interacts with PPAR-γamino acid residue TYR473(Fig.5A).The side chain of TYR473 forms a hydrogen bond with the OH group of quercetin(Fig.5B).The docking score of the other phytocompound,gallic acid,is lower than that of quercetin,but it also has good binding affinity(Fig.6).
The electrostatic energy of the binding interface is important for protein-ligand complex formation.The major electrostatic interactions include hydrogen bonds(both from side chains and back bones),salt bridges,and π-π stacking.Hydrogen bonding is one of the most important interactions for biological macromolecular interactions,crucial for conferring stability to protein molecules and selected protein-ligand interactions.
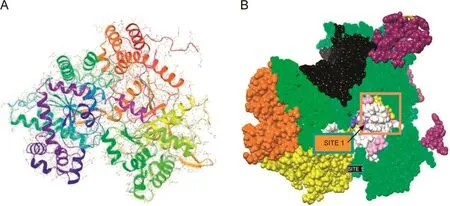
Fig.1.Template of glycogen phosphorylase(1NOI)with ligand binding site.(A)3D structure of the target molecule;(B)the active site.
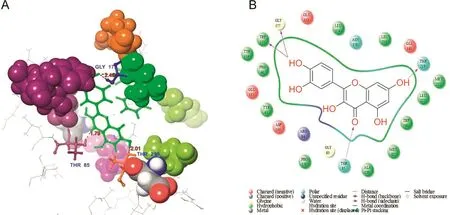
Fig.2.Molecular structure represents the interaction of quercetin and GP.(A)3D interaction shows residue interaction and their distance values;(B)The 2D interaction plot shows the contacts types.
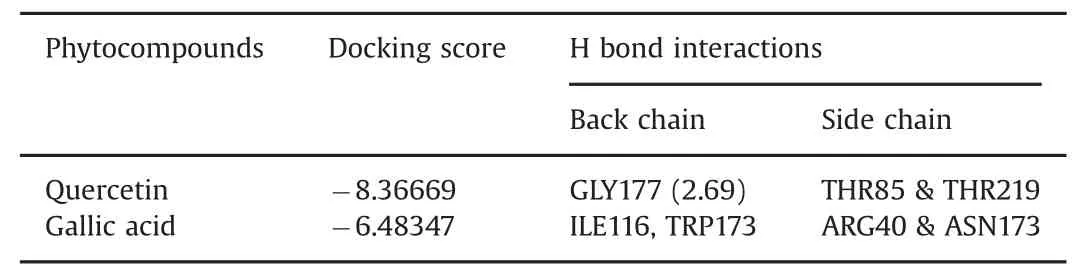
Table 2 Docking score and H bond interaction of various phytocompounds with glycogen phosphorylase molecule(1NOI).
Metformin has the lowest docking score when compared with that of the phytocompounds.Metformin interacts with PPAR-γ amino acid residues SER42 and GLU291(Fig.7A).The backbone of SER342 formed a hydrogen bond with the ligand NH group.The side chain of GLU291 formed hydrogen bonds with the ligand NH/NH2groups(Fig.7B).
According to reports,commercial antidiabetic drugs stimulate insulin production from the pancreas,with the other treatment being insulin injection.Various synthetic diabetes drugs are available on the market,including the insulin secretagogues,sulfonylurea and meglitinides.Several insulin sensitizers,such as biguanides,thiazolidinediones,and metformin,as well asα-glycosidase inhibitors(e.g.,acarbose)are also available.These medications have many side-effects such as hypoglycemia,idiosyncratic liver cell injury,lactic acidosis,digestive discomfort,permanent neurological def i cit,headache,dizziness,and even death[23,24].As mentioned previously,these drugs are often expensive[25]
3.3.Isolation of the phytocompound
The potential phytocompound was isolated from P.emblica fruit extracts.The identity of the purified sample was elucidated by13C NMR,1H NMR,mass spectroscopy,and UV absorption spectroscopy.The functional groups of quercetin were analyzed by Fourier-transform infrared(FTIR)spectroscopy.
3.3.1.Structural determination and characterization
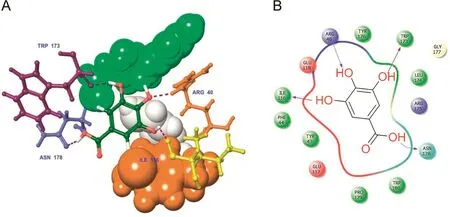
Fig.3.Docked complex of gallic acid with GP.(A)3D interaction shows the residue interaction and their distance values;(B)The 2D interaction plot shows contacts types.
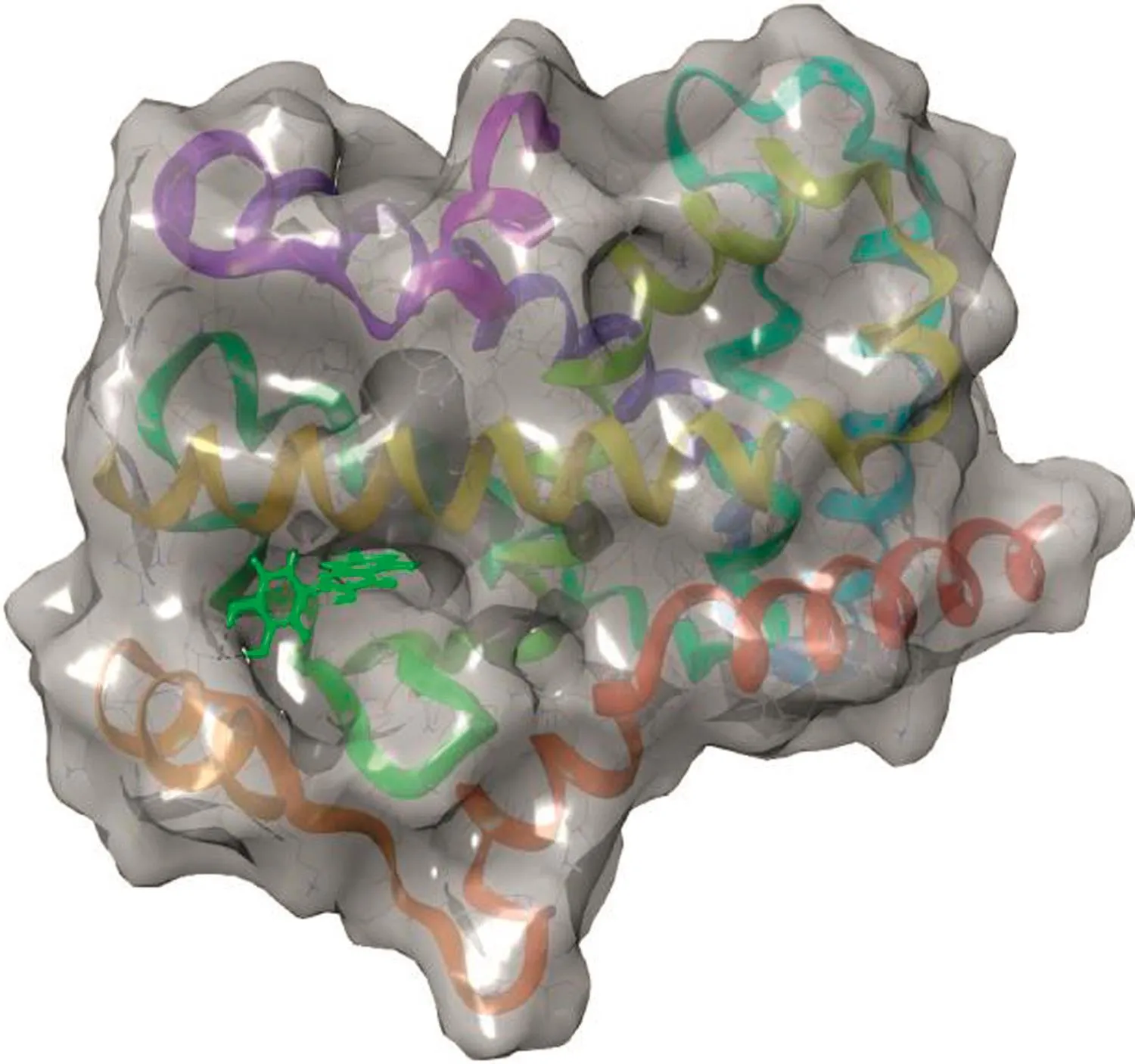
Fig.4.Ligand binding cavity of PPAR-γ.
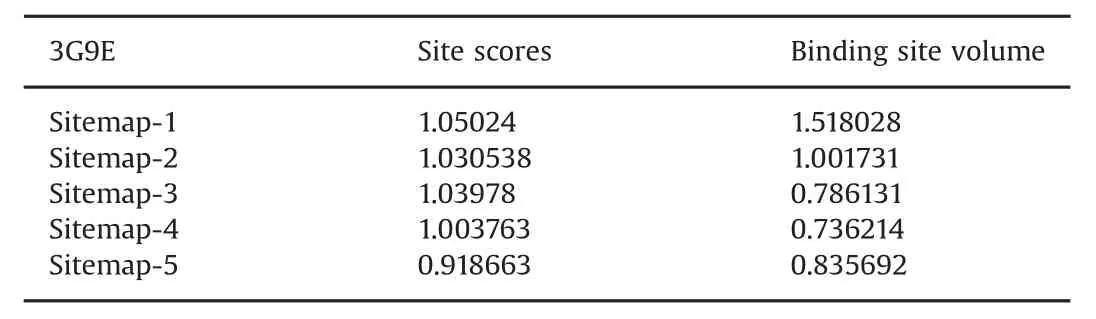
Table 3 Target site score and ligand contact volume in PPAR-γ(3G9E).
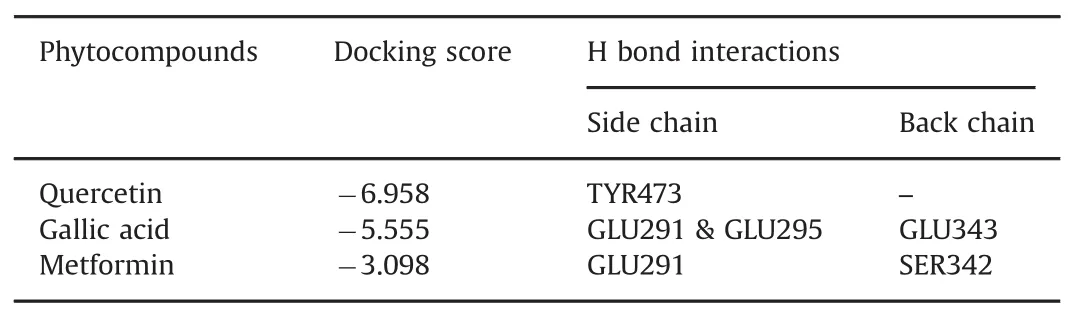
Table 4 Docking score and H bond interaction of various phytocompounds with PPAR-γ(3G9E).
Different spectral analysis techniques were used to interpret the structure of the purified molecule.Precisely,the FTIR spectrum revealed peaks at 709 cm-1(C-H rocking),1549 cm-1(C-C stretching(Ar)),1709 cm-1(C-O stretching),3502 cm-1(O–H stretching(bending)),3066 cm-1(C-H stretching(Ar)),and 3640 cm-1(H-O stretching–free OH group present)(Fig.S1 and Table5).1H NMR revealed the aromaticC-H proton at δ7.00–7.24(S)-Ar(Fig.S1A),13C NMR revealed a carbonyl(C=O)atδ170,C–H carbon atδ112,tertiary cation atδ124,C–OH atδ136,and C–OH(L)at δ147(Fig.S1B).Mass spectroscopy revealed the molecular ion peak of the compound at 302(m+).The mass spectroscopy indicates the base is obtained at 115.The identified product is accomplished by determination of elemental physical constants(Fig.S1C).UV absorption spectroscopy was used to identify the presence of the conjugatedπe.As shown in Fig.S1D,Peak I is the conjugatedπe at 285 nm,and Peak II is the enone with external conjugated group at 356 nm(Fig.S1D).The product was yellow colored,with an attached chromophoric=O ring in the compound(Fig.8).
3.3.2.Product characterization
After separating the extract by column chromatography,preliminary screening of the various fractions was accomplished by a thin layer chromatographic technique.However,different spectral analyses(i.e.,FTIR,1H NMR,13C NMR,mass spectroscopy,and UV absorption)helped confirm that the compound was2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H- chromen-4-one(quercetin).Its molecular formula is C15H10O7and its molecular weight is 302.235.The compound is yellow crystalline,with a melting point of 316°C.It is insoluble in water but soluble in methanol(Table 6).In computer modeling,the structure of the compound,2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one,strongly suggested its usefulness as an antidiabetic,so we tested it in an STZ-induced diabetic rat model.
3.4.Effect of quercetin on body weight and blood glucose
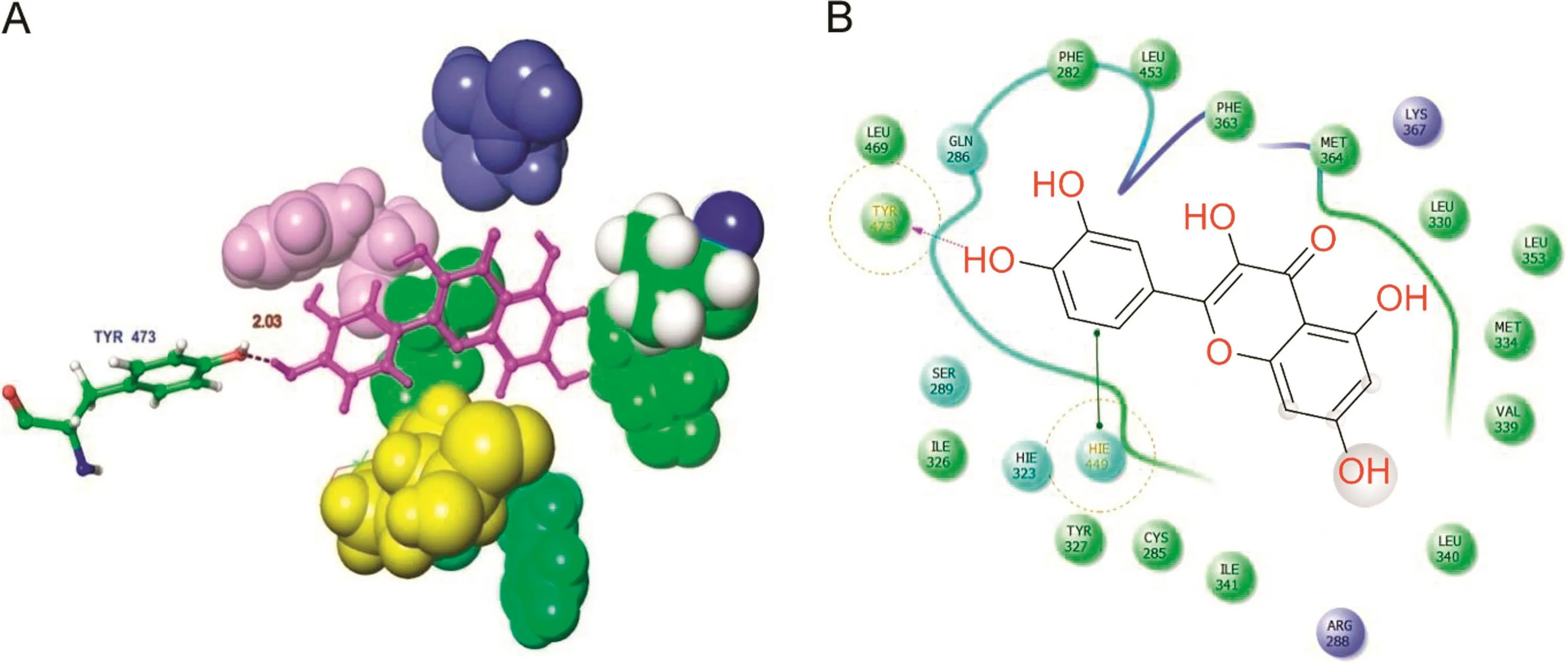
Fig.5.Docked complex of quercetin with PPAR-γ.(A)3D interaction shows the residue interaction and their distance values.(B)The 2D interaction plot shows contacts types.
The experimental animals were treated with various concentrations of the potential lead compound,quercetin.In the present study,this phytocompound was selected via molecular docking analysis.Quercetin was orally administered at different doses(25,50,and 75 mg/kg)in rats with experimentally induced hyperglycemia.The body weights of the rats are shown in Table 7.The baseline weight of the rats at the beginning of the study was similar among all groups.At the end of the treatment(after 4 weeks),the diabetic animals had lost weight due to the catabolism of protein in muscle tissue caused by insulin deficiency[26].However,the quercetin-treated diabetic rats had not lost body weight(Table 7).It is possible that the phytocompound was able to protect the pancreaticβ-cells.Similar results were obtained by another group,and collectively,suggest that quercetin treatment protects against diabetic weight loss[27].The use of medicinal plants is increasing due to the extraction and development of many successful drugs and chemotherapeutic agents from plants and their use as traditional rural herbal remedies[28].STZ causes selective destruction ofβ-cells of the pancreatic islets,which leads to an increase in blood glucose levels.It is evident from the present investigation that STZ administration at a dose of 60 mg/kg causes significant diabetogenic response in the rats(Table 8).Blood glucose levels were measured randomly at baseline and on the 7th,14th,21st,and 28th days of the study.The increased glucose levels in the diabetic control group were found to be significant(P<0.05)compared to that of the normal control group.The elevated blood glucose levels declined sharply after oral administration of quercetin(25,50,and 75 mg/kg)or metformin(10 mg/kg).When comparisonswere made between baseline and the 28th day in the treated groups,there was a statistically significant(P<0.01)decline in blood glucose levels.If the decline in blood glucose levels is the only index to be considered,then treatment with quercetin or metformin produced a highly effective antihyperglycemic response in STZ-induced diabetic rats.Considering the relative efficacy,although all tested concentrations of quercetin produced antihyperglycemic activity,quercetin of 75 mg was shown to produce the highest antihyperglycemic activity among all tested agents including metformin(Table 8).The reduction in blood glucose levels is the principal therapeutic goal of diabetes.According to Torres-Piedra et al.[29],quercetin significantly reduced the glucose levels in STZ-induced diabetic rats.The effective control of blood glucose levels by increasing the secretion of insulin and regeneration ofβ-cells in the pancreas is a vital step in preventing cardiovascular and diabetic complications and improving the quality of life in type 2 diabetic patients[30].Based on the results of our experimental analysis,it is now well-established that quercetin is a plant-based natural drug that is sufficiently potent to reduce elevated glucose levels in diabetic rats.
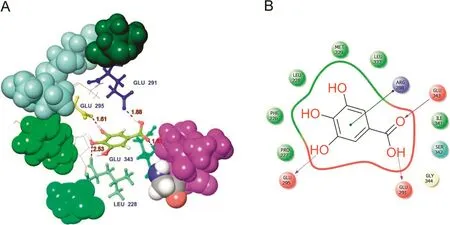
Fig.6.Docked complex of gallic acid with PPAR-γ.(A)3D interaction shows the residue interaction and their distance values.(B)The 2D interaction plot shows contacts types.
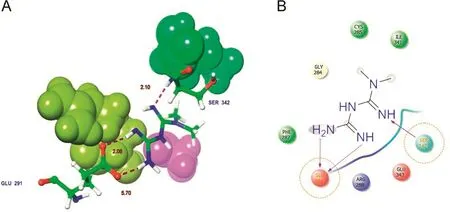
Fig.7.Docked complex of synthetic drug molecule Metformin with PPAR-γ.(A)3D interaction shows the residue interaction and their distance values.(B)The 2D interaction plot shows contacts types.
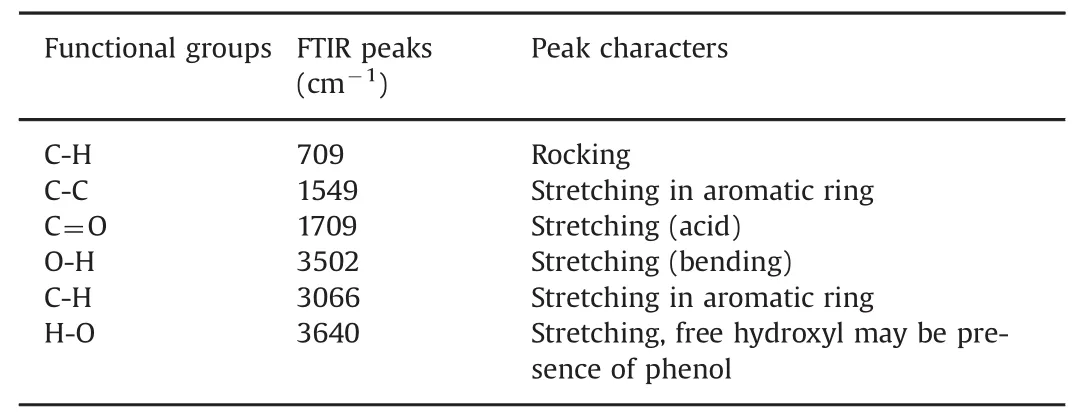
Table 5 Functional groups and their peak characters.
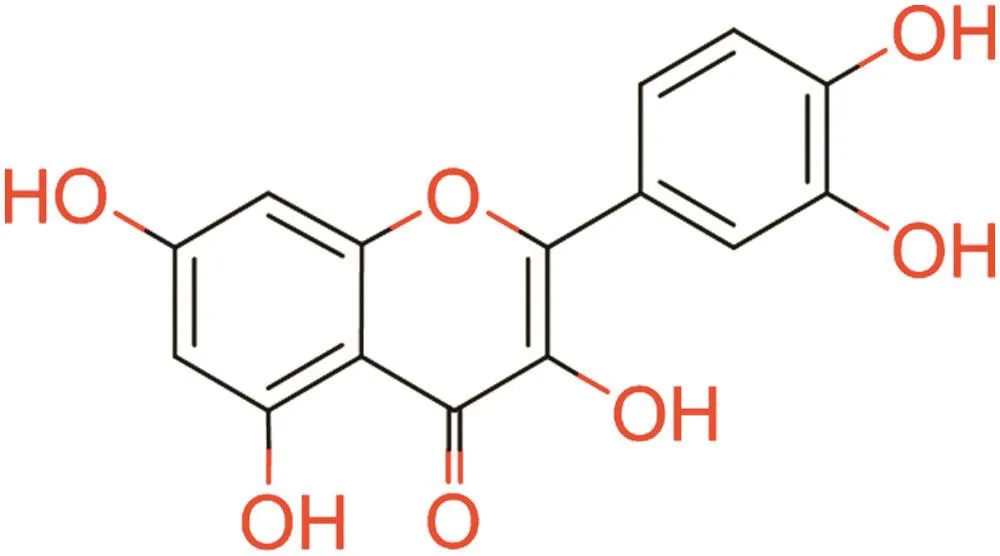
Fig.8.Structure of 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one(Quercetin).
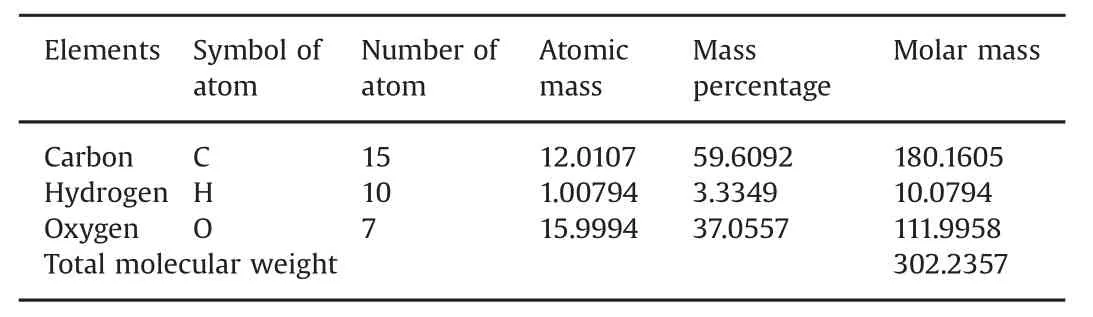
Table 6 Elemental analysis.
3.5.Effect of quercetin on hemoglobin,plasma insulin,and urine sugar
The levels of hemoglobin,plasma insulin,and urine sugar in normal control and quercetin treated diabetic rats were measured.Increased hemoglobin,plasma insulin,and lack of urine sugar were observed in the treated diabetic rats.These values were significantly improved close to normal after treatment with quercetin(25,50,and 75 mg/kg)or metformin(10 mg/kg)(Table 9).The effect of quercetin on glycogen synthase and glycogen phosphorylase activities in the liver of diabetic rats is shown in Table 10.Diabetic rats showed increased activity of glycogen phosphorylase and decreased activity of glycogen synthase.These enzyme activities were significantly(p<0.05)reverted to near normal levels after treatment with quercetin.In addition,it has been suggested that quercetin can improve insulin signaling and insulin sensitivity in rats with insulin resistance[31].Quercetin could improve fasting hyperglycemia by enhancing insulin sensitivity viaα-glucosidase inhibition and enhanced insulin signaling in diabetic rats[32].
3.6.Effect of quercetin on plasma lipid pro fi les
Diabetic rats showed significant increases in the blood levels of TC,TG,LDL,and VLDL.In contrast,HDL was significantly reduced compared to the values of normal control rats.Regular administration of quercetin for 4 weeks nearly normalized the lipid profiles in the diabetic rats.Oral administration of metformin significantly decreased the blood levels of TC,LDL,and VLDL.However,metformin did not significantly change the blood levels of TG compared to those of the diabetic control rats.In diabetic rats treated with quercetin (75 mg/kg),theblood levelsofTC(24.02%↓),LDL(31.75%↓),and VLDL(48.23%↓)decreased significantly by the end of study(after 28 days);however,the blood level of HDL was significantly increased compared to the value in the diabetic rats(78.88%↑)(Table 11).In diabetes,LDL and VLDL carry cholesterol to the peripheral tissues where it is deposited,while HDL transports cholesterol from peripheral tissues to the liver to facilitate its excretion and metabolism.It has been reported that elevated serum LDL and VLDL are indicative of the atherogenic process[33].As suggested by previous studies,an active phytocompound,such as quercetin,can decrease the levels of cholesterol and LDL in diabetic rats[34].
3.7.Histopathology observation
Representative histopathological changes in the pancreas are shown in Fig.9.STZ administration elicited severe injury of the
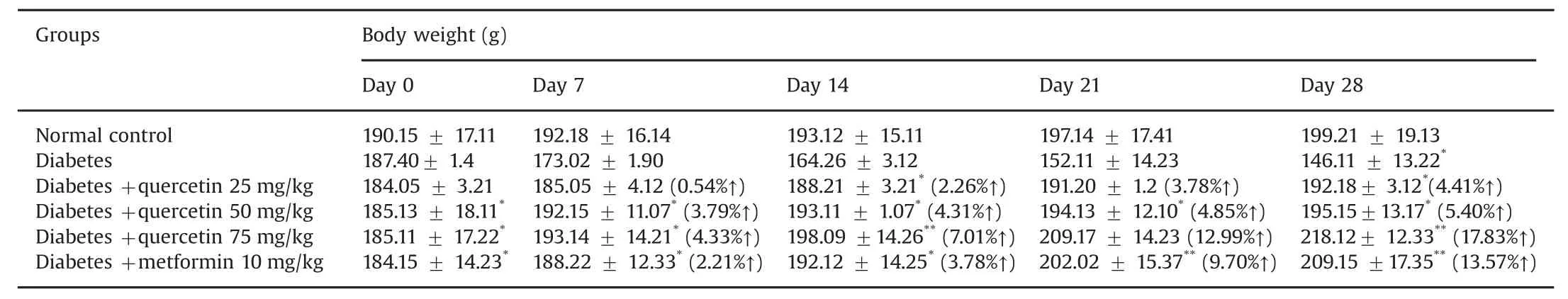
Table 7 Effects of quercetin on the changes of body weight in normal control and experimental rats observed at weekly interval during the 28 days of study.
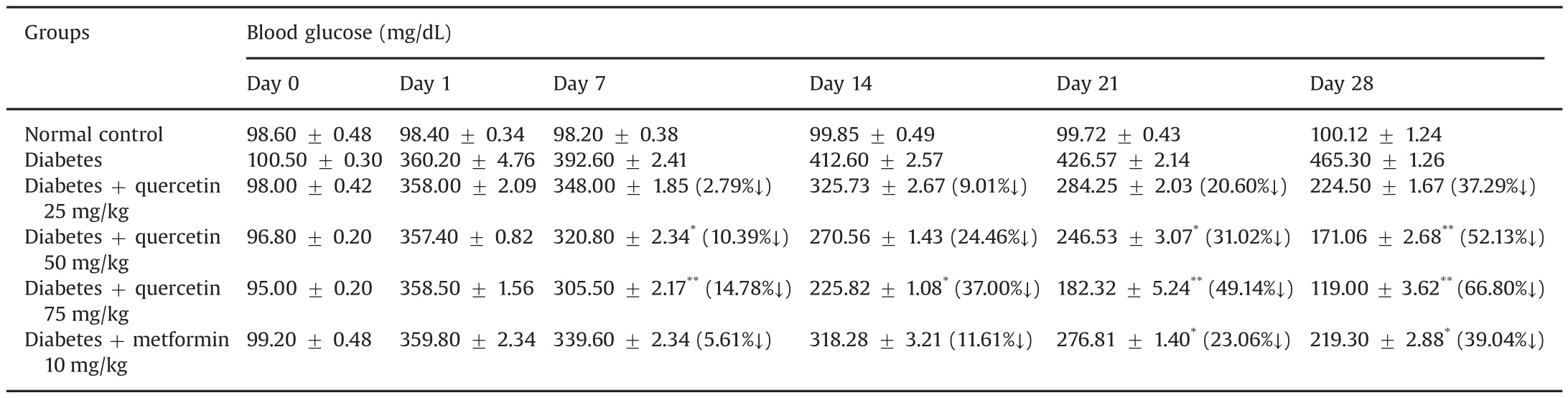
Table 8 Effects of quercetin on blood glucose levels in streptozotozin-induced diabetic rats.
when compared to diabetes group.pancreas,as indicated by decreasing numbers of islet cells and diminishing diameters of the pancreas islets(Fig.9).The islets were smaller in diabetic rats than in normal control rats(Fig.9B).Administration of quercetin(25,50,and 75 mg/kg)or metformin(10 mg/kg)reduced pancreatic injury.The pancreas is a vital organ of metabolism and macronutrients digestion,which also secretes different digestive enzymes and pancreatic hormones[35].Many studies have indicated that quercetin has the capacity to prevent pancreatic injury,promoting regeneration of the pancreatic islets and increasing its ability to maintain normal blood glucose levels in diabetes-induced rats[32,36].
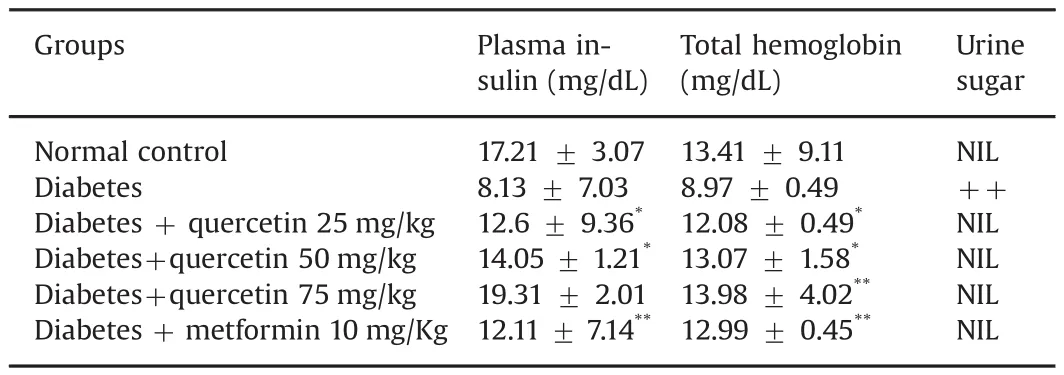
Table 9 Effects of quercetin on the levels of plasma insulin,total hemoglobin and urine sugar.
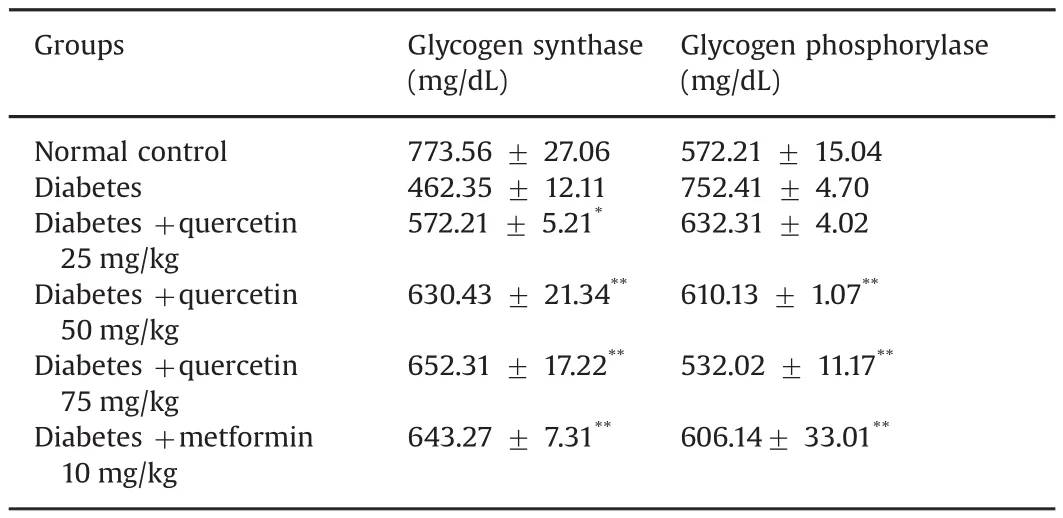
Table 10 Effect of quercetin on activity of glycogen synthase and glycogen phosphorylase in the liver.
4.Conclusion
The present molecular docking analysis showed a better binding affinity and lead docking score to glycogen phosphorylase with quercetin than with gallic acid.Quercetin was isolated from P.emblica fruit extract after the in silicofinding.Different concentrations of quercetin showed remarkable antihyperglycemic effects and potent defense mechanisms in STZ-induced diabetic rats.Metforminis more commonly associated with gastrointestinal side effects than most other antidiabetic drugs.Finally,we conclude that quercetin is a remarkable antihyperglycemic
agent,which can be considered as a potential drug candidate for diabetes mellitus with fewer side effects than metformin.
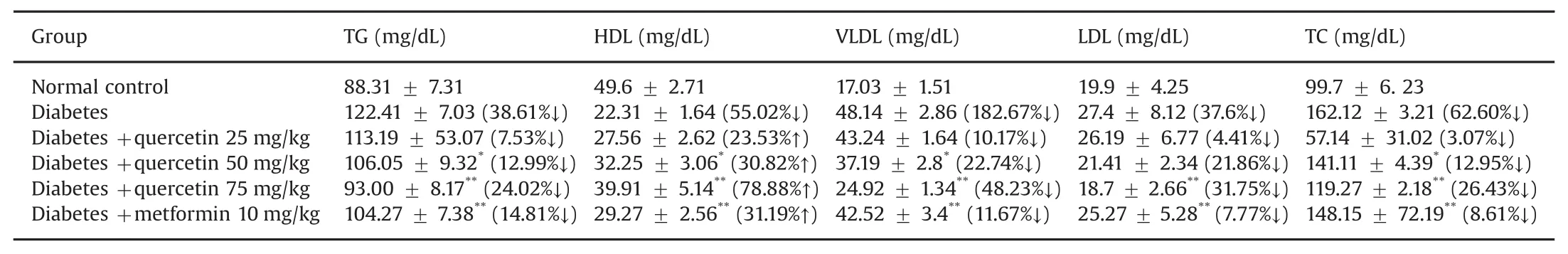
Table 11 Effect of quercetin on serum lipid profile.
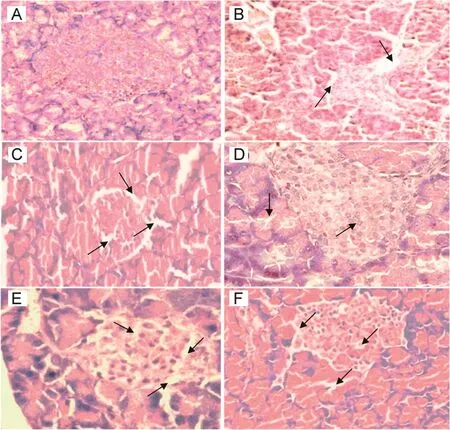
Fig.9.Histopathology of pancreas at the end research with hematoxylin and eosin stain in different groups.(A)Normal control group:typical histological structure of pancreas and normal islets;(B)Streptotozocin induced diabetes model group showing severe nectrotic changes,β-cells injury and breakdown of the islets after 72 h;(C)Diabetes+25 mg/kg quercetin group showing slightly restored the necrotic changes and β cells with shrunken islets;(D and E)Diabetes+50 mg/kg and Diabetes+75 mg/kg groups presenting near normal histological structure;(F)Diabetes+10 mg/kg metformin group showing significantly reduced pancreas injury and slightly restored the necrotic change.
Conflicts of interest
The authors declare that there are no conflicts of interest.
The authors are grateful to the DST-SERB Major Research Project,New Delhi,India[Project File No.SB/YS/LS-109/2014]for funding this project.We especially express our thanks to the Management of A.V.V.M.Sri Pushpam College(Autonomous),Poondi,and Sharmila Institute of Medicinal Products Research Academy(SIMPRA),Thanjavur,India,for providing necessary facilities and support in carrying out this work.
Appendix A.Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2017.10.005.
[1]M.Riaz,M.Zia-Ul-Haq,B.Saad,The role of anthocyanins in obesity and diabetes,Anthocyanins and Human Healthy:Biomolecular and therapeutic aspects,Springer Briefs in food,Health,and Nutrition,Springer,2016:109–123.
[2]T.Kuzuya,S.Nakagawa,J.Satoh,et al.,Report of the Committee on the classification and diagnostic criteria of diabetes mellitus,Diabetes Res.Clin.Pract.1(2002)65–85.
[3]S.Wild,G.Roglic,A.Green,et al.,Global prevalence of diabetes:estimates for the year 2000 and projections for 2030,Diabetes Care 27(2004)1047–1053.
[4]Y.Liu,J.Sun,S.Rao,Y.Su,et al.,Antihyperglycemic,antihyperlipidemic and antioxidant activities of polysaccharides from Catathelasma ventricosum in streptozotocin-induced diabetic mice,Food Chem.Toxicol.57(2013)39–45.
[5]T.Vilsboll,J.J.Holst,lncretins,insulin secretion and Type 2 diabetes mellitus,Diabetologia 47(2004)357–366.
[6]World Health Organization,Chronicle,1985.
[7]M.S.Valiathan,Healing plants,Curr.Sci.75(1998)1122–1127.
[8]J.Santosh kumar,M.S.Devarmani,M.M.Sajjanar,et al.,Study of anti-inlf ammatory activity of fruit of Emblica of ficinalis(Amla)in Albino rats,Med.Innov.2(2013)17–25.
[9]W.Duan,Y.Yu,L.Zhang,Anti-atherogenic effects of Phyllanthus emblica associated with corilagin and its analogue,Yakugaku Zasshi 125(2005)587–591.
[10]S.Madhuri,G.Pandey,K.S.Verma,Antioxidant,immunomodulatory and anticancer activities of Emblica of ficinalis:an overview,Int.Res.J.Pharm.2(2011)38–42.
[11]M.Krishnaveni,S.Mirunalini,Therapeutic potential of Phyllanthus emblica(amla):the ayurvedic wonder,J.Basic.Clin.Physiol.Pharmacol.21(2010)93–105.
[12]D.W.Unander,G.L.Webster,B.S.Blumberg,Records of usage or assays in Phyllanthus(Euphorbiaceae).I.Subgenera Isocladus,Kirganelia,Cicca and Emblica,J.Ethnopharmacol.30(1990)233–264.
[13]Schrödinger,LLC,New York,NY,2015.
[14] 〈http://www.chemspider.com〉.
[15] 〈http://www.rcsb.com〉.
[16]Glide v5.7,Schrödinger,LLC,New York,2011.
[17]LigPrep v2.5,Schrödinger,LLC,New York,2011.
[18]A.Kemp,A.J.Van Heijningen,A colorimetric micro-method for the determination of glycogen in tissues,Biochem.J.56(1954)646–648.
[19]J.C.McCormack,N.Westergaard,M.Kristiansen,et al.,Pharmacological approaches to inhibit endogenous glucose production as a means of anti-diabetic therapy,Curr.Pharm.Des.7(2001)1451–1474.
[20]J.L.Treadway,P.Mendys,D.J.Hoover,et al.,Glycogen phosphorylase inhibitors for treatment of type 2 diabetes mellitus,Expert.Opin.Investig.Drugs 10(2001)439–454.
[21]R.Sarabu,J.Tilley,Recent advances in therapeutic approaches to type 2 diabetes,Annu.Rep.Med.Chem.40(2005)167–181.
[22]R.Paramaguru,P.M.Mazumder,D.Sasmal,et al.,Antidiabetic activity of Pterospermum acerifolium flowers and glucose uptake potential of bioactive fraction in L6 muscle cell lines with its HPLC f i ngerprint,BioMed.Res.Int.(2014)1–10.
[23]H.Hui,G.Tang,V.L.Go,et al.,Hypoglycemic herbs and their action mechanisms,Chin.Med.4(2009)11–14.
[24]J.Neustadt,S.R.Pieczenik,Medication-induced mitochondrial damage and disease,Mol.Nutr.Food Res.52(2008)780–788.
[25]S.Bibi,S.Kalsoom,H.Rashid,Ligand based approach for pharmacophore generation for identification of novel compounds having anti-diabetic activity,Int.J.Pharm.Sci.4(2013)303–314.
[26]R.Sundaram,R.Naresh,P.Shanthi,et al.,Modulatory effect of green tea extract on hepatic key enzymes of glucose metabolism in streptozotocin and high fat diet induced diabetic rats,Phytomedicine 20(2013)577–584.
[27]O.Coskun,M.Kanter,A.Korkmaz,et al.,Quercetin,a flavonoid antioxidant,prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas,Pharmacol.Res.51(2005)117–123.
[28]A.K.Tiwari,J.M.Rao,Diabetes mellitus and multiple therapeutic approaches of phytochemicals:present status and future prospects,Curr.Sci.83(2002)30–38.
[29]M.Torres-Piedra,R.Ortiz-Andrade,R.Villalobos-Molina,et al.,A Comparative study of flavonoid nalogues on streptozotocin-nicotinamide induced diabetic rats:quercetin as a potential antidiabetic agent acting via 11beta-hydroxysteroid dehydrogenase type 1 inhibition,Eur.J.Med.Chem.45(2010)2606–2612.
[30]R.A.DeFronzo,Pharmacologic therapy for type 2 diabetes mellitus,Ann.Intern.Med.131(1999)281–303.
[31]N.Arias,M.T.Macarulla,L.Aguirre L,et al.,Quercetin can reduce insulin resistance without decreasing adipose tissue and skeletal muscle fat accumulation,Genes.Nutr.9(2014)361.
[32]J.H.Kim,M.J.Kang,H.N.Choi,et al.,Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus,Nutr.Res.Pract.5(2011)107–111.
[33]J.Mohammadi,P.R.Naik,Evaluation of hypoglycemic effect of Morus alba in an animal model,Indian J.Pharmacol.40(2008)15–18.
[34]L.Nuraliev,G.A.Avezov,The efficacy of quercetin in alloxan diabetes,Exp.Clin.Pharmacol.55(1992)42–44.
[35]C.Guo,C.Zhang,L.Li,Hypoglycemic and hypolipidemic effects of oxymatrine in high-fat diet and streptozotocin-induced diabetic rats,Phytomedicine 21(2014)807–814.
[36]M.Vessal,M.Hemmati,M.Vasie,Antidiabetic effects of quercetin in streptozotocin induced diabetic rats,Comp.Biochem.Physiol.Toxicol.Pharmacol.135(2003)357–364.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Surrogate potency assays:Comparison of binding profiles complements dose response curves for unambiguous assessment of relative potencies
- Eco-friendly reduced graphene oxide for the determination of mycophenolate mofetil in pharmaceutical formulations
- Ultrasensitive electrochemical determination of metronidazole based on polydopamine/carboxylic multi-walled carbon nanotubes nanocomposites modified GCE
- Quantitative determination of erlotinib in human serum using competitive enzyme-linked immunosorbent assay
- Structural confirmation of sulconazole sulfoxide as the primary degradation product of sulconazole nitrate
- Physicochemical characterization,the Hirshfeld surface,and biological evaluation of two meloxicam compounding pharmacy samples
