Ni/bentonite catalysts prepared by solution combustion method for CO2 methanation☆
2018-04-08YuexiuJiangTongxiaHuangLihuiDongZuzengQinHongbingJi
Yuexiu Jiang ,Tongxia Huang ,Lihui Dong ,Zuzeng Qin ,*,Hongbing Ji ,2,*
1 School of Chemistry and Chemical Engineering,Guangxi Key Laboratory of Electrochemical Energy Materials,Guangxi University,Nanning 530004,China
2 School of Chemistry,Sun Yat-sen University,Guangzhou 510275,China
Keywords:CO2 methanation Ni/bentonite catalyst Solution combustion synthesis Impregnation method
A B S T R A C T A 20 wt%Ni/bentonite catalyst was prepared by a solution combustion synthesis(SCS),which exhibited higher activity for the CO2 methanation than that of an impregnation method(IPM),and the catalyst prepared by SCS showed a CO2 conversion of 85%and a CH4 selectivity of 100%at 300°C,atmospheric pressure,and 3600 ml·(g cat)-1·h-1,and the catalyst exhibited stable within a 110-h reaction.The results showed higher metallic Ni dispersion,smaller Ni particle size,larger specific surface area and lower reduction temperature in the Ni/bentonite prepared by SCS than that of IPM.And the Ni/bentonite prepared by the SCS moderated the interaction between NiO and bentonite.
1.Introduction
In the past few decades,the CO2concentration in the atmosphere has increased from the combustion of fuels and rapid development of modern industry,which causes the global warming and even climate change[1].Therefore,the conversion and capture of the CO2have attracted most of the attention in recent years.CO2methanation is a simple and environment-friendly reaction,the product,CH4,is the main constituents of the natural gas with high calorific value,and the environmental protection and the growing demands have been increasing in recent years[2,3].At present,the catalysts used for the catalytic reduction of CO2to methane have been widely studied,such as Rh/γ-Al2O3[2],Ni/Al2O3[4],and Ni/La2O3[5].Among them,the noble metal-based catalysts were more active in the CO2methanation than the non-noble metal catalysts,however,the cost of the noble metalbased catalysts restricts its large-scale industrial application[4,6,7].In another side,the Ni-based catalysts have been widely studied in CO2hydrogenation due to its low cost,high catalytic properties and easy availability.However,to obtain a higher CO2conversion,the reaction requires higher temperatures above 350°C,which has an adverse influence on the stability/lifetime of the catalysts as well as increased energy consumption[6].In addition,it is a challenge that CO2is an inert molecule and difficultly activated at low temperature,as well as the Ni-based catalysts are easy to sinter deactivation at high temperature[8].Therefore,low-temperature activity Ni-based catalysts should be developed to reduce the reaction temperature.
The solution combustion synthesis(SCS)contains metal nitrates(as oxidizers)and fuel(as reducers)to generate small particle oxides,and was used to prepare the nanosized catalysts with a high metal dispersity,uniform distribution even precise stoichiometric radio[3,9],and the catalysts prepared by SCS exhibited many advantages,including the simply prepared devices,low-cost,rapid reaction rates,and relatively low preheating temperatures[3],furthermore,the fuel in SCS provided energy in the combustion and as a complexing agent to form complex with metal ions increasing its solubility.The different gases are released during the rapid exothermic reaction,which promotes the formation of the nanosized material with high specific areas and inhibiting particle size growth[10].
Bentonite is a porous clay mineral with a layered structure,and is an abundant mineral resource,and exhibits the characterization of low cost and environmental compatibility,which is widely used in the field of catalysis[11].Based on the previous research about the Nibased catalysts[12–14],the Ni/bentonite catalysts were prepared by SCS using urea as a fuel,and were applied in the CO2catalytic hydrogenation to CH4in the present study.Furthermore,the Ni/bentonite catalysts were characterized by the X-ray diffraction(XRD),H2temperature-programmed reduction(H2-TPR),scanning electron microscope(SEM),N2-adsorption/desorption,ICP optical emission spectrometers(ICP-OES),and X-ray photoelectron spectroscopy(XPS).And as a comparison,the Ni/bentonite catalyst was prepared by a traditional impregnation method(IPM).
2.Experimental
2.1.Catalyst preparation
The Ni supported bentonite catalyst was prepared according to the literature[15],typically,the ratio of the fuel(F,urea)/oxidizer(O,nickel nitrate)was 3,and the Ni/bentonite catalysts were prepared by SCS with a metallic Ni loading amount of 20 wt%based on the weight of bentonite[12].Firstly,2.97 g Ni(NO3)2·6H2O and 3.0 g bentonite were dissolved in 20 ml deionized water to obtain a suspension,and the urea was added to the suspension.The mixture was heated to 70°C at 200 r·min-1for 4 h,followed by a process of heating to 400°C with a heating rate of 5°C·min-1and kept at 400°C for 4 h.The obtained catalyst with a Ni loading amount of 20 wt%was marked as Ni/Bn-SCS.As a comparison,the catalysts were prepared by a traditional IPM(marked as Ni/Bn-IPM),which was the same process as mentioned above without using urea as a fuel;as well as the Ni/γ-Al2O3-SCS prepared by combustion method was used as another comparative catalyst.
2.2.Catalytic hydrogenation of CO2
The catalytic hydrogenation of CO2to CH4over the Ni/bentonite catalyst was conducted at atmospheric pressure in a fixed-bed reactor,which consisted of a stainless-steel tube with an inner diameter of 8 mm and a tube length of 300 mm.0.50 g catalyst was added to the tube and was reduced at 400 °C for 2.0 h accompanied by a 30 ml·min-1H2(99.999%).After cooling to 250°C,the feed gas(a mixture of H2/CO2=4:1(volume ratio))was introduced into the reactor at a 30 ml·min-1flow rate,and the catalytic hydrogenation of CO2to methane was conducted at 250–450°C with a gaseous hourly space velocity(GHSV)of 3600 ml·(g cat)-1·h-1.The products were analyzed with an Agilent 4890D gas chromatograph equipped with a TCD detector.The CO2conversion(XCO2)and CH4selectivity(SCH4)were determined by using a peak area normalization method.
2.3.Characterization of catalysts
The XRD was conducted in a Bruker D8 Advance X-ray diffractometer with a Cu Kαradiation at 40 kV,40 mA,by using a graphite monochromator,and the average crystal size of the NiO crystallite was estimated from the(200)plane of NiO by the Scherrer equation.N2adsorption–desorption was conducted on a TriStar II 3020(Micromeritics Instrument Co.Ltd.),and the surface areas were calculated by the Brunauer–Emmett–Teller(BET)method and the pore size distribution was evaluated from adsorption branches of the nitrogen isotherms using the Barret–Joyner–Halenda(BJH)method.The Ni contents in the catalysts were precisely determined by an Agilent 725 ICP-OES after the samples dissolved by using the nitric acid solution.The SEM images were observed in a Hitachi SU8220 scanning electron microscope.The XPS profile was measured by an ESCSALAB 250X multifunction imaging electron spectrometer(Thermo Fisher Scientific Co.,Ltd.)with an Al Kαradiation at 150 W and a pass energy of 30 eV,and the binding energy values were calibrated by C 1s(284.6 eV).H2-TPR was applied on a DAS-7000 adsorption instrument(Hunan Huasi Instrument Co.Ltd.,China),the detail was as described in the literature[12].
3.Results and Discussion
3.1.XRD analysis
The XRD patterns of the bentonite,the calcined Ni/Bn-SCS and the Ni/Bn-IPM were shown in Fig.1(A).Obvious diffraction peaks were found at 2θ = 19.8°, 34.9°, and 61.9°, corresponding to the montmorillonite(JCPDS card NO.29-1499),and the diffraction peaks at 2θ=21.8°,26.6°,and 36.5°corresponded to the SiO2(JCPDS card NO.46-1045),which indicated that the support was a typical bentonite[12].The diffraction peaks of NiO were detected in Ni/bentonite at 2θ=37.2°,43.3°,62.8°,75.4°,and 79.3°,which ascribed to the plane(111),(200),(220),(311),and(222)of the cubic phase NiO,respectively.The diffraction peak intensity of NiO in Ni/Bn-SCS was remarkably weaker than that of Ni/Bn-IMP,indicating that NiO particles had a good dispersity and corresponding to the size of metallic nickel particle shrinks[16,17].And the average crystallite size of NiO in Ni/Bn-IPM(18.9 nm)was bigger than that of Ni/Bn-SCS(7.5 nm),calculated by the Scherrer's equation according to the diffraction plane(200),which demonstrated that the NiO in Ni/Bn-SCS catalyst exhibited a good dispersion.The XRD pattern of the reduced Ni/Bn-IPM and Ni/Bn-SCS was shown in Fig.1(B).No NiO peaks were found in the reduced catalysts,and the metallic Ni was found in Ni/Bn-IPM and Ni/Bn-SCS at 2θ=44.6°,52.0°and 76.6°,which ascribed to the planes(111),(200),and(220)of the metallic Ni(JCPDS card NO.87-0712),respectively.The diffraction peak intensity of Ni in the Ni/Bn-SCS was weaker than that of the Ni/Bn-IMP,indicating that the metallic Ni particles had a good dispersity and the shrinking of the metallic nickel particle size[16,17].
3.2.SEM analysis
Fig.2 showed the SEM images of the Ni/Bn-IPM and Ni/Bn-SCS catalysts before and after the reduction.In Fig.2(A),the calcined Ni/Bn-IPM catalyst presents an irregularly-shaped bulk morphology NiO particle aggregation,and the NiO particle size was about 0.16 μm.While the calcined Ni/Bn-SCS catalyst(in Fig.2(B))with the spherical NiO particles was uniformly dispersed with a smaller particle size of 0.05–0.10 μm on the layered bentonite surface.From Fig.2(C)and(D),obviously,the reduced Ni/Bn-SCS catalyst exhibited a smaller Ni particle size and narrower Ni particle size distribution than the reduced Ni/Bn-IPM catalyst,and the metallic Ni particles were highly dispersed on the bentonite surface on the Ni/Bn-SCS catalysts,which was caused by the formation of the large amount of gaseous byproducts in the Ni/Bn-SCS preparation process,and leading to a significant expansion of the product and rapid cooling after the reaction,which makes the solid products better dispersed.The results indicated that the solution combustion method was a useful method to prepare smaller Ni particle of the Ni-based catalysts,which would benefit to the CO2methanation.
3.3.Textural properties
The N2adsorption–desorption isotherm and pore size distribution of bentonite,Ni/Bn-IPM and Ni/Bn-SCS were shown in Fig.3,and texture properties of the three samples are summarized in Table 1.
From Fig.3(A),the N2adsorption isotherm of the catalysts and bentonite was a IV-type isotherm according to the IUPAC classification,which indicated that the catalysts were of mesoporous structure.In the low-pressure region(P/P0=0–0.4),the N2adsorption capacity increased slowly with the increase of relative pressure,which indicated that the N2adsorption on the surface of the catalyst converts single layer into multilayer[18].In the medium and high-pressure region(P/P0>0.4)the adsorption and desorption isotherms were inconsistent,an H3-type hysteresis loop was generated by the capillary condensation,indicating that the Ni/Bn-IPM and Ni/Bn-SCS catalysts were the layered structure[19].The pore size distribution curve was presented in Fig.3(B)and the textural property data was showed in Table 1,which showed a narrow pore size range between 2 and 20 nm in all samples,indicating a good homogeneity porous structure.In Table 1,the BET surface area of the bentonite,calcined Ni/Bn-SCS and the calcined Ni/Bn-IPM were 75.7,79.8,and 48.9 m2·g-1,respectively.The specific surface area of the Ni/Bn-IPM catalyst was smaller than that of the bentonite,which was caused by the active component Ni blocking the pores of the bentonite[20].And the specific surface area of the Ni/Bn-SCS catalyst was slightly higher than that of the bentonite,which is due to the active component of Ni that was well-dispersed on the support and further increased the total area of the Ni/Bn-SCS,which was the sum of the active components,carriers and impurities on the surface.In addition,the specific surface area of the Ni/Bn-SCS was higher than that of the Ni/Bn-IPM,which would favor the dispersion of active components on the catalyst surface and further increased the mass transfer of the reactants[21].It is interesting to note that the pore volume increases from 0.158 to 0.228 ml·g-1with Ni/Bn prepared by SCS method,which was probably because the Ni particles could block some micropores of bentonite to decrease the pore volume of the Ni/bentonite,and partly because some pores were formed in the Ni particles to increase the pore volume of the Ni/bentonite catalyst[3,22].Combined with the SEM results,the particle size of Ni in Ni/Bn-SCS catalyst was the smaller,which indicated that the catalysts prepared by SCS would be favorable for the preparation of Ni-based catalysts with smaller Ni particle size,which not only increased the surface areas of catalyst,and also led to a better dispersion of the Ni particles and less blocked holes of the catalyst.

Fig.2.SEM images of calcined Ni/Bn-IPM(A),and Ni/Bn-SCS(B),and reduced Ni/Bn-IPM(C),and Ni/Bn-SCS(D).
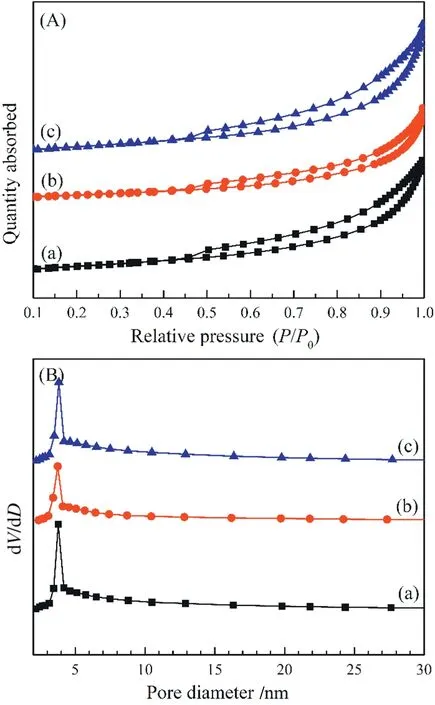
Fig.3.N2-adsorption/desorption isotherms(A)and pore diameter distributions profiles(B)of the bentonite(a),Ni/Bn-IPM(b),and Ni/Bn-SCS(c).
3.4.H2-TPR analysis

Fig.4.H2-TPR profiles of Ni/Bn-IPM(a)and Ni/Bn-SCS(b)catalyst.
H2-TPR was carried out for the reducibility of the NiO,the amount of the Ni species reduction,and the interaction between the Ni species and the support of the Ni/bentonite prepared by the SCS and the IPM,the results were shown in Fig.4.There are four Gaussian fitting peaks in the Ni/Bn-IPM and Ni/Bn-SCS catalysts,peaks α,β,γ,and δ.In the two samples,the reduction peak α at 330–380°C corresponds to the reduction of NiO located at the outermost layer of bentonite;the peak β at 390–460°C was attributed to the reduction of the NiO particles confined within the mesopores,which had a relatively strong interaction with the bentonite support[16,17];the higher temperature peak γ at 490–530°C was assigned to the reduction of the greater dispersion NiO particles,which had a strong interaction with the bentonite;the small δ peak centered at 708 and 696°C,corresponding to Ni/Bn-IPM and Ni/Bn-SCS catalyst,was ascribed to the stable nickel aluminate phase with a spinel structure[17,23].For the Ni/Bn-SCS catalyst,the reduction peaks α and β centered at 340°C and 393°C,respectively,which were lower than those of Ni/Bn-IPM catalyst(371°C and 455°C,respectively),indicating the much higher dispersion of NiO particles on the bentonite surface for the Ni/Bn-SCS catalyst[24],which would have increased the catalytic activity of Ni/bentonite in the hydrogenation of CO2.
In general,the catalyst reduction is closely related to the activity of the catalyst,and the larger reduction peak area of the samples can provide more active sites for the reactant,which results in higher catalytic activity.From Table 2,the NiO species of peaks α and β were the main sources of low-temperature activity after reduction,and the corresponding total reduction peak areas of the peaks α and β of the Ni/Bn-SCS(13268)were higher than those of Ni/Bn-IPM catalyst(11420),indicating more active sites in the Ni/Bn-SCS and would be favorable for the CO2methanation reaction at low temperature.In addition,the appearance of reduction peak area of the peaks α was shifted to the higher temperature when the catalyst was prepared by SCS,the peak α of the Ni/Bn-SCS catalyst was weaker,indicating the high dispersion of the NiO on the surface of the bentonite[17].

Table 1 Texture properties of Ni/bentonite catalysts prepared by IPM and SCS

Table 2 Temperatures and area distributions of reduction peaks of Ni/bentonite catalyst prepared by IPM and SCS①
3.5.XPS analysis
Fig.5 showed the XPS profiles of the Ni 2p and O 1s for the Ni/bentonite catalyst prepared by IPM and SCS.Two peaks of Ni 2p 1/2 and Ni 2p 3/2,which were located at 874.2 eV and 856.3 eV in the Ni/Bn-IPM and located at 874.4 eV and 856.7 eV in the Ni/Bn-SCS,respectively,were the typical binding energies of Ni2+,indicating that the Ni existed in the form of Ni2+in the two catalysts before reduction[25].And the peaks centered at 880.6 eV and 862.5 eV in the Ni/Bn-IPM and 880.9 eV and 874.4 eV in the Ni/Bn-SCS ascribed to the satellite peaks of the Ni 2p 1/2 and 2p 3/2,respectively,which produced by the orbital spin splitting[26].In the Al 2p spectra,the peak located at 68.3 eV for Ni/Bn-IPM and 68.2 eV for Ni/Bn-SCS,corresponded to the Ni 2p 3/2 in Ni(OH)2,which showed that a small amount of Ni(OH)2exists in the catalysts[27,28].Combined with the XRD results,the Ni in the catalyst exists mainly in the form of NiO,and the Ni 2p spectra of the two catalysts are basically the same,indicating that the presence of Ni in the two catalysts is NiO.Compared with the Ni/Bn-IPM,the peak intensity of the Ni 2p in Ni/Bn-SCS was much stronger,which indicated that the Ni species of Ni/Bn-SCS was uniformly dispersed in the benonite[26].In another hand,the binding energies of the Ni species in the Ni/Bn-SCS were 0.3–0.4 eV higher than the Ni/Bn-IPM catalyst,which was due to the SCS method that weakened the interaction between the NiO and the bentonite support,and decreased the electron cloud density of Ni atoms.
In addition,in the O 1s spectra,the peaks located at 534.0,532.0,and 529.1 eV for Ni/Bn-IPM were lower with 0.1–0.3 eV than the Ni/Bn-SCS with 534.1,532.1,and 528.6 eV,corresponding to the three types of O on the SiO2,Al2O3,and NiO[28],respectively.It shows that the electron cloud around the O atom in the SiO2and Al2O3of the Ni/Bn-IPM catalyst was more inclined to NiO,which reduces binding energies of Ni2+.Therefore,the electron cloud density around Ni of the Ni/Bn-SCS catalyst was decreased,and a weak interaction between NiO and bentonite after the catalyst prepared by SCS method,and the reduction temperature of NiO decreases,which is consistent with the results of H2-TPR analysis[29].Meanwhile,the peaks of Al 2p 1/2 and Si 2p 3/2 located at 74.4 eV and 102.4 eV for Ni/Bn-IPM were higher with 0.1–0.3 eV than the Ni/Bn-SCS with 74.1 eV and 102.3 eV,respectively,indicating the weak interaction between metal NiO and bentonite support species of the Ni/Bn-SCS to decrease the binding energies of Al 2p and Si 2p.
3.6.CO2 methanation on the Ni/Bn catalysts
3.6.1.Effect of reaction temperature
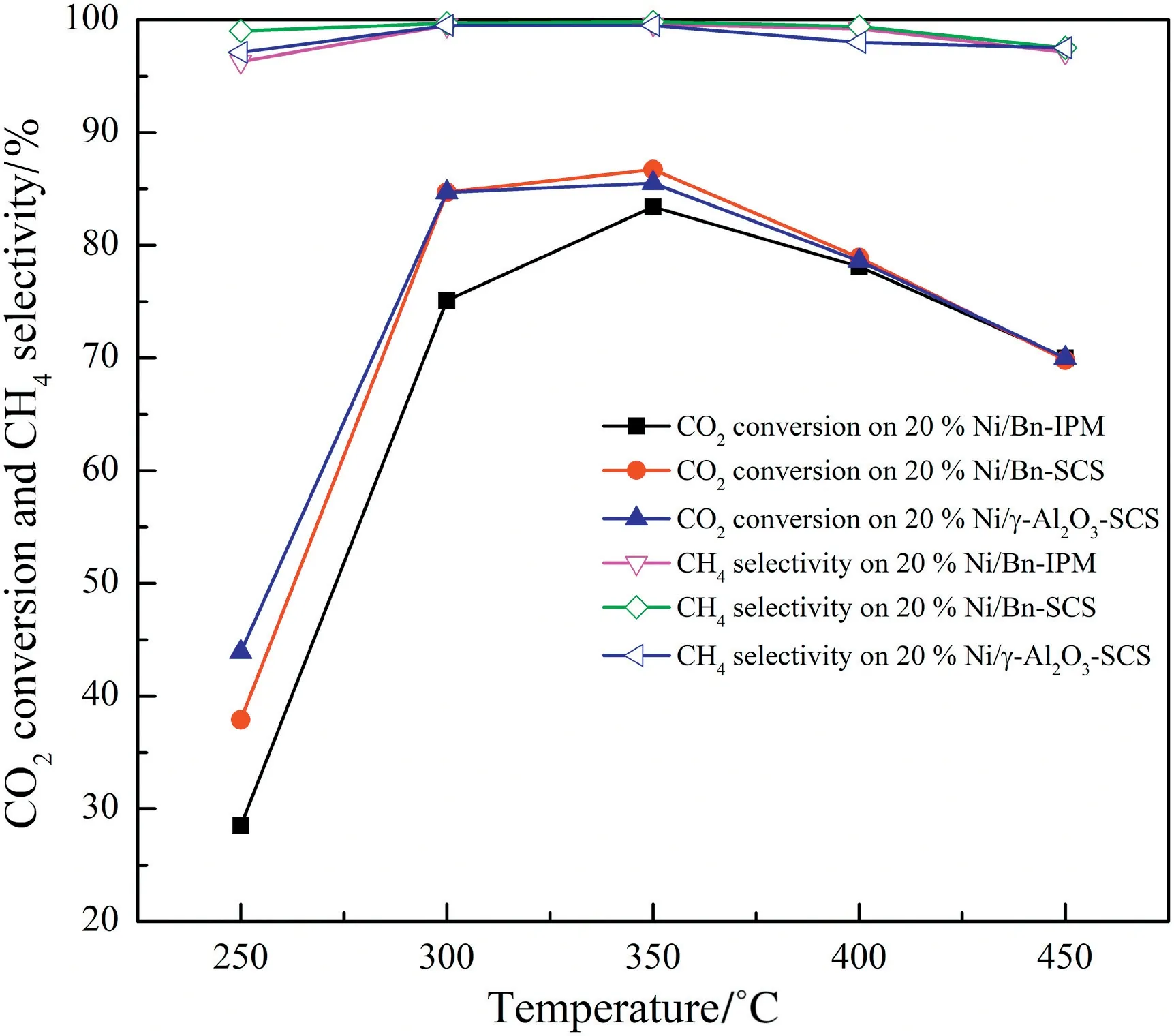
Fig.6.The catalytic activity of Ni/Bn-IPM,Ni/Bn-SCS and Ni/γ-Al2O3 catalyst was for CO2 methanation.Reaction conditions:V(H2)/V(CO2)=4:1,atmospheric pressure,GHSV=3600 ml·(g cat)-1·h-1.

Fig.5.XPS profiles of the Ni 2p,O 1 s,Al 2p and Si 2p regions for Ni/Bn-IPM(a)and Ni/Bn-SCS(b)catalyst.
The catalytic CO2methanation was tested at 250 to 450°C,the results showed in Fig.6.With the increase of the reaction temperature,the CO2conversion rapidly increased and reached the maximum of 83%and 86%at 350°C when using Ni/Bn-IPM and Ni/Bn-SCS as catalysts,respectively.However,further increasing the reaction temperature up to 450°C,the CO2conversion was decreased.The CO2methanation is an exothermic reaction with the Gibbs free energy △G<0 at 298.15 K,therefore,the △G increases with the increase of reaction temperature,which would promote the reverse reaction and decrease the CO2conversion in a higher temperature of 450°C.In the present study,the CO2conversion at 250°C on the Ni/Bn-SCS was 43.9%,which was nearly two times higher than that on the Ni/Bn-IPM(28.5%).At 300°C on the Ni/Bn-SCS and the Ni/Bn-IPM catalyst,the CO2conversion was 85%and 75%,respectively.Moreover,the CH4selectivity of both was approximately 100%at the temperature higher than 300°C.The better catalytic performance of the Ni/Bn-SCS catalyst especially at low-temperature regions can be due to the availability of lowtemperature reducible metal species that is evidenced from H2-TPR results and weak interaction between metal Ni and bentonite support species that is shown from XPS results.The SCS processes released a large amount of gaseous byproducts,such as CO2,H2O,and N2,which not only makes the solid products porous and increases the specific surface area of the solid oxide,but also makes the NiO better dispersed in the bentonite[30,31].These results indicated that the Ni/Bn-SCS catalyst exhibited higher CO2conversion than the Ni/Bn-IPM catalyst,especially at low temperature,and the catalyst was prepared by SCS promoting the catalytic activity.Finally,the Ni/γ-Al2O3-SCS as a comparative catalyst,its activity was almost the same as Ni/Bn-SCS and better than that of Ni/Bn-IMP catalyst.
3.6.2.Effect of gas hourly space velocity
The effects of the gas hourly space velocity(GHSV)of 2400–7200 ml·(g cat)-1·h-1on the CO2methanation are shown in Fig.7.When the GHSV increased from 2400 to 7200 ml·(g cat)-1·h-1,the CO2conversion decreased from 87.7%to 75.7%,and the CH4selectivity was still kept above 99%,which was caused by the shortening of the contact time between the catalyst and the CO2/H2mixing gases,leading to a lower catalytic activity.Although the CO2conversion decreased in the process,3600 ml·(g cat)-1·h-1was chosen as the optimal GHSV for the high CO2conversion and CH4selectivity for the other reactions to provide a comparison of the data.
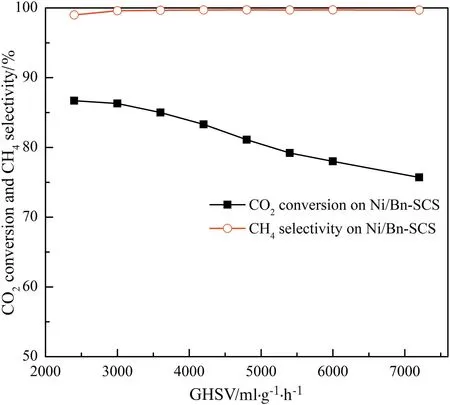
Fig.7.Effect of gas hourly space velocity on the CO2 methanation over the Ni/Bn-SCS catalyst.Reaction conditions:V(H2)/V(CO2)=4:1,atmospheric pressure,T=300°C.
3.6.3.Stability of the Ni/Bn catalysts

Fig.8.Lifetime tests of 20%Ni/Bn-IPM and 20%Ni/Bn-SCS catalysts for CO2 methanation.Reaction conditions:T=300°C,V(H2)/V(CO2)=4:1,atmospheric pressure,GHSV=3600 ml·(g cat)-1·h-1.
The stability of the Ni/Bn-SCS and Ni/Bn-IPM catalyst was studied at V(H2)/V(CO2)=4:1,atmospheric pressure,300°C,and GHSV=3600 ml·(g cat)-1·h-1,the results were shown in Fig.8.During a 110-h reaction,the CO2conversions on the Ni/Bn-SCS catalyst remained at about 85%,and the CH4selectivity was approximately 100%.For the Ni/Bn-IPM catalyst,the CO2conversions decreased from 75.2%to 68.9%during the 100-h reaction.These results indicated that the Ni/Bn-SCS exhibited a good catalytic stability than the Ni/Bn-IPM,and showed a higher catalytic activity at low temperatures.
4.Conclusions
The hydrogenation of CO2to CH4was conducted on the Ni/Bn-SCS and Ni/Bn-IPM catalysts in the present study.Characterization studies based on XRD,SEM,H2-TPR and XPS indicated that the highlydispersed NiO was formed during the SCS process,which subsequently contributes to the formation of a high metallic Ni dispersion in the supported catalyst.N2adsorption–desorption revealed that the catalysts prepared by the SCS would be beneficial for the increase of the surface area and the decrease of NiO particle size,and more uniform particle size distribution and better dispersion.Superior behavior with a CO2conversion of 85%and CH4selectivity of 100%was obtained on the Ni/Bn-SCS catalyst at a low temperature of 300°C.Compared with Ni/Bn-IPM catalyst,the Ni/Bn-SCS catalyst exhibited higher catalytic stability at low temperatures during the 110-h stability test.In a word,the present work provides a simple method for the preparation of a supported Ni-based catalyst with high dispersion,and demonstrates its enhanced low-temperature activity for CO2methanation.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- The CO2 absorption and desorption performance of the triethylenetetramine+N,N-diethylethanolamine+H2O system☆
- Modelling of a post-combustion carbon dioxide capture absorber using potassium carbonate solvent in Aspen Custom Modeller
- Mass transfer correlations for membrane gas-solvent contactors undergoing carbon dioxide desorption
- Carbon deposition and catalytic deactivation during CO2 reforming of CH4 over Co/MgO catalyst☆
- Recent developments and consideration issues in solid adsorbents for CO2 capture from flue gas☆
- High-efficiency and pollution-controlling in-situ gasification chemical looping combustion system by using CO2 instead of steam as gasification agent
