Quantitative determination of erlotinib in human serum using competitive enzyme-linked immunosorbent assay
2018-04-17YutaYamamotoTetsuyaSaitaYutaroYamamotoMasashiShin
Yuta Yamamoto,Tetsuya Saita,Yutaro Yamamoto,Masashi Shin
Applied Life Science Department,Faculty of Biotechnology and Life Science,Sojo University,4-22-1 Ikeda,Kumamoto 860-0082,Japan
1.Introduction
Erlotinib(Fig.1),an epidermal growth factor receptor-tyrosine kinase inhibitor(EGFR-TKI),is widely used to treat non-small cell lung cancer and unresectable pancreatic cancer[1,2].However,it is associated with a higher incidence of interstitial lung disease than other anticancer drugs,and many fatal cases have been reported[3].It also frequently causes acneiform rashes by inhibiting the expression of EGFR in the stratum basale and skin appendages.These reactions are frequently reported as a reason for dose reduction or interruption of treatment in patients receiving erlotinib[4,5].
Erlotinib is metabolized by CYP3A4,CYP1A2,and CYP3A5 in the liver,and the induction of CYP3A4 via tobacco smoking has been shown to decrease the blood concentration of erlotinib by about 50%[6].Food also influences the absorption of erlotinib.Taking erlotinib after consuming a high-fat,high-calorie meal has been shown to increase maximum blood concentrations by about 1.5-fold and the AUC about 2-fold[7].The severity of skin damage caused by erlotinib has been shown to be correlated with the concentration of erlotinib in the blood[8],which indicates that therapeutic drug monitoring(TDM)is needed to minimize the toxicity of erlotinib and improve the response to treatment.
O-Desmethyl erlotinib is known as a major metabolite in humans(Fig.1)[9].During long-term therapy,the plasma concentration of O-desmethyl erlotinib is about 10%of that of erlotinib[10].Therefore,an analytical method specific to erlotinib must be developed for TDM or pharmacokinetic studies.Previous TDM and pharmacokinetic studies of erlotinib have used high-performance liquid chromatography(HPLC)[11]and liquid chromatography with tandem mass spectrometry(LC–MS/MS)[12–17].However,no immunoassay technique that compares favorably with these analytical methods has been developed.The reason for this is that it is very challenging to produce an anti-erlotinib antibody that is not cross-reactive with O-desmethyl erlotinib,the major metabolite of erlotinib.We previously created a specific antibody,which is not cross-reactive with the major metabolite,and changed part of the structure of the drug to a hapten antigenic structure,by considering the structure of the major metabolite,to develop an enzyme immunoassay technique that could be used for pharmacokinetic studies of the antibody[18–20].Similarly,we created a specific anti-erlotinib antibody by changing part of the structure of erlotinib to a hapten antigenic structure to develop enzyme immunoassay techniques that could be used for pharmacokinetic studies on erlotinib.
In this study,we successfully developed the first specific and sensitive competitive enzyme-linked immunosorbent assay(ELISA)for erlotinib using a polyclonal antibody against part of the structure of erlotinib and herein report the technique.The initial application of the assay for the measurement of erlotinib levels in mice demonstrates its usefulness for the assessment of basic pharmacokinetic parameters.
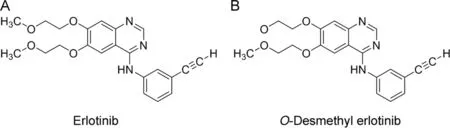
Fig.1.Chemical structures of erlotinib and its major metabolite.
2.Materials and methods
2.1.Chemicals and reagents
Erlotinib hydrochloride and O-desmethyl erlotinib were obtained from AdooQ BioScience LLC(Irvine,CA,USA).Ethyl 3,4-bis(2-methoxyethoxy)benzoate(EBMB)was obtained from AK Scientific,Inc(Union City,CA,USA).2,4,6-Trinitrobenzene sulfonic acid was obtained from Tokyo Chemical Industry Co.,Ltd.(Tokyo,Japan).Horseradish peroxidase(HRP)and 3,3,5,5-tetramethylbenzidine(TMB)were obtained from Boehringer Ingelheim Pharma GmbH(Ingelheim,Germany).Sodium hydroxide,hydrochloric acid,sulfuric acid,sodium dihydrogenphosphate dehydrate,disodium hydrogenphosphate 12-water,sodium azide,tris(hydroxymethyl)aminomethane (Tris),1-ethyl-3,3-dimethylaminopropyl carbodiimide hydrochloride(EDC),N-hydroxysuccinimide,dioxane,ethyl acetate,N,N-dimethylformamide and bovine serum albumin(BSA)were purchased from Wako Pure Chemical Industries,Ltd.(Osaka,Japan).Hydrogen peroxide(30%in water)was obtained from Nacalai Tesque Inc.(Kyoto,Japan).
2.2.Preparation of the immunogen for erlotinib
The erlotinib immunogen was prepared with part of the structure of erlotinib(EBMB)as shown in Fig.2.EBMB(10 mg,33.5 μmol)was dissolved in 500 μL of 1 M NaOH and the resulting solution was left to stand at 60°C for 1 h.The resulting EBMB carboxylate was acidified by the addition of 550 μL of 1 M HCl and then extracted with ethyl acetate.The organic layer was separated and evaporated underreduced pressure.Theresiduewas dissolved in 95%dioxane(1 mL).EDC(12.7 mg,67μmol)and N-hydroxysuccinimide(7.7 mg,67μmol)were added to the dioxane solution,and the solution was left to stand at room temperature for 2 h.The reaction mixture containing succinimidyl EBMB was immediately mixed with BSA(20 mg)in 1 mL of 0.1 M phosphate buffer(pH 7.0)and incubated at room temperature for 2 h.The reaction solution was dialyzed in 1 mM phosphate buffer(pH 7.0)for 24 h.The purified conjugate was lyophilized and used as an immunogen for erlotinib.The trinitrobenzene sulfonic acid method was used to determine the primary amine[21],and about 18.1 EBMB molecules were found to be coupled with each molecule of BSA based on the reduction of the primary amine.
2.3.Preparation of erlotinib antibody
Five 5-week-old,female BALB/c mice(Kyudo Exp.Animals,Kumamoto,Japan)were injected intraperitoneally with 0.1 mg of EBMB-BSA conjugate emulsified in complete Freund’s adjuvant.The mice received 3 injections of the con-jugate(0.05 mg)alone at 2-week intervals.Seven days after the final injection,the mice were euthanized and sera were collected,separated by centrifugation,heated at 55 °C for 30 min,and then stored at-30 °C.The anti-erlotinib serum obtained was directly used as the anti-erlotinib antibody for ELISA.The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Sojo University.
2.4.Preparation of the erlotinib-HRP conjugate
Erlotinib was labeled by binding to HRP,essentially by the same method as that used for the preparation of erlotinib immunogen.EBMB(10 mg,33.5 μmol)was dissolved in 500 μL 1 M NaOH and the solution was left to stand at 60°C for 1 h.The resulting EBMB carboxylate was acidified by the addition of 550 μL of 1 M HCl and then extracted with ethyl acetate.The organic layer was separated and evaporated under reduced pressure.The residue was dissolved in 95% dioxane(1 mL).EDC (12.7 mg,67 μmol)and N-hydroxysuccinimide(7.7 mg,67 μmol)were added to the dioxane solution.The resulting solution was left to stand at room temperature for 2 h,and a 50 μL aliquot of the reaction mixture,containing succinimidyl EBMB,was then added directly to HRP(0.5 mg,12.5 nmol)in 0.5 mL of 0.1 M phosphate buffer(pH 7.0),followed by incubation at room temperature for a further 2 h.The mixture was chromatographed on a column of Sephadex G-75(2.0 cm×30 cm)using PBS containing 0.1%BSA to remove any remaining small molecules.Fractions(4 mL)were collected and fractions 8 and 9,corresponding to the main peaks showing enzyme activity,were used as a label for ELISA.
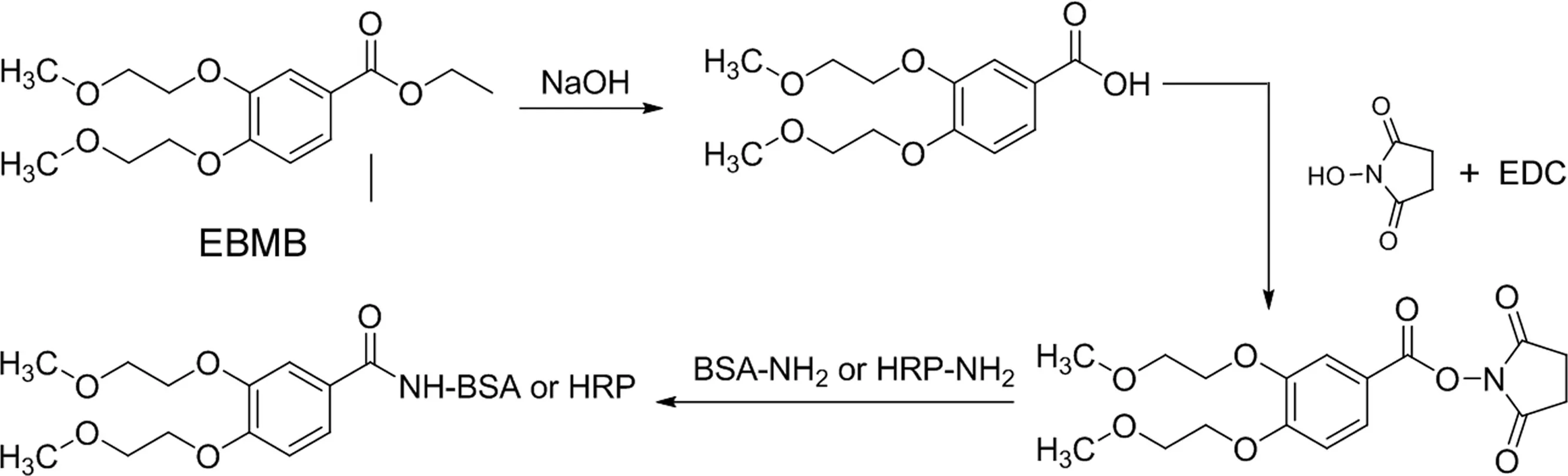
Fig.2.Scheme showing the preparation of the immunogen and enzyme conjugate.
2.5.ELISA procedure
ELISA is based on the principle of competition between enzyme-labeled and unlabeled drugs for an immobilized antibody,followed by measurement of the marker enzyme activity of the immunocomplex bound to the solid phase.Briefly,microtiter plate wells(Nunc F Immunoplates I;Nunc,Roskilde,Denmark)were coated with 100 μL of anti-mouse-IgG antibody(2 μg/mL)(Bethyl Laboratories,Montgomery,TX)in 10 mM Tris-HCl buffer(pH 8.5)containing 10 mM NaCl and 10 mM NaN3and left to stand for 1 h at 37°C.Plates were washed twice with PBS containing 0.1%BSA and incubated with 100 μL of anti-erlotinib serum diluted 2000-fold with PBS containing 1%skim milk for 30 min at 37°C.The anti-erlotinib antibody-coated wells were then f i lled with 50 μL of either erlotinib-treated samples or PBS containing 0.1%BSA as a control,followed immediately by 50 μL of the pooled erlotinib-HRP conjugate(diluted 1:20 in PBS containing 0.1%BSA for erlotinib).The wells were incubated overnight at 4°C and once again washed thoroughly with PBS containing 0.1%BSA.The activity of the enzyme conjugate bound to each well was measured by the addition of 100 μL of 0.42 mM TMB in 0.05 M acetate-citric acid buffer(pH 5.5)containing 3%N,N-dimethylformamide and 0.01%hydrogen peroxide,followed by incubation of the wells at 37°C for a suitable period.The enzyme reaction was stopped by the addition of 100 μL of 2.0 M H2SO4to each well,and the resulting color intensity was measured spectrophotometrically at 450 nm using an ELISA analyzer(ImmunoMini NJ-2300,Nalge Nunc Int.Co.,Ltd.,Tokyo,Japan).Concentrations were calculated from the standard curve using semi-logarithmic graph paper.
2.6.Pharmacokinetic evaluation
Three 8-week-old female BALB/c mice(Kyudo Exp.Animals;Kumamoto,Japan)weighing 25–30 g were used in this study.Erlotinib was orally administered at a dose of 30 mg/kg using a sonde after an overnight fast.The drug was suspended in methyl cellulose(0.5%,m/v water)at 5 mg/mL concentration.Peripheral blood(5 μL)was collected from the tail vein pre-administration and at 0.25,0.5,1,2,4,8,12,and 24 h after administration,diluted 25 times with PBS containing 0.1%BSA,and immediately vortexed for several seconds.Diluted blood samples were centrifuged at 3000g for 10 min at 4°C,and each resulting supernatant was stored at-30°C until the erlotinib concentration was assayed.The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Sojo University.
3.Results and discussion
3.1.Preparation of the immunogen and enzyme conjugate for erlotinib
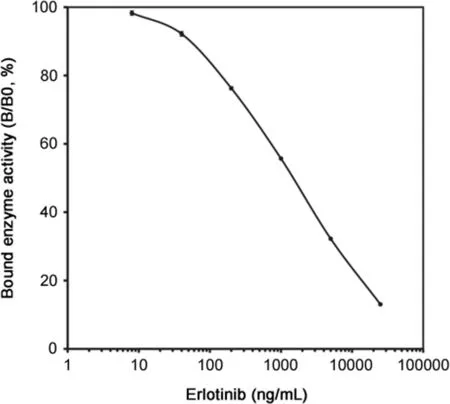
Fig.3.Standard curve of the developed ELISA for erlotinib in human serum.The curve shows the bound enzyme activity(%)for various doses of erlotinib(B)as a ratio to that bound using erlotinib-HRP alone(B0).Each point represents the mean±S.D.(n=3).
In long-term therapy,the plasma concentration of O-desmethyl erlotinib,the major metabolite of erlotinib in humans,is about 10%of that of erlotinib[10].Therefore,an anti-erlotinib antibody that does not show cross-reaction with O-desmethyl erlotinib must be produced to develop an ELISA that can specifically measure the concentration of erlotinib in human plasma.Namely,antibodies against erlotinib must have high affinity for the bis(2-methoxyethoxy)group,which is the primary site of metabolism.In general,antibody specificity on the hapten appears to be towards the group farthest away from the region of conjugation to the carrier protein in the immunogen structure[22,23].Therefore,when creating erlotinib antigens,it must be ensured that the bis(2-methoxyethoxy)group within the hapten structure is as far away as possible from the carrier protein binding domain.Moreover,erlotinib has no suitable reactive structure for making immunogens such as the erlotinib-BSA conjugate.Based on these findings,erlotinib immunogen was prepared from part of the structure of erlotinib(EBMB)(Fig.2).The EBMB carboxylate was coupled to BSA using the hydroxysuccinimide ester method[24],and the resulting EBMB-BSA conjugate(erlotinib immunogen),with about 18 mol of EBMB per mol of BSA,induced the formation of specific antibodies in each of the fi ve mice immunized.EBMBHRP conjugate(as a tracer)was also prepared by the same procedure.The conjugate was stable for more than 6 months in eluted buffer(pH 7.0)at 4°C without any loss of the enzyme or immunoreactive enzyme activity.
3.2.Validation of method
The optimal quantities and incubation times for each reaction were established.The standard dose-response curve of erlotinib obtained in the human serum system is shown in Fig.3.The ELISA detection range was 8–25,000 ng/mL of erlotinib.The curve was essentially linear on a semilogarithmic plot between 40 and 25,000 ng/mL.For practical purposes,the working range was arbitrarily set to 40–5000 ng/mL based on the findings of precision and accuracy for the ELISA(Table 1),which showed this ELISA to be a reproducible technique.The recoveries of four different levels of erlotinib ranging from 40 to 5000 ng/mL were satisfactory at96.6%–107.5%(n=5).The coefficients of variation(CV)for intraand inter-assays of erlotinib at four different level concentrations in the range of 40–5000 ng/mL were 1.9%–9.2%and 2.3%–7.3%(n=5 for each),respectively.The detection limit of erlotinib in the ELISA was 40 ng/mL(student’s t-test,n=3,P < 0.01 compared with the B0 value).The plasma concentration range for the usual clinical dose of erlotinib is about 0.6–2.5 μg/mL[25].Therefore,this ELISA may be sensitive enough to quantify erlotinib in TDM and pharmacokinetic studies.
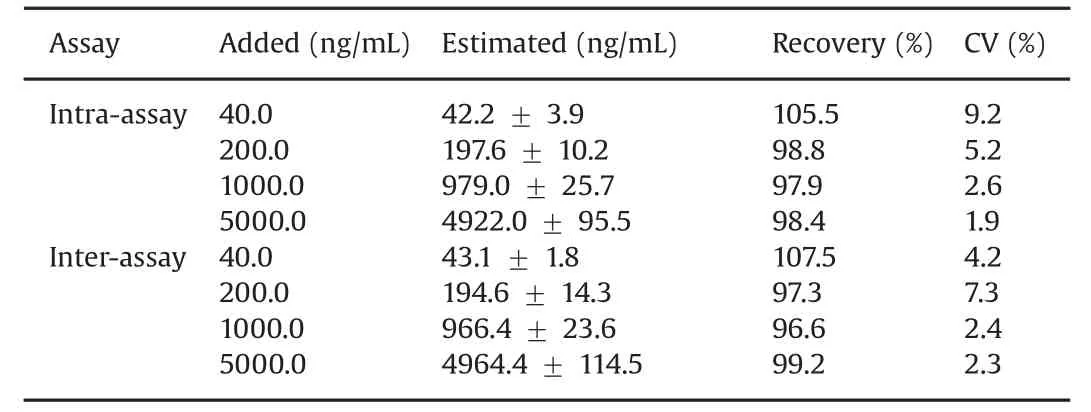
Table 1 Recoveries of erlotinib from human serum and precision of ELISA for erlotinib.
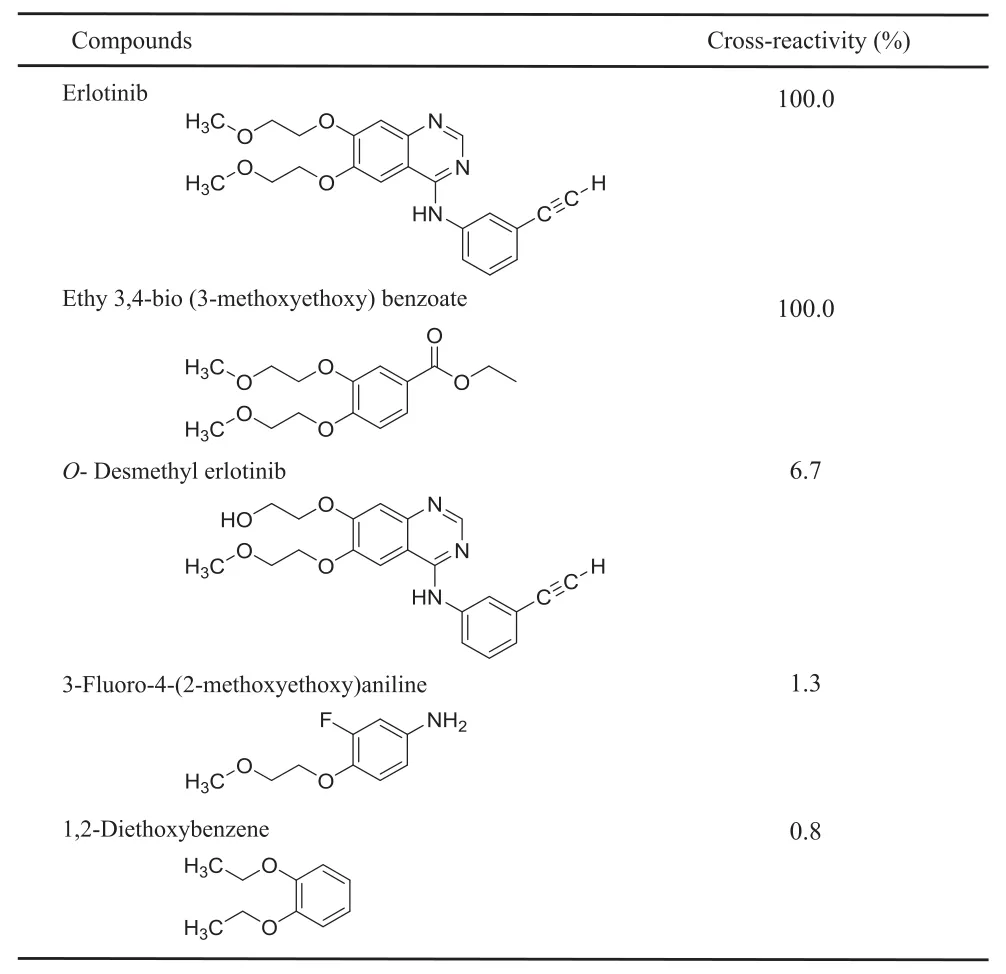
Table 2 Percent cross-reactivity of metabolite and analogs measured by ELISA.
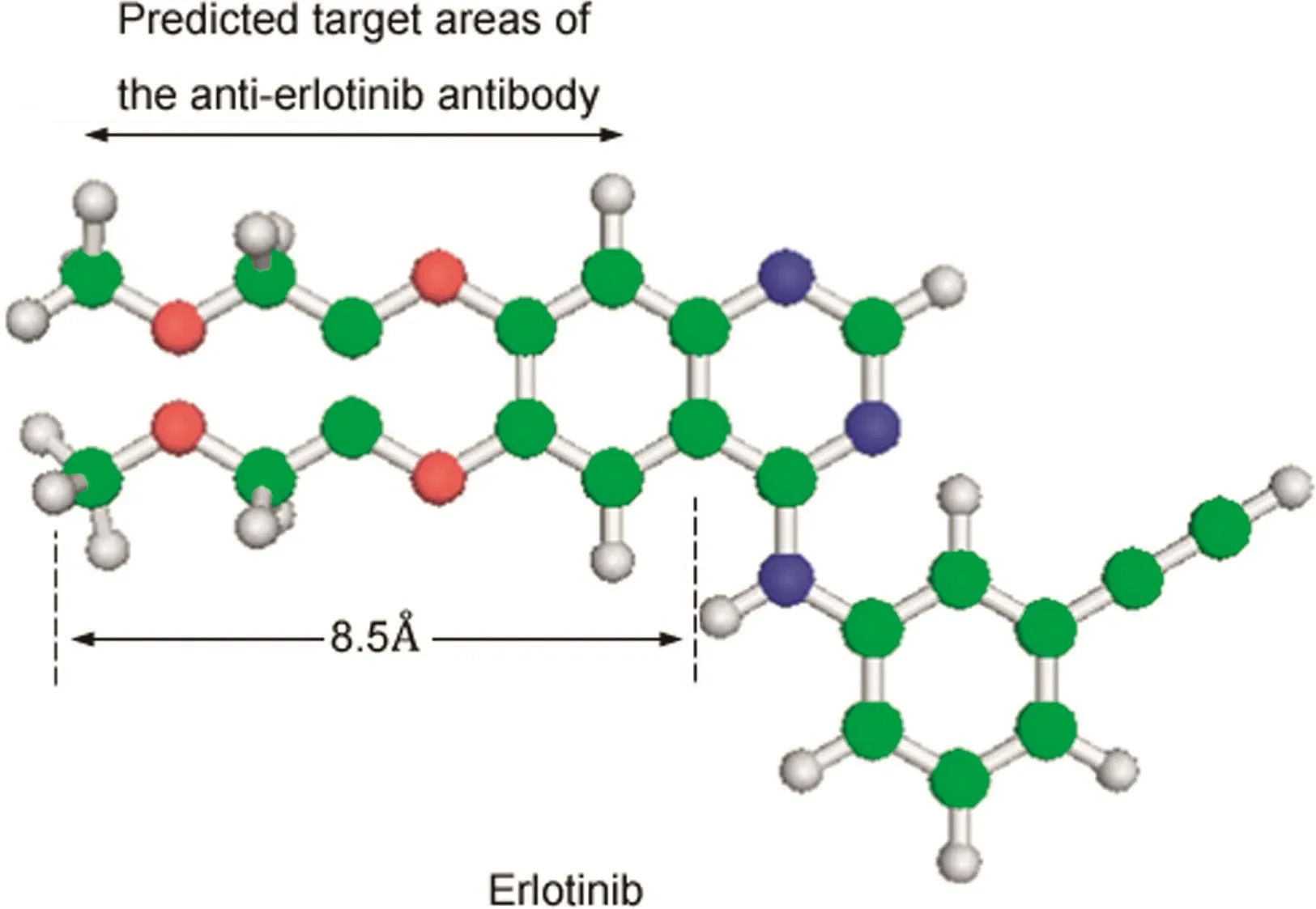
Fig.4.The predicted target areas of the anti-erlotinib antibody.The length of epitope was approximately 8.5 Å.
3.3.Specif i city
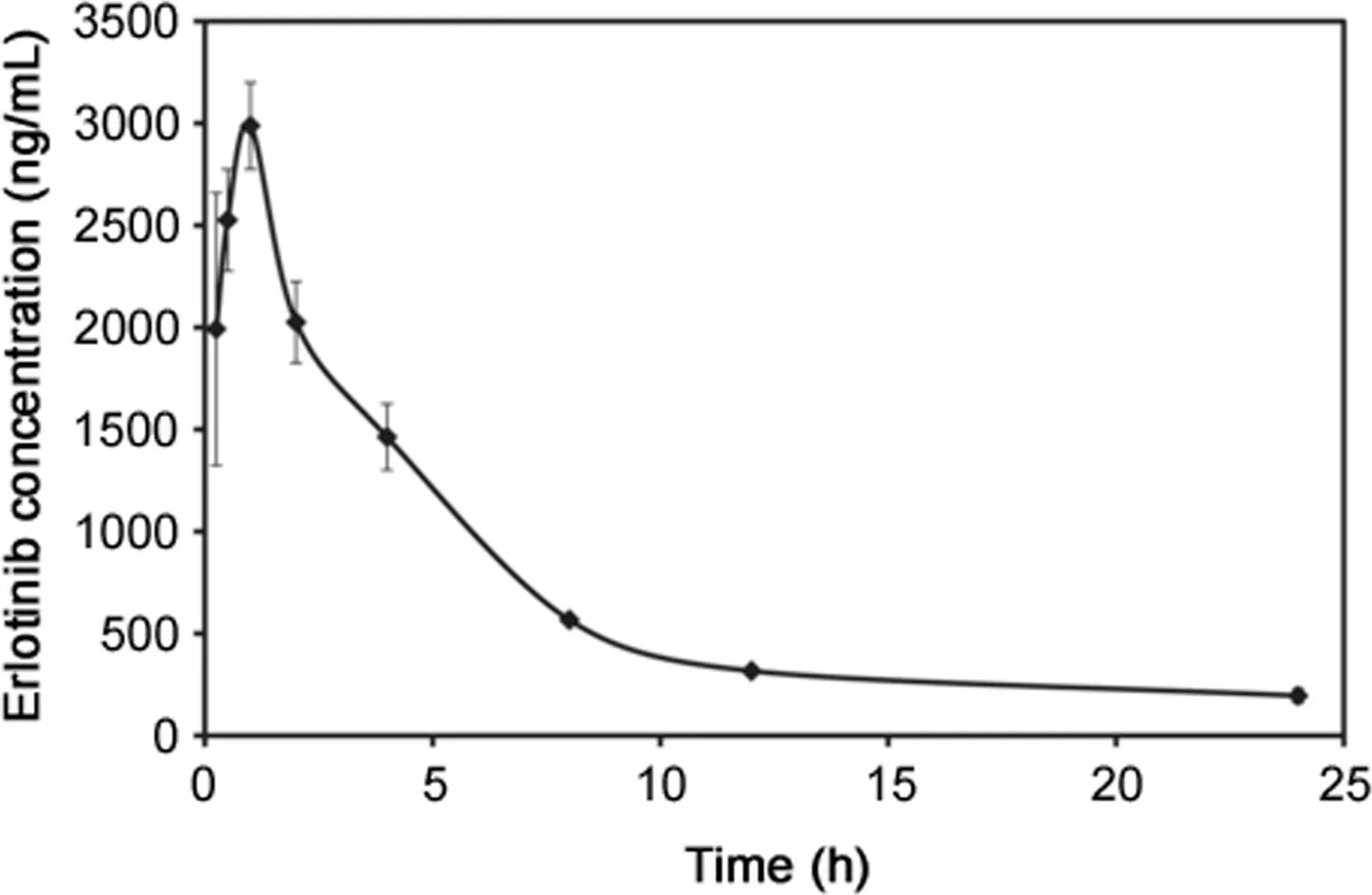
Fig.5.Blood erlotinib levels in mice after a single oral administration of erlotinib.Three mice weighing 25–31 g were injected with 30 mg/kg erlotinib.At each interval,blood was collected and the levels of erlotinib were measured by ELISA.Each point represents the mean±S.D.(n=3).
Antibody cross-reactivity was determined by the displacement of bound erlotinib-HRP by similar compounds.Cross-reactivity values were defined as the ratio of each compound to erlotinib in the concentrations required for 50%inhibition of erlotinib-HRP binding to the antibody.The anti-erlotinib antibody showed 100%cross-reactivity with EBMB used as a hapten antigen,6.7%with the major erlotinibmetabolite,O-desmethyl erlotinib,1.3% with 3-f l uoro-4-(2-methoxyethoxy)aniline,and 0.8%with 1,2-diethoxybenzene(Table 2).Furthermore,the antibody showed high affinity for erlotinib and EBMB was used as a hapten antigen.However,it showed limited reactivity with the major metabolite O-desmethylerlotinib and erlotinib analogs.Thesefindings suggest that the antibody recognizes almost the whole EBMB structure,and thus is suitably specif i c to the structure of erlotinib.In addition,based on the predicted target area of the anti-erlotinib antibody,we estimated the length of the epitope to be approximately 8.5 Å(Fig.4).
3.4.Pharmacokinetic study of erlotinib in mice
To demonstrate the potential of the ELISA,a preliminary pharmacokinetic study of erlotinib in mice was performed(Fig.5).Erlotinib was rapidly absorbed,reached peak concentrations in the blood of 2987.5 ng/mL 60 min after oral administration,and then slowly decreased.The erlotinib levels in the blood samples were similar to those of the LC–MS results reported by Smith et al.[26].Using the ELISA,erlotinib levels were easily measured in the blood of mice after a single oral dose of erlotinib.The ELISA determinations may have measured the total amount of erlotinib immunoreactivity and included erlotinib metabolites that cross-reacted with the anti-erlotinib antibody,such as the major metabolite in humans,O-desmethyl erlotinib[9].However,during long-term therapy,the plasma concentration of O-desmethyl erlotinib is only about 10%of that of erlotinib[10].Therefore,considering the specif i city of the antibody and the plasma concentration of O-desmethyl erlotinib,there might not have any interference.The cross-reactivity of the other metabolites has not yet been confirmed,and their maximum concentrations detected in human plasma are relatively low[9].Therefore,this ELISA may be specif i c enough to quantify erlotinib for TDM and pharmacokinetic studies in humans.
4.Conclusion
We have described the preparation of the first antibody against erlotinib by preparing immunogens from part of the structure of erlotinib.The generated antibody against erlotinib was used to develop the ELISA,which was shown to be sensitive,specific,and adaptable for high-throughput analyses.This ELISA will be a valuable tool in TDM and pharmacokinetic studies of erlotinib.Finally,our approach is expected to be invaluable in the development of new immunoassays for low-molecular-weight drugs that produce a variety of metabolites.
Conflicts of interest
The authors declare that there are no conflicts of interest.
[1]F.A.Shepherd,J.Rodrigues Pereira,T.Ciuleanu,et al.,Erlotinib in previously treated non-small-cell lung cancer,N.Engl.J.Med.353(2005)123–132.
[2]A.M.Czarnecka,P.Korzeń,A.Nowak-Dement,et al.,Prolonged complete response following gemcitabine-erlotinib combined therapy in advanced pancreatic cancer,Oncol.Lett.11(2016)1101–1104.
[3]R.ter Heine,R.T.van den Bosch,C.M.Schaefer-Prokop,et al.,Fatal interstitial lung disease associated with high erlotinib and metabolite levels.A case report and a review of the literature,Lung Cancer 75(2012)391–397.
[4]S.Sato,K.Kurishima,K.Miyazaki,et al.,Efficacy of tyrosine kinase inhibitors in non-small-cell lung cancer patients undergoing dose reduction and those with a low body surface area,Mol.Clin.Oncol.2(2014)604–608.
[5]K.Yamada,K.Azuma,M.Takeshita,et al.,Phase II trial of erlotinib in elderly patients with previously treated non-small cell lung cancer:results of the Lung Oncology Group in Kyushu(LOGiK-0802),Anticancer Res.36(2016)2881–2887.
[6]A.N.Hughes,M.E.R.O'Brien,W.J.Petty,et al.,Overcoming CYP1A1/1A2 mediated induction of metabolism by escalating erlotinib dose in current smokers,J.Clin.Oncol.27(2009)1220–1226.
[7]J.Ling,S.Fettner,B.L.Lum,et al.,Effect of food on the pharmacokinetics of erlotinib,an orally active epidermal growth factor receptor tyrosine-kinase inhibitor,in healthy individuals,Anticancer Drugs 19(2008)209–216.
[8]J.F.Lu,S.M.Eppler,J.Wolf,et al.,Clinical pharmacokinetics of erlotinib in patients with solid tumors and exposure-safety relationship in patients with non-small cell lung cancer,Clin.Pharmacol.Ther.80(2006)136–145.
[9]J.Ling,K.A.Johnson,Z.Miao,et al.,Metabolism and excretion of erlotinib,a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase,in healthy male volunteers,Drug Metab.Dispos.34(2006)420–426.
[10]Y.Togashi,K.Masago,M.Fukudo,et al.,Pharmacokinetics of erlotinib and its active metabolite OSI-420 in patients with non-small cell lung cancer and chronic renal failure who are undergoing hemodialysis,J.Thorac.Oncol.5(2010)601–605.
[11]L.Faivre,C.Gomo,O.Mir,et al.,A simple HPLC-UV method for the simultaneous quantification of gefitinib and erlotinib in human plasma,J.Chromatogr.B Anal.Technol.Biomed.Life Sci.879(2011)2345–2350.
[12]T.Ishida,T.Naito,J.Kawakami,Simultaneous determination of erlotinib and its isomeric major metabolites in human plasma using isocratic liquid chromatography-tandem mass spectrometry and its clinical application,Biomed.Chromatogr.29(2015)643–646.
[13]H.Hayashi,Y.Kita,H.Iihara,et al.,Simultaneous and rapid determination of gefitinib,erlotinib and afatinib plasma levels using liquid chromatography/tandem mass spectrometry in patients with non-small-cell lung cancer,Biomed.Chromatogr.30(2016)1150–1154.
[14]H.M.Maher,N.Z.Alzoman,S.M.Shehata,Simultaneous determination of erlotinib and tamoxifen in rat plasma using UPLC-MS/MS:application to pharmacokinetic interaction studies,J.Chromatogr.B Anal.Technol.Biomed.Life Sci.1028(2016)100–110.
[15]R.W.Sparidans,H.Rosing,J.J.M.Rood,et al.,Liquid chromatography-tandem mass spectrometric assay for therapeutic drug monitoring of the B-Raf inhibitor encorafenib,the EGFR inhibitors afatinib,erlotinib and gefitinib and the O-desmethyl metabolites of erlotinib and gef i tinib in human plasma,J.Chromatogr.B Anal.Technol.Biomed.Life Sci.1033–1034(2016)390–398.
[16]H.M.Maher,N.Z.Alzoman,S.M.Shehata,et al.,UPLC-ESI-MS/MS study of the effect of green tea extract on the oral bioavailability of erlotinib and lapatinib in rats:potential risk of pharmacokinetic interaction,J.Chromatogr.B Anal.Technol.Biomed.Life Sci.1049–1050(2017)30–40.
[17]M.W.Ni,J.Zhou,H.Li,et al.,Simultaneous determination of six tyrosine kinase inhibitors in human plasma using HPLC-Q-Orbitrap mass spectrometry,Bioanalysis 9(2017)925–935.
[18]T.Saita,M.Shin,H.Fujito,Development of a specific and sensitive enzymelinked immunosorbent assay for the quantification of imatinib,Biol.Pharm.Bull.36(2013)1964–1968.
[19]T.Saita,Y.Yamamoto,S.Noda,et al.,Quantif i cation of sorafenib in human serum by competitive enzyme-linked immunosorbent assay,Biol.Pharm.Bull.38(2015)1788–1793.
[20]T.Saita,Y.Yamamoto,K.Hosoya,et al.,An ultra-specif i c and sensitive sandwich ELISA for imatinib using two anti-imatinib antibodies,Anal.Chim.Acta 969(2017)72–78.
[21]A.F.Habeeb,Determination of free amino groups in proteins by trinitrobenzenesulfonic acid,Anal.Biochem.14(1966)328–336.
[22]B.K.van Weeman,A.H.Schuurs,The influence of heterologous combinations of antiserum and enzyme-labeled estrogen on the characteristics of estrogen enzyme-immunoassays,Immunochemistry 12(1975)667–670.
[23]K.Fujiwara,H.Saikusa,M.Yasuno,et al.,Enzyme immunoassay for the quantific.ation of mitomycin Cusingβ-galactosidase as a label,Cancer Res.42(1982)1487–1491.
[24]H.Hosoda,Y.Sakai,T.Nambara,A direct enzyme immunoassay of 6 betahydroxycortisol in human urine,Chem.Pharm.Bull.29(1981)170–175.
[25]N.A.Lankheet,E.E.Schaake,S.A.Burgers,et al.,Concentrations of erlotinib in tumor tissue and plasma in non-small-cell lung cancer patients after neoadjuvant therapy,Clin.Lung Cancer 16(2015)320–324.
[26]N.F.Smith,S.D.Baker,F.J.Gonzalez,et al.,Modulation of erlotinib pharmacokinetics in mice by a novel cytochrome P450 3A4 inhibitor,BAS 100,Br.J.Cancer 98(2008)1630–1632.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Surrogate potency assays:Comparison of binding profiles complements dose response curves for unambiguous assessment of relative potencies
- Eco-friendly reduced graphene oxide for the determination of mycophenolate mofetil in pharmaceutical formulations
- Ultrasensitive electrochemical determination of metronidazole based on polydopamine/carboxylic multi-walled carbon nanotubes nanocomposites modified GCE
- Anti-diabetic activity of quercetin extracted from Phyllanthus emblica L.fruit:In silico and in vivo approaches
- Structural confirmation of sulconazole sulfoxide as the primary degradation product of sulconazole nitrate
- Physicochemical characterization,the Hirshfeld surface,and biological evaluation of two meloxicam compounding pharmacy samples
