Recent developments and consideration issues in solid adsorbents for CO2 capture from flue gas☆
2018-04-08LijuanNieYuanyuanMuJunsuJinJianChenJianguoMi
Lijuan Nie ,Yuanyuan Mu ,Junsu Jin ,Jian Chen ,Jianguo Mi
1 Beijing Key Laboratory of Membrane Science and Technology,Beijing University of Chemical Technology,Beijing 100029,China
2 State Key Laboratory of Chemical Engineering,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
Keywords:CO2 capture Adsorption Adsorbents Inorganic materials Organic materials
A B S T R A C T The increase in energy demand caused by industrialization leads to abundant CO2 emissions into atmosphere and induces abrupt rise in earth temperature.It is vital to acquire relatively simple and cost-effective technologies to separate CO2 from the flue gas and reduce its environmental impact.Solid adsorption is now considered an economic and least interfering way to capture CO2,in that it can accomplish the goal of small energy penalty and few modifications to power plants.In this regard,we attempt to review the CO2 adsorption performances of several types of solid adsorbents,including zeolites,clays,activated carbons,alkali metal oxides and carbonates,silica materials,metal–organic frameworks,covalent organic frameworks,and polymerized high internal phase emulsions.These solid adsorbents have been assessed in their CO2 adsorption capacities along with other important parameters including adsorption kinetics,effect of water,recycling stability and regenerability.In particular,the superior properties of adsorbents enhanced by impregnating or grafting amine groups have been discussed for developing applicable candidates for industrial CO2 capture.
1.Introduction
CO2is one of the major greenhouse gases,and its concentration has been on the rise from 280 μl·L-1to 370 μl·L-1,such that the rising amplitude of the global earth temperature varies from 0.6°C to 1.0°C during the same period of time[1].Nowadays 37%of the anthropogenic released CO2are given by the combustion of fossil fuels in the power plants[2].Moreover,the fossil fuels will remain the major sources of energy for the next several decades.Faced with such severe reality,the reduction of CO2concentration in atmosphere becomes a global concern.Carbon capture and storage(CCS)is a promising technique for significant reduction of CO2emission from large point sources[3,4].CO2capture needs about three quarters of the total cost for the CCS system[5].Therefore,it is necessary to employ a cost-effective technology for CO2capture.
In the past years,liquid amine absorption was used in many power plants and becomes a mature technology to capture CO2from the flue gas[6,7].Nevertheless,the drawbacks such as high regeneration energy requirement and equipment corrosion rate limit its further development[8–10].In consideration of the advantages of more cost-effective and no corrosion to the equipment,solid adsorption has been developed as a substitute to the traditional solution absorption.Solid adsorbents are superior to the liquid amine absorbents because the formers require relatively lower CO2partial pressure,less energy for regeneration,and less amine loss during the recycling process.An ideal adsorbent should has the following characteristics:high working capacity and CO2/N2selectivity,tolerance to impurities,fast adsorption/desorption kinetics,morphological and chemical stability,favorable abrasive resistance in fixed or fluidized beds,low regenerate energy requirement and equipment cost[11,12],etc.Unfortunately,nowadays few adsorbents satisfy these comprehensive requirements.
There have been a multitude of researchers devoted to develop highefficient adsorbents.One possible method is the physical adsorption with the microporous adsorbents that have high porosity and surface area,such as zeolites[13,14],activated carbons[15,16],and metal–organic frameworks[17,18].Compared with liquid amine absorption,physical adsorption has the advantage of significant energy saving.Meanwhile,these physical adsorbents also have shortcomings,such as low tolerance to impurities and low CO2uptakes.In addition,these adsorbents require the incoming gas stream to be completely dehydrated because water can induce a reduction of adsorption capacity and promote their decomposition.To solve these problems,various amines have been considered to load on the pore surface,forming chemical adsorbents for CO2capture[19].As a consequence,these adsorbents combine the advantages of both amine species(strong affinity to CO2)and porous materials(large surface area and large pore volume).Another method is chemical adsorption.Carbonates, alkali metal oxides,amine-functionalized porous materials are three typical kinds of chemical adsorbents.CO2molecules can be adsorbed on the surface of these adsorbents by forming a chemical bond,predominantly a covalent bond.Compared with physical adsorption,chemical adsorption not only has high CO2uptake and CO2/N2selectivity but also leads to high adsorption and desorption temperature.
Herein we present a review on zeolites,clays,activated carbons,alkali metal oxides and carbonates,silica materials,metal–organic frameworks, covalent organic frameworks, and amine-functionalized polymerized high internal phase emulsions.The purpose of this review is to update the adsorption characteristics and the enhancement strategies.The adsorption mechanisms and kinetic behaviors of various adsorbents are discussed,and the basic researches on adsorption performances are also emphasized to evaluate their comprehensive properties.In addition,we try to introduce the advantages and disadvantages of the enhancement methods.It is expected to be helpful for future investigations.In general,the development of solid adsorbents for CO2capture is still in its initial stage,whereas there exists a huge opportunity in the near future for solid adsorbents applied in industrial CO2capture.
2.Zeolites
Zeolites are naturally typical materials with microporous crystalline aluminosilicate skeletons and can be synthesized in laboratory[20].The empirical molecular formula of zeolites can be expressed as M2/nO·Al2O3·xSiO2·yH2O,where n is the valence of the cation,and x and y are integers.They are made of silicate[SiO4]4-and aluminate[AlO4]5-tetrahedrons connected by oxygen atoms,forming a box-like structure(Fig.1)with a molecular size of 0.1–2.0 nm.

Fig.1.The structure of box-like zeolite[21].
Zeolites have been extensively applied in separation technologies due to their unique ability of molecular sieving[22,23].A gas with a high quaternary moment,such as CO2,interacts with the electron field formed by the cationic structure of zeolite,which is conducive to the adsorption.The adsorption characteristics of zeolites are influenced by alkalinities,porosities and electron field intensities.The focus of current studies is to optimize these factors by changing the Si/Al ratio and/or exchange with alkali and alkaline earth cations.For instances,Yang et al.[24]prepared the small“molecular gate”adsorbent ZK-5-n-K by adjusting the K+/Cs+ratio in the extra-framework and the Si/Al ratio in the zeolite of ZK-5.Fig.2 shows the K+and Cs+cations residing in different sites in ZK-5.The increase of K+ions can prevent the entry of larger molecules such as CH4and N2,while allowing smaller molecule CO2to enter.The adsorption selectivities of CH4or N2to CO2were enhanced by 105times.Pham et al.[25]conducted a detailed study on Li+-,Na+-,K+-,and Mg2+cation exchange sites in the ZK-5 zeolite,and determined the CO2adsorption sites within the framework.It was shown that the Li-ZK-5 sample possesses the highest CO2uptake at 101.325 kPa.Meanwhile,Li-,K-,and Na-ZK-5 revealed high adsorption isosteric heats(Qst)in low CO2concentration,and Na-ZK-5 displayed the maximum Qstof 49 kJ·mol-1.Mg2+was located in the cationic center of the hexagonal prism,which is difficult to contact with CO2molecules and leads to lower Qst(30 kJ·mol-1)and CO2adsorption capacity.The results on the ion exchange effect at the molecular level provide a basis for further study of novel zeolite adsorbents in CO2-related applications.
Water vapor has serious adverse effect on the CO2adsorption capacity and support stability of zeolites.As the physical adsorbents,zeolites are often restricted at low or ambient temperature.The low CO2selectivity toward N2is another limit for their application in CO2capture from the flue gas.In order to solve these problems,many researchers were committed to adding amines onto the pore surfaces of zeolites to obtain the composite materials.Madden et al.[26]synthesized the novel amine modified β-25 zeolite via physical impregnation.Physical impregnation was applied by mixing of amines with solvent(such as methanol and water)at a specific proportion,with a few hours,and at ambient temperature.Then the solvent were removed from the slurry by filtration or evaporation.The adsorbent displayed excellent CO2adsorption/desorption capacity with a CO2uptake up to 4.705 mmol·g-1and desorption occurring at 60°C.Moreover,the added moisture enhanced the CO2adsorption capacity by 19%(Fig.3).These results indicated that the wet CO2gas is beneficial to increase adsorption.Chen et al.[27]similarly prepared mesoporosity zeolite(Mesa-13X)and functionalized it with polyethylenimine(PEI)by wet impregnation. Compared with Meso-13X, Meso-13X-PEI showed higher CO2/N2adsorption selectivity along with higher CO2adsorption capacity at a relatively high temperature(100°C)and low CO2concentration.
Previous studies also found that most zeolites have perfect CO2adsorption kinetics performance,which can achieve adsorption equilibrium state within few minutes.They also have good cyclic stability and mechanical stability to shape them into pellets.However,the temperature and pressure have a great influence on the CO2capture performances.Zeolites require high regeneration temperature(above 573 K),thus the regeneration can cause large energy consumption.In addition,it is also necessary to reduce the high cost of zeolite materials.
3.Clays

Fig. 3. CO2 temperature-programmed desorption profiles for 40%aminopropyltriethoxysilane/β-25, following CO2 adsorption in dry and humid conditions[26].
Clay is a major component of soil minerals and is one of the most abundant natural materials at a very low cost(about$50 per ton).Different clay minerals usually consist of layered silicate,aluminum and other cations(iron,potassium,sodium,calcium,magnesium and zinc),and have been regarded as the potential adsorbent supports due to their low cost,rich natural abundance as well as high mechanical and chemical stability.However,the application is limited by their poor textural properties(e.g.low pore volume,<0.2 cm3·g-1).This defect can be overcome at some extent by acid or alkali modification,since they possess high cation exchange capacity and quick reaction rate with acid and alkali,leading to the improvement of structure performance,especially the pore volume and surface area.Therefore,the application of clays in the field of adsorption and separation can be largely extended.Many authors studied clay minerals such as bentonite,montmorillonite and kaolinite as supporting materials for CO2capture.For example,Wang et al.[28]employed an inexpensive and commercially available bentonite modified by sulfuric acid(Fig.4)as a support to prepare the amine functionalized adsorbent for CO2capture,and the maximum CO2uptake of 2.95 mmol·g-1was determined at 75°C under a dry condition.In another work,they used the wet impregnation method to immobilize PEI onto the porosity-enhanced kaolinite and montmorillonite to develop a new type of inexpensive adsorbent for CO2capture[29]. The maximum CO2uptakes were achieved at 75 °C with 2.55 mmol·g-1under dry condition and 3.23 mmol·g-1with the moisture addition.Irani et al.[30]prepared a low cost CO2adsorbent by immobilizing tetraethylenepentamine(TEPA)onto the acid-modified nanosepiolite(Fig.5),and the CO2uptake was raised to 3.8 mmol·g-1for 1 vol.%CO2in N2along with 1 vol.%H2O at 60°C.Vieira et al.[31]developed a layered silicate adsorbent via impregnating PEI,and the maximum CO2uptake reached 6.11 mmol·g-1at 75°C.To save the cost of material and energy,the development clay materials for CO2capture are imperative,since they have the advantages of low cost,easy availability,high mechanical and chemical stability.
4.Activated Carbons
Carbonaceous materials are the main components of physical adsorbents,including activated carbons,carbon molecular sieves,carbon nanotubes and graphene[32,33].Though carbonaceous materials only consist of carbon element,they have many advantages including fine thermal and chemical stability,extraordinary thermal conductivity and low cost;thus it is not surprising that carbonaceous materials were extensively investigated for CO2capture[34–36].Among various carbonaceous materials reported in the literature,activated carbons are primarily reviewed here,since they are the most utilized adsorbents for post-combustion CO2capture.Activated carbons possess mesoporous and microporous structure and have an advantage over other adsorbents in the cost of raw materials.The cheap and readily available carbon sources and industrial scale production can extensively reduce the preparation cost.Despite their advantages,different raw materials bring about great variation in textural properties,resulting in highly variable CO2adsorption characteristics[37].Carbonization and activation are two basic steps for preparing activated carbons from raw materials[38,39].In the carbonization process,the precursor materials discharge a majority of the non-carbon elements,such as H,O and N in the form of gases and tars,leaving behind a rigid carbon skeleton.Different kinds of precursor materials result in variable adsorption characteristics[40].After the activation stage,the modified carbonaceous materials can produce appropriate porosity and active sites,and high surface area.Both the functional groups and structural properties(surface area,pore size distribution and pore volume)of activated carbons can be organized via the carbonization or activation treatments.By optimizing carbonization process,Sethia et al.[35]developed a strictly microporous activated carbon with nitrogen content as high as 22.3 wt%.This adsorbent displayed the highest CO2uptake about 5.39 mmol·g-1at 25°C and 101.325 kPa.The result demonstrates that both the nitrogen content and pore structure in activated carbon play important roles in CO2adsorption.Alabadi et al.[16]employed a direct carbonization method by mixing the carbon precursors and activating agents in the tubular furnace in nitrogen or argon atmosphere to form a high-performance activated carbon.The obtained adsorbent yielded significantly high CO2uptake of 7.49 mmol·g-1at 0°C,which can be attributed to the exceptional microstructure and the introduction of oxygen and nitrogen functional group by surface modification.Furthermore,the researchers added various basic groups onto the activated carbon supports.For example,Alhassan et al.[41]reported the performance of base functionalized bagasse activated carbon for CO2capture.The base functionalized adsorbent was characterized with improved CO2uptake and CO2/N2selectivities due to the enhanced affinity of adsorbent to CO2molecules.

Fig.4.Simplified illustration of bentonite modification by sulfuric acid.

Fig.5.A proposed mechanism for the acid dissolution of sepiolite and modified sepiolite/TEPA.
Due to the competitive adsorption performance of water vapor and CO2molecules,the CO2adsorption capacities of activated carbons are negatively affected by water vapor[42].Even though the hydrophobic performance of majority activated carbons diminish this adverse effect compared to zeolites,storage under humid surroundings also lead to a decline of CO2adsorption capacity.This unfavorable aging effect on the adsorption capacity of activated carbons under vapor environment is due to a gradual oxidation of the adsorbent surfaces.
In conclusion,activated carbons offer some advantages such as low desorption temperature(<373 K),easy-to-regenerate and low energy consumption,competitive kinetics performance(reaching their equilibrium CO2capacity is less than 10 min)with zeolites,and high thermal and cyclic stability,but most of all,the adsorption capacities increase with the CO2partial pressure[1,43–45].The main drawbacks of active carbons include:the low CO2uptakes under relatively low CO2partial pressure because of the inert surface chemistry,the adsorption capacities decreased dramatically with temperature,they are more susceptible to impurities such as NOx,SOxand H2O in the flue gas[46],and the strong effect of Hg0on CO2adsorption[47].As mentioned above,in order to overcome these disadvantages,many studies paid more attention on the modification and activation of carbon materials to strengthen the surface affinity toward CO2[48].Moreover,active carbons are relatively soft,which may lead to high attrition in a fluidized bed and high adsorbent replacement in the whole CO2capture system.At present,the studies on active carbons are still concentrated on improving CO2uptake and selectivity at low CO2partial pressure.The main approaches include employing the novel precursors to obtain materials with high surface area and tunable pore structure and structural functionalization by either amine exterior impregnation or combination of basic nitrogen group interior of the supports.
5.Carbonates and Alkali Metal Oxides
There is other category of CO2adsorbent made up of alkali metal oxides and alkali metal carbonates.Among various adsorbents studied in this group,the representative CaO-based and K2CO3-based adsorbents are mainly considered and reviewed in this part.
5.1.CaO-based adsorbents
The CaO-based adsorbents have been regarded as favorable candidates compared with other currently used materials in consequence of inexpensive and readily available of precursors such as marble or dolomites[49,50].Calcium oxide operates by carbonation-calcination cycles(Eq.(1)).The process diagram of capturing CO2using calcium circulation process is shown in Fig.6.During a carbonation stage,CO2molecules react with CaO molecules at 600–650°C to form the corresponding alkali metal carbonate(CaCO3)molecules while the reverse calcination reaction takes place at 800–850°C to regenerate the CaO and release CO2[52]:

Fig.6.Schematics of the applications of calcium looping process in CO2 capture[51].

where the produced CO2is compressed and sequestered subsequently.At meantime,the adsorbents could be deactivated due to the high temperature in the calcination process,thus those deactivated parts should be removed from the calcination process and fresh limestone adsorbent needs to be supplied.The carbonation–calcination reaction is often referred as high-temperature CO2adsorption/desorption cyclic process.The main advantage of this process is that the overall cost is significantly lower than that of the competing technologies,since the heat from the exothermic carbonation reaction in the carbonator can be used to generate extra energy.Furthermore,there is no need to modify the existing power plant,which is another advantage of the capturing process.
According to the adsorption stoichiometric carbonization reaction,the theoretical adsorption capacity is 17.8 mmol·g-1.With regard to this,it should be pointed out that even though most of CO2uptakes described in the studies are lower than the theoretical value,they are still higher than other inorganic chemical adsorbents.However,one of the major drawback of the natural CaO–CaCO3process is its rapidly capacity fading during the repeated adsorption/desorption cycles.The decrease of working-capacity is mainly due to pore blockage and surface sintering(Fig.7).Fortunately,the presence of water steam can promote the production of mesopores,even in favorable conditions for sintering.Many researchers suggested that,for the CaO–CaCO3looping system,water steam and adsorbent interact in two ways[54,55].On the one hand,steam seems to affect the evolution of the pore structure,which is crucial to the cyclic working-capacity.On the other hand,it is a possibility that steam affect the reaction rate constant and the mobility of CO2molecules and ionic species(e.g.O2-and Ca2+)on the surface and in the matrix.Generally,steam have been proven to enhance CO2capture performance in the CaO–CaCO3cycles.

Fig.7.Schematic description of the transformation of a calcium oxide adsorbent in recarbonation/decomposition cycles.The CaCO3 phase is shown by dark gray,and calcium oxide is shown by light gray.The first decomposition of the calcium carbonate produces highly dispersed reactive calcium oxide,being followed by incomplete recarbonation.The amount of unreacted calcium oxide increases from cycle to cycle until the rigid interconnected calcium oxide skeleton is formed that prevents further sintering of the adsorbent[53].

Fig.8.Comparison of typical conversion data with the conversion curves expected from chemical control at the CaO/CaCO3 interface and diffusion control through the product layer with constant diffusivity,respectively[56].
The CO2adsorption kinetics of CaO-based adsorbents are controlled by the rates of the carbonation reaction,which is governed by surface reaction and product-layer diffusion(Fig.8)[57].Through the study of carbonization kinetics of CaO,many researchers demonstrated that there exist two stages of carbonation reaction rate[58].The adsorption rate on the first and rapid stage is affected by the temperature and CO2partial pressure,and the reaction rate increased with temperature or pressure.The carbonization reaction rate on the second stage is almost uninfluenced by CO2pressure,which is gradually reduced due to pore filling during the carbonization reaction.Many adsorption kinetic models were used to describe the dynamic characteristics of these two reaction stages(Fig.9).For examples,Johnsen et al.[60]applied the shrink core model to analysis the carbonation reaction and assumed the adsorbent is a sphere without porous.Sun et al.[57]applied a grain model to study the rate-controlling steps of the carbonation reaction,and the results indicated that the reaction rate with a first-order reaction turn into zero-order dependence when the CO2partial pressure surpasses 10132.5 Pa.Benedetti et al.[61]utilized a random pore model to describe the carbonation process,and the computed data indicated that this model can accurately predict the whole conversion-time curves.
An excellent review paper regarding the CaO-based adsorbents for CO2capture was published by Wang et al.[46].All the research efforts can be briefly summarized in Fig.10.The CaO particles with large surface area,small particle size or special microstructures can be obtained by different synthesis methods or acid pretreatment.To further improve its performance,a more feasible approach is to prepare the mixed adsorbents.For instance,the durability of CaO particles can be improved significantly by adding CaO particles into inert materials which are used as structural support or matrix.Because the CaO-based adsorbents have a good prospect in practical application,their abrasion resistance,adsorbent reactivation and the effect of SO2have also been investigated.
Overall,the advantages of CaO-based adsorbents include inexpensive,high theoretical CO2adsorption capacity and high attrition resistance.However,the kinetic factors and CO2adsorption capacities are strongly influenced by sintering during continuous carbonation/calcination,and their practical application may be also influenced by higher adsorption and regeneration energy.

Fig.10.A brief summary of all efforts for improving CO2 capture capacity and sinteringresistant properties of CaO-based sorbents[46].
5.2.K2CO3-based adsorbents
Alkali metal carbonates such as K2CO3and Na2CO3can adsorb CO2in the presence of water to form the corresponding hydrogen carbonates at 40–80°C and discharge CO2above 120°C[62,63].The reaction mechanism can be represented by:

Former studies showed that the adsorption capacity of the K2CO3-based adsorbents is higher than that of the Na2CO3-based ones[64,65],such that we pay more attention to the K2CO3-based type.Deliquescent potassium carbonate needs to be supported on active carbon or other porous supports such as silica aerogels,γ-Al2O3and MgO with an optimal loading.Various types of K2CO3-based adsorbents in literature are shown in Table 1.One can see that different supports are applied to improve the surface areas and porosities of the adsorbents,at the same time to improve the abrasive resistance of adsorbents in fluidized bed[75,76].The reported adsorption capacities of the K2CO3-based adsorbents vary from 0.25 to 4.49 mmol·g-1.These different capacities arise from different specific surface areas,pore volumes and the synergistic effects on CO2adsorption.

Fig.9.Gas–solid reaction models[59].

Table 1 Adsorption capacities of various K2CO3-based adsorbents reported in the literature
The CO2uptakes of K2CO3-based adsorbents were reported in the range of 0.25–4.49 mmol·g-1.It was shown that the CO2adsorption capacity relies mainly on the structure of the supports and the percentage of water vapor in the flue gas[68,71,72].Zhao et al.[77]studied the influence of CO2adsorption capacities with different potassium-based supports.The results indicated that the K2CO3/coconut activated charcoal,K2CO3/coal active carbon and K2CO3/Al2O3yield high CO2uptakes,while the K2CO3/silica gel exhibits low CO2uptake.Lee et al.[78]prepared K2CO3/activated carbon,K2CO3/TiO2,K2CO3/Al2O3,K2CO3/MgO,K2CO3/SiO2and K2CO3/zeolite adsorbents,and evaluated the influence of CO2adsorption and desorption capacity in the company of water vapor in a fixed-bed react system.The results suggested that the K2CO3/MgO possess the maximum CO2uptake of 2.70 mmol·g-1,and the K2CO3/active carbon and K2CO3/TiO2can be wholly regenerated under 150°C.When Al2O3,MgO and CaO are used for the K2CO3supports,the initial CO2capture capabilities are acceptable,but the observed byproducts cannot be completely converted to their original stage under the temperature below 200°C.
During the adsorption process,K2CO3·1.5H2O plays an important role in the K2CO3-based adsorbents,which can react quickly with CO2molecules:

such that the water vapor concentration is crucial in creating K2CO3·1.5H2O in chemical reaction.The CO2uptake of the K2CO3/molecular sieve can be reduced when the vapor content in the flue gases is as high as 8–17 vol%[79].In addition,the deliquescent character of K2CO3results in the formation of aqueous solution in the support pores,which controls the adsorption temperature of CO2[80].
In summary,the advantages of the alkali metal carbonates include higher abrasion resistance,acceptable CO2uptake and high bulk density.Their disadvantages include low adsorption rate,higher regeneration temperature,difficulty in heat control,susceptible to impurities and weak cycling stability.The latest research direction is to develop composite materials using different kinds of adsorbent supports such as Al2O3,activated carbon,TiO2,SiO2,ZrO2,and so on.
6.Silica Materials
Silica materials have the advantages of high surface area,large pore volume,adjustable pore size and excellent mechanical stability[81,82].So far,there were many published papers using silica materials for CO2capture.The researches focused mainly on the following three aspects:using the new preparation methods to synthesize amine modified silica composite materials;developing silica adsorbents with new pore structures;and evaluating CO2adsorption performances of various silica composites[83,84].
There are many kinds of silica adsorbents,and the latest investigations focused on the synthesis and modification of mesoporous silica materials for efficient CO2capture[85–91].Wang et al.[92]synthesized a series of PEI modified glass fibers via physical impregnation.The CO2uptakes of these adsorbents were higher than that of pure PEI,and the best had the highest CO2uptake of 1.36 mmol·g-1.In contrast,MCM-41 had the lowest CO2uptakes.All PEI modified materials exhibited reversible and stable CO2adsorption/desorption behaviors in the cycling tests.Jones CW groups contributed great work in grafted aminosilica adsorbents in the past two decades[93–97].They prepared the hyperbranched aminosilica CO2adsorbents by the ring-opening polymerization of aziridine from mesoporous silica and amines.The advantage of this adsorbent over the previously reported adsorbents is the stability of the organic groups covalently bound to the silica support compared to those made by physisorbed methods.To understand the effects of amine species and load methods on CO2adsorption performances,Sanz et al.[98]used four organic amines to synthesize the amine modified SBA-15 adsorbents by co-condensation,chemical grafting and physical impregnation.The results indicated that both the adsorbents obtained by co-condensation and physical impregnation have poor CO2adsorption performances owing to the weak distributions of CO2accessible sites on the external surface of the support.They found that the ideal adsorbent is SBA-15-supported aminosilane,which possesses fine spreading of amino sites and enhanced diffusivity of CO2molecules in the pores.Niu et al.[99]used pristine halloysite nanotubes to produce a novel nanocomposite CO2adsorbent.This material exhibited both acceptable CO2uptake(2.75 mmol·g-1at 85°C)and good cycling stability,indicating that the obtained novel mesoporous silica nanotubes display the potential of application in CO2capture.Khdary et al.[87]prepared mesoporous silica composite containing Cu nanoparticles.They found that the CO2adsorption capacity of the composite is 40%higher than that of the pristine mesoporous silica materials.
Previous researches not only synthesized amine or metal modified silica composites,but also created silica materials with tunable pore structures.As a result,the CO2capture performances were significantly improved.It is worth mentioning that the“molecular basket”adsorbents.The concept of CO2“molecular basket”was firstly proposed by Song CS group for developing a high CO2uptake and selective adsorbent[100–104].In order to prepare the“molecular basket”adsorbent,a sterically branched PEI with numerous CO2-affinity sites and low adsorption heat is loaded into the large-pore-volume mesoporous molecular support.Fig.11 shows the schematic diagram of PEI loaded in the mesoporous molecular sieve of MCM-41.The MCM-41-PEI adsorbent can selectively“pack”CO2in a condensed form in the mesoporous molecular sieve,thus functioning like a CO2“molecular basket”and therefore show a high CO2adsorption capacity and a high CO2selectivity.On the other hand,Sanz-Pérez et al.[85]employed several amines as structure-directing agents to prepare different pore structures of HMS mesoporous silica materials.The consequences revealed that the large pores can increase the grafting degree of organosilane,while the narrow pores improve the physical adsorption performance by enhancing the interaction between CO2and adsorbent.Jahandar Lashaki et al.[105]studied the influence of pore structure of the SBA-15 adsorbent on CO2capture performance.These results indicated that the adsorbents with macropore structure have the highest amine loading on the pore surface,the highest CO2adsorption capacity and CO2/N2selectivity,and the shortest adsorption time.

Fig.11.Schematic diagram of PEI loaded in the mesoporous molecular sieve of MCM-41.(A)MCM-41 support;(B)low PEI loading;(C)high PEI loading;(D)extremely high PEI loading[101].
In conclusion,it has been demonstrated that silica materials have highly interconnected meso/macropores and high CO2/N2ratio[63,81],thus have high CO2uptakes at ambient temperature[106],and need low regeneration energy.Under the condition of dry flue gas,the amine-based silicon material is quite stable at the actual concentration of SO2gas stream,and the presence of NOximpurities at low concentration do not significantly affect the CO2adsorption capacity[107].However,because of the hydrolysis of Si--O--Si bonds,many problems with these materials are caused by their low hydrolytic stability.The latest research direction is to develop amine modified silica composite adsorbents.
7.Metal–Organic Frameworks
Metal–organic frameworks(MOFs)are a subclass of coordination polymers and have tunable pore structure[108].These materials are usually made of three-dimensional organic–inorganic hybrid networks formed by multiple metal–ligand bonds.Their structural properties having many advantages such as good thermal stability,adjustable chemical functionality and pore structure,high porosity and so on.Several MOFs and composites were testified as promising CO2adsorbents[109–111].Recently,Bhatt et al.[17]prepared a set of physical MOF adsorbents used for CO2capture at relatively low CO2concentrations,and the adsorbents showed extraordinary high CO2selectivities and uptakes.Ghahramaninezhad et al.[112]fabricated the novel Li-trapped polyoxometalate/MOF porous nanocomposite material named ZIF-8@{Mo132}@Li+,which possessed noticeably higher CO2uptake than the pristine zeolitic imidazolate framework-8(ZIF-8)(Fig.12).This study is the first report on the creation of MOF-type CO2adsorbent using both functionalization and combination method with other materials.Xu et al.[113]prepared the MOF(HKUST-1)and graphite oxide(GO)composite materials for CO2capture.The 2GrO@HKUST-1 adsorbent achieved 9.0 mmol·g-1CO2uptake at 1 bar and 0°C,which is 32%higher than that of HKUST-1 adsorbent(6.85 mmol·g-1).However,the presence of impurities leaded to poor CO2capture performance of MOFs.In particular,water molecules destroyed crystal lattices of MOFs by replacing the ligands[114,115].
On the other hand,MOFs modified with amine groups not only increase CO2uptake and selectivity,but reduce negative influence of water in the flue gas[116].Martínez et al.[117]reported the incorporation of tetraethylenepentamine(TEPA)in ZIF-8 by wet impregnation,and found that the appropriate TEPA loading weight in the material yields a suitable CO2adsorption capacity of 1.52 mmol·g-1at 0.15 bar and 45°C.Under the simulated flue gas condition,the CO2adsorption capacity of the TEPA-impregnated ZIF-8 was significantly enhanced to 2.36 mmol·g-1in the presence of water.
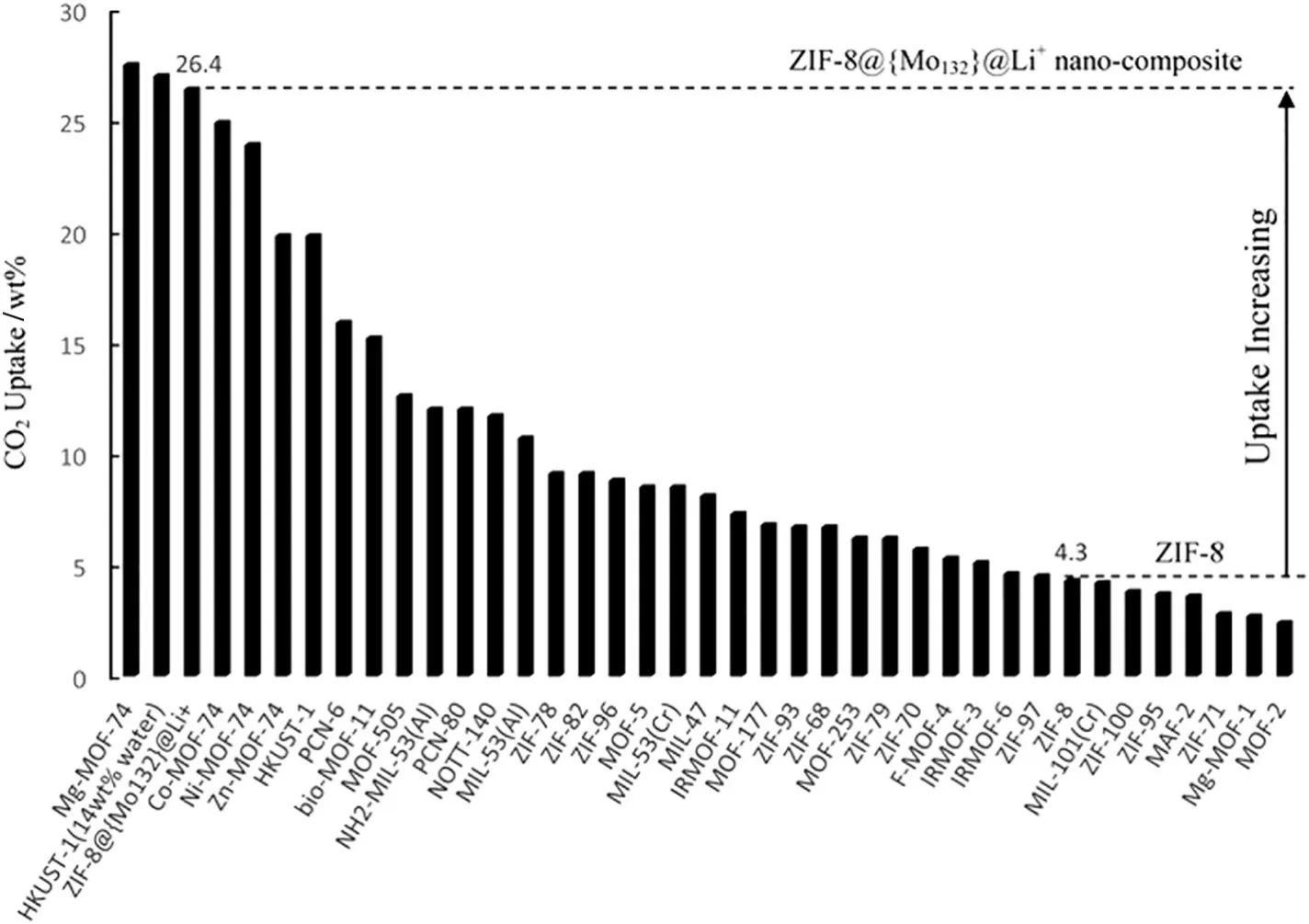
Fig.12.CO2 adsorption capacity(wt%)of the ZIF-8@{Mo132}@Li+nanocomposite in comparison with other MOFs at 298 K and 0.1 MPa[112].
However,the CO2uptakes of the pristine MOFs are lower than other solid adsorbents under low CO2partial pressure[118,119].At the pressure of 0.1 bar,the influence of structure characteristics(pore volume or surface area)on the adsorption capacity is larger than that of adsorption heat,which was identified by Yazaydin et al.[120].When desorption is performed at high temperature,MOFs are prone to collapse,resulting in the loss of porosity and order,and forming amorphous solid.Furthermore,the composition of flue gas also has great influence on the structure of MOFs.Even at low moisture level,the oxygen-metal coordination bonds in MOFs are readily hydrolyzed,which irreversibly destroy the frameworks.Luz et al.[121]found that,in the presence of SO2,amine-based MOF materials show a clear deactivation of the CO2adsorption capacity.This deactivation is due to the irreversible reaction of SO2and amines during the adsorption process,which has no further activity for CO2capture.Besides,the difficulty in large-scale production and high cost are additional disadvantages of MOFs.Nowadays,the research tendency of MOFs in CO2capture is to find the adsorbent with high CO2uptake,good kinetics and cyclic stability,and invulnerable to impurities under real flue gas condition.It is necessary to further assess the advantages and disadvantages of MOFs for CO2capture[115].
8.Covalent Organic Frameworks
Covalent organic frameworks(COFs)are two/three-dimensional porous crystal macromolecules,entirely composed of light elements including C,H,O,N,and B,which are connected with strong covalent bonds[8,122].Because of their excellent physical and chemical stability,high surface area,and easy functionality,COFs have a promising prospect in CO2capture[123].There are several organic reactions for constructing COFs,including the formation of B--O(boronate,boroxine and borosilicate),C=N(imine,hydrazine,and squaraine),C--N(triazine and imidization),B--N(borazine)and N--N(azodioxides)bond linkages[124,125].Several organic reactions are shown in Fig.13.
Boron-based COFs are the earliest known organic covalent materials discovered in 2005[126].The basic building blocks of the most boronbased COFs are organic molecules having multiple boronic acid sites.On the one hand,this particular skeleton structure results in high thermal stability.On the other hand,they are vulnerable to nucleophilic attack by water molecules at the electron-deficient boron sites,even slightly humid air could cause the damage of framework.Low hydrolytic stability is a major limitation on the use of B-based COFs for CO2capture from the flue gas.Some enhancements in the water stability have been achieved by pyridine doping of the COF pore walls.Du et al.[127]found that adding less than 1%pyridine to COF-5 and COF-10 can stabilize these basic building blocks upon exposure to humid gas.The unmodified COF-10 decomposed within 24 h under the water vapor environment,while the COF-10 modified by pyridine lasted for several weeks without decomposing under the same condition.Later,they studied the effects of higher pyridine doses on COF-5 and COF-10,and the effect of pyridine doping on the stability of the boroxine ring based COF-1[128].They concluded that the low doses of pyridine doping can trigger neutral reaction with the Brønsted acid sites and give hydrolytic stability.However,excess of pyridine could convert all of the remaining B[3]in COF-5/-10 to Lewis B[4]and cause the total and irreversible structural decomposition of the two materials.High dose of pyridine modification brings about a decrease in the gas capture performance,despite it enhances the hydrolytic stability to small extent.Therefore,the defect of low hydrolytic stability in B-based COFs prevents their usage for CO2capture from the flue gas.
The imine-based COFs is another class of COFs,which were widely studied for the CO2capture.Compared with the B-based COFs,the imine-based COFs are more stable in acid and base treatments and insensitive to water,which are beneficial to hydrolytic stability.Kandambeth et al.[129]combined reversible and irreversible organic reactions(Fig.14)to synthesize two novel imine-based COFs(TpPa-1 and TpPa-2).They displayed special resistance to boiling water and acid treatment,and TpPa-2 also possessed brilliant stability in a basic medium.They believed that,by introducing a large alkyl group near the center of secondary nitrogen,the problem of basic stability could be solved to some extent at the expense of reduced surface area.Due to the enhanced interaction between nitrogen atoms and CO2molecules,the nitrogen-rich COFs are an ideal candidate for CO2capture.Kaleeswaran et al.[130]synthesized four kinds of nitrogen-rich COFs,with different surface areas and permanent porosities,leading to different CO2uptakes.It is worth noting that the TAPB-TFP adsorbent has the highest CO2uptake of 4.09 mmol·g-1(273 K,101.325 kPa).In the application of CO2capture,the imine-based COFs with high CO2selectivity,large CO2uptake and moderate adsorption temperature make them be the most promising adsorbent.Nevertheless,the synthesis of COFs require complex monomers,expensive catalysts,and/or severe operating condition,which lead to the highly preparation cost,complex production process and hard to industrialize.

Fig.13.Several reversible organic reactions used for successful construction of COFs[124].

Fig.14.Schematic representation of the synthesis of TpPa-1 and TpPa-2 by the combined reversible and irreversible reaction of 1,3,5-triformylphloroglucinol with p-phenylenediamine and 2,5-dimethyl-p-phenylenediamine,respectively.The total reaction proceeds in two steps:(1)reversible Schiff-base reaction and(2)irreversible enol-to-keto tautomerism[129].
Compared with B-based COFs,the triazine-based COFs exhibited low adsorption heat,high CO2uptake and CO2/N2selectivity.Even in the presence of water,the adsorbents still maintained high CO2capture performance during repeated regeneration process.Zhao et al.[131]created a perfluoro-covalent triazine-based CO2adsorbent.The adsorbent has a hydrophobic nature of the C--F bonds and an excellent hydrolytic stability.Meanwhile,the triazine-based COFs showed excellent CO2/N2selectivity and thermal/chemical stability.However,only few triazinebased COFs possess high crystallinity and their structure depends largely on synthesis conditions,and other disadvantages such as poor structural integrity and small porosity are also unavoidable issues during their further development.
9.Porous Polymer Materials
Porous polymer materials,another type of CO2adsorbents,have high physical and chemical stability due to the covalent bonding of network structures.Compared with inorganic porous adsorbents such as zeolites,activated carbons and silica materials,porous polymer adsorbents exhibit permanent porosity with high surface areas and can be prepared via diversified chemical synthetic routes.These characteristics provide several chances to control the porous structure as well as framework and surface functionality to meet the demands of CO2capture.There are several relevant classes of porous polymer adsorbents[132–135].Here we focus on the materials via high internal phase emulsion polymerization(polyHIPE).Emulsion is a mixture of two highly immiscible liquids,and when the volume fraction of the dispersed phase is greater than 74%,it is called a high internal phase emulsion.The polymerization of monomers in the continuous phase followed by the removal of the emulsified droplets in the dispersed phase yields a highly porous polyHIPE[136].An obvious approach to modify a synthesized polyHIPE is to incorporate functional groups onto the pore surface,and the obtained composite combine the properties of both material.The CO2adsorption property of the functionalized adsorbent is affected by the nature of the polyHIPE support and the introduced functional groups.The functional groups such as amino and imine can provide the basic sites to react with the acidic CO2molecules,while the polyHIPEs matrix controls the mechanical properties of the adsorbents.Thus,recent researches are mainly focus on the polyHIPEs supports and functionalization strategy to improve CO2adsorption.
As mentioned above,the amine-functionalized adsorbents can enhance the affinity to CO2molecules and improve the CO2adsorption performance.At meantime,the huge pore volume,interconnected voids and holes in polyHIPE supports render the functionalized groups highly permeability and good compatibility,which is favorable for increasing mass transport and thus the CO2capture performance.Compared with the pristine polyHIPE supports,the amine-functionalized adsorbents possess relatively lower surface area,pore size and volume.Different types of amines result in different amine loadings on the polyHIPE supports due to the varying steric hindrance and partial blockage of the amines in the pores.However,the amine-polyHIPE materials still exhibit the open porous structure with interconnectivity similar to the pristine polyHIPE supports.These amine-modified adsorbents have been reported to possess high CO2adsorption capacity and water tolerance as compared to pristine porous adsorbents[137,138].Furthermore, the decomposition temperature of the pristine polyHIPE supports and amine-functionalized materials are higher than 300°C and 200°C,respectively[139].The adsorbents own a relatively good thermal stability and can be acceptable for practical application in CO2capture progress.All these advantages of amine-modified adsorbents as well as great CO2/N2selectivity and reasonable adsorption heat,to a great extent make them an extremely competitive class of adsorbents for CO2capture.Previous studies focused on loading liquid amines(such as ethanolamine,tetraethylenepentamine,3-aminopropyltriethoxysilane,and so on.)on multiple polyHIPE supports such as polystyrene[140],polydivinylbenzene[141],poly(styrene-co-divinylbenzene)[142],poly(vinylbenzyl chlorideco-divinylbenzene) [143, 144], poly(4-vinylbenzyl chloride and divinylbenzene)[136],poly(divinylbenzene-co-ethylene glycol dimethyl acrylate)[145],and so on.
Three methods were engaged in preparing amine-modified polyHIPE adsorbents.The first one is composed of monomeric or polymeric active amines physically impregnated into/onto porous supports.In this way,amines can be uniformly scattered on inner and outer surface of the supports by the Van Der Waals forces.These materials can be easily synthesized and can introduce a large amount of amines to the polyHIPEs supports.Liu et al.[145]synthesized a hypercrosslinked polymeric support using the suspension polymerization method,subsequently impregnated with tetraethylenepentamine(TEPA).The CO2uptake and amine efficiency of the TEPA-impregnate adsorbent were influenced by TEPA loading weight(Fig.15).In detail,as the TEPA loading weight increased from 10 wt%to 30 wt%,the CO2uptake and amine efficiency increased gradually.The highest CO2uptake(3.11 mmol·g-1)and amine efficiency(0.46 mmol CO2/mmol N)of the adsorbent appeared at 30 wt%TEPA loading.However,with the further increase of TEPA loading to 50 wt%,the CO2uptake decreased significantly.The reason was referred to the appropriate amount of TEPA,which provides more available active sites for CO2capture.While excess TEPA not only covered the outer surfaces of support,but also blocked the pore channels,which prevents the reaction of CO2molecules with internal amine groups.Wang et al.[142]prepared the polyHIPEs with highly interconnected and hierarchical pore structure.When the supports were impregnated with PEI,the obtained adsorbents displayed high CO2uptake and CO2/N2selectivity,fast adsorption kinetics,and improved CO2capture performance in moist gas.In general,the physical impregnation method is easy to operate and can accommodate the highest amount of active amines,but the amines do not provide the strong interaction with the supporting surface,leading to poor cycling stability.

Fig.15.(a)Breakthrough curves and(b)amine efficiencies of the hyper-cross-linked resin with different TEPA contents(TEPA@PDVBpc:1 g,C(CO2):10 vol%,T:25°C,V:30 ml·min-1)[141].
The second type of adsorbent is on the basis of polyHIPE supports covalently tethered with amines.Up to now,diverse chemical grafting approaches have been used to prepare amine-modified polyHIPE adsorbents for CO2capture.Duan et al.[140]synthesized cyanuric chloride cross linked polystyrene polyHIPE supports via free radical polymerization (Fig. 16). The highest specific surface area of the adsorbents reaches up to 1226 m2·g-1and the maximum CO2capacity is 3.20 mmol·g-1at 0°C and 101.325 kPa.He et al.[136]produced a series of porous polyHIPEs with different pore volumes and sizes.Later,the quaternary ammonium salt groups were introduced into the pore surface through further modification of the porous supports by quaternization and ion exchange reaction(Fig.17).Thus obtained adsorbents with highly interconnected meso/macroporous showed fast CO2adsorption kinetics.Han et al.[146]produced a novel CO2adsorbent by injecting PEI hydrogel into the porous poly(glycidyl methacrylate)support.The PEI molecules were grafted onto internal pore surface by a ring opening reaction between the amino groups and epoxy groups.These results indicated that the porous polymer modified with PEI displays high CO2uptake and good regeneration stability despite in the presence of water vapor.One advantage of grafting method is to establish the strong bond interaction between the amine functional groups and the supports,hence enhancing cycling stability of the functionalized adsorbents.Nevertheless,the amount of loading amines are limited by the number of functional groups existent on the support surface,and it seems that the actual amine groups grafting ratio are relatively low,which inevitably leads to small CO2uptakes.
The third type of amine-modified adsorbents can be prepared by one-step method to directly polymerize the amines into the polyHIPE matrix.After the polymerization reaction,the hydrophobic part of the functional groups is embedded into the polyHIPE matrix and the hydrophilic amines groups can be suspended on the surface of the pores to react with CO2molecules.Such reaction ensures high CO2adsorption capacity and prevents amine leaching and volatilization at high temperature.Wang et al.[147]synthesized such a PEI functionalized polyHIPE by one-step polymerization method.The adsorbent possessed high CO2uptake of 5.25 mmol·g-1,good moist-resistance performance,and excellent cycling stability(Fig.18),providing a promising adsorbent for actual CO2capture process.Because of this kind of amine functionalized method can affect the stability of the HIPEs and prevent polymerization process,thus only a small number of amine-polyHIPE adsorbents can be obtained from this directly synthesize method,which should be exploited more in the future.

Fig.16.Construction of nitrogen-containing hierarchical porous polymers and its application on carbon dioxide capturing[140].

Fig.17.HIPEs as templates for the preparation of functional porous polymeric materials[136].

Fig.18.50 adsorption/desorption circles of the polyHIPE/nano-TiO2/PEI-50 adsorbent under two conditions:moist CO2,adsorption at 75°C with CO2/H2O/N2(1:1:8)gas mixture and desorption at 150°C with CO2/H2O(9:1)gas mixture;dry CO2,adsorption at 75°C with CO2/N2(1:9)gas mixture and desorption at 150°C with pure CO2[147].
All these three kinds of amine modified methods have their advantages and disadvantages.Further improvements of the adsorbent CO2capture performance are necessary such as long-term stability and CO2adsorption capacity when the functional groups are added.In this regard,combination of two or three amine functionalization methods could be suitable to avoid any shortcomings of each method.
In conclusion,the polyHIPE based adsorbents used in CO2capture from the flue gas is still in its initial stage,but have a promising future owing to their merits such as hierarchical pore structure,excellent physicochemical and moisture stability,and mild preparation conditions.The optimal performance of these polyHIPE adsorbents is still more or less lacking in some practical CO2capture situations,such as their relatively poor mechanical strength.In spite of the fact that the crush strength of the polyHIPEs can be partially enhanced by replacing some or all of the soft monomer with the rigid monomer,by increasing the continuous phase content,by incorporating inorganic nanoparticles into the organic matrix,or by increasing weight content of surfactant in HIPEs,the mechanical properties of polyHIPEs are still necessary for further enhancement.
10.Conclusions
CO2capture technologies have been extensively concerned by scientific researchers.It is obvious that the synthetic routes of the adsorbents are crucial for high capture efficiency.Various solid adsorbents such as zeolites,clays,activated carbons,alkali metal oxides and carbonates,silica materials,metal–organic frameworks,covalent organic frameworks and porous polymer materials have been well studied for their application in CO2capture.Many typical features of the adsorbents have been clarified,including CO2adsorption capacity,adsorption thermodynamics and kinetics,the influence of water and impurities,regeneration energy,cyclic stability,and mechanical strength.Unfortunately,no single perfect adsorbent can meet all of these demands so far.The advantages and disadvantages of each type of adsorbents should be systematically evaluated in the actual adsorption process so as to effectively separate CO2from the flue gas.
The ultimate goal of studies in CCS is to reduce the cost of CO2capture by minimizing the energy requirements and improving the efficiency of the energy conversion.Hence,the most effective method is to explore new and cheap adsorbents and technologies so that the capture process can operate reliably and the adsorbents can be reused many times in a real flue gas situation.In addition,it is also necessary to extend the new technologies from laboratory to industrial scale.These challenges will be the focuses of future research.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Ni/bentonite catalysts prepared by solution combustion method for CO2 methanation☆
- Mass transfer correlations for membrane gas-solvent contactors undergoing carbon dioxide desorption
- Carbon deposition and catalytic deactivation during CO2 reforming of CH4 over Co/MgO catalyst☆
- The CO2 absorption and desorption performance of the triethylenetetramine+N,N-diethylethanolamine+H2O system☆
- Modelling of a post-combustion carbon dioxide capture absorber using potassium carbonate solvent in Aspen Custom Modeller
- High-efficiency and pollution-controlling in-situ gasification chemical looping combustion system by using CO2 instead of steam as gasification agent
