The CO2 absorption and desorption performance of the triethylenetetramine+N,N-diethylethanolamine+H2O system☆
2018-04-08YaoyaoLiChangjunLiuRichardParnasYingyingLiuBinLiangHoufangLu
Yaoyao Li ,Changjun Liu ,Richard Parnas ,Yingying Liu ,Bin Liang ,Houfang Lu ,*
1 School of Chemical Engineering,Sichuan University,Chengdu 610065,China
2 Institute of New Energy and Low Carbon Technology,Sichuan University,Chengdu 610207,China
3 Institute of Materials Science,University of Connecticut,Storrs,CT 06269-3136,USA
Keywords:CO2 capture Post-combustion Amine mixtures Biphasic absorbent
A B S T R A C T Post-combustion CO2 capture(PCC)process faces significant challenge of high regeneration energy consumption.Biphasic absorbent is a promising alternative candidate which could significantly reduce the regeneration energy consumption because only the CO2-concentrated phase should be regenerated.In this work,aqueous solutions of triethylenetetramine(TETA)and N,N-diethylethanolamine(DEEA)are found to be efficient biphasic absorbents of CO2.The effects of the solvent composition,total amine concentration,and temperature on the absorption behavior,as well as the effect of temperature on the desorption behavior of TETA–DEEA–H2O system were investigated.An aqueous solution of 1 mol·L-1 TETA and 4 mol·L-1 DEEA spontaneously separates into two liquid phases after a certain amount of CO2 is absorbed and it shows high CO2 absorption/desorption performance.About 99.4%of the absorbed CO2 is found in the lower phase,which corresponds to a CO2 absorption capacity of 3.44 mol·kg-1.The appropriate absorption and desorption temperatures are found to be 30°C and 90°C,respectively.The thermal analysis indicates that the heat of absorption of the 1 mol·L-1 TETA and 4 mol·L-1 DEEA solution is-84.38 kJ·(mol CO2)-1 which is 6.92 kJ·(mol CO2)-1 less than that of aqueous MEA.The reaction heat,sensible heat,and the vaporization heat of the TETA–DEEA–H2O system are lower than that of the aqueous MEA,while its CO2 capacity is higher.Thus the TETA–DEEA–H2O system is potentially a better absorbent for the post-combustion CO2 capture process.
1.Introduction
A large number of carbon capture and storage(CCS)programs have been put forward to mitigate CO2emissions[1].The Chinese government is investing in this strategy and has declared intentions to reduce CO2emissions by 40%–45%and 60%–65%per unit of GDP from 2005 levels by 2020 and 2030,respectively.Carbon dioxide capture,utilization,and storage(CCUS)technology is the most promising way to achieve these goals[2].CO2capture is the initial step of these processes.A low energy-consuming CO2capture process will greatly benefit the economic feasibility of CCUS technologies.
Post-combustion capture technology is the most plausible way to retrofit existing large CO2emitting facilities[3–5].Various aminebased CO2absorption processes such as the classic monoethanolamine(MEA)-based process,methyldiethanolamine(MDEA)-based process,and methyldiethanolamine(MDEA)–piperazine(PZ)-based process were adapted to capture CO2from flue gas due to their availability and maturity[6–12].Many efforts focused on process optimization and energy integration were performed to reduce the energy demand[13].However,the improvement achieved by process optimization and energy integration is limited by the thermodynamics of the solvent system.The absorption heat of the MEA system is around 90 kJ·(mol CO2)-1[14,15].Recent research has led to industry claims of capturing CO2from coal based exhaust with a reboiler energy requirement of about 2.8 GJ·(t CO2)-1,or even less[16].The optimized energy demand of the MEA system is still too high to be affordable for most industrial facilities such as power plants.Tertiary amines of lower reaction heat(e.g.61 kJ·(mol CO2)-1for MDEA)[17–19]can absorb CO2in the form of bicarbonates,which decompose at a much lower temperature to release CO2than the MEA carbamate does.Reaction heat occupies around 60%of the total regeneration energy requirement in PCC.Therefore,considering their lower reaction heat,tertiary amine based absorption solvents could lead to much lower energy demands in PCC[5,20].On the other hand,the energy consumed by heating-up and vaporizing the solvent in the regeneration process can be minimized if the CO2-binding products can be concentrated and separated from the bulk solution.Biphasic absorbents show great potential in this aspect.Certain combinations of tertiary amine and primary/secondary amine can form a biphasic absorbent of CO2which would separate into two immiscible phases after absorbing CO2and most of the CO2will be concentrated in one phase.Only the CO2-rich phase is necessarily sent to a regenerator.In this way,smaller stripper and less energy are needed.At the same time,primary/secondary amine as an activator could enhance the absorption rate and tertiary amine as a promoter could improve the desorption rate and regenerability. Therefore, such tertiary and primary/secondary amine combinations are expected to be promising CO2capture solvents due to their possibility to efficiently capture CO2with lower energy demand[21].

Fig.1.Molecular structure of various amines.
Several biphasic absorbents for CO2capture were reported[22–27].IFP Energies Nouvelles developed the biphasic DMX™absorbent which shows much higher CO2cyclic loading than that of the 30%(5 mol·L-1)aqueous MEA absorbent(2.4 mol CO2·(kg solvent)-1vs.1.3 mol CO2·(kg solvent)-1)[22].The minimum energy demand for the DMX™absorbent regeneration is about 2.1 GJ·(t CO2)-1which is 1.6 GJ·(t CO2)-1lower than the 3.7 GJ·(t CO2)-1typical of the 30%MEA process[28,29].
N,N-diethylethanolamine(DEEA)is found to be a critical component of biphasic solvents.It can form homogeneous aqueous solution with various primary/secondary amines,and the resultant solutions will separate into two liquid phases upon CO2absorption[23–26].Primary/secondary amines such as 3-(Methylamino)propylamine(MAPA)and 1,4-butanediamine(BDA)are important activators to enhance the CO2absorption.The cyclic CO2loading of a MAPA–DEEA system is much higher than that of MEA[23].However,its absorption heat is still too high(about-93.5 kJ·(mol CO2)-1)[27].It was found that 97.4%of the total absorbed CO2was enriched in the lower phase of the BDA+DEEA system[24,25].However,the absorption capacity and absorption rate of MAPA or BDA based mixtures are still not good enough since the number of amine groups in the absorbent molecule positively affects desorption amount,absorption capacity,and absorption rate[30–32].Ye et al.[26]compared the CO2capacity of a series of linear aliphatic primary monoamines,secondary monoamines,saturated cyclic primary monoamines,heterocyclic secondary amines,linear diamines,and linear polyamines as the second amine component combined with either DEEA or DMCA.The aqueous mixture of the triethylenetetramine(TETA)and DEEA was found to be the most promising biphasic system.Furthermore,increasing the secondary amine groups led to a lower absorption heat due to the formation of the secondary carbamates[31].Thus,TETA is expected to have less energy demand and better CO2absorption/desorption performance(Fig.1).Besides,the TETA is less volatile than its counterparts such as MAPA,BDA,and MEA(Table 1),which could benefit the absorbent recovery.As a tertiary amine,DEEA can also form carbonate and bicarbonate with CO2,which can facilitate the regeneration of absorbent.However,the CO2absorption and desorption behaviors of the TETA–DEEA system,as well as the effects of operation parameters on its performance have not been well documented yet.

Table 1 Summary of the basic properties of the chemicals
This work focused on the CO2absorption and desorption performance of the TETA–DEEA–H2O system and compared it with that of the 30%MEA solution.In addition,the specific heat capacity of the absorbent,the heats of absorption,and the regeneration heat duty were measured and analyzed.
2.Materials and Methods
2.1.Chemicals
MEA(≥99.0 wt%),TETA(≥98.0 wt%),and DEEA(≥99.0 wt%)were purchased from Chengdu Kelong reagent chemical plant.N2(≥99.99%,volume fraction)and CO2(≥99.99%,volume fraction)were purchased from Chengdu Dongfeng Gas Co.,Ltd.All the chemicals and gases were used without further purification.The relevant physical properties are shown in Table 1.
2.2.CO2 absorption/desorption and thermal analysis experiments
The CO2absorption and desorption experiments were carried out in a bubbling gas–liquid contacting system equipped with a FN316B online infrared CO2analyzer from Shaanxi Fei'ente Instrument Technology Co.,Ltd.(Fig.2).
2.2.1.Absorption experiment
A clean gas was used in this investigation to evaluate the amine chemistry,whereas industrial flue gas contains many other compounds such as SO2,ash,and residual O2[33].A 12 vol%CO2flow was controlled by passing pure CO2and N2through mass flow controllers to simulate industrial flue gas.Both the N2and CO2were dried with silica desiccant before mixing.A flask was put in a water bath at a given temperature and a chilling condenser was adopted to minimize solvent vaporization.The system was purged with pure N2prior to each experiment.The absorption was initiated by injecting a given amount(25 ml)of the prepared fresh solvent into the sealed flask.The gas stream leaving the absorption flask was washed with dilute sulfuric acid and dried with silica desiccant before it entered the online CO2analyzer.The CO2concentration of the outgoing gas was recorded by the online CO2analyzer continuously.The absorption was considered complete when the CO2concentration of the outgoing gas reached 99%of the inlet gas and kept constant.
2.2.2.Desorption experiment
The process of desorption was carried out at atmospheric pressure.A given amount of the CO2saturated solution was introduced into the flask and kept at a given desorption temperature by a water bath.Pure N2was introduced into the flask at a given flowrate as the purge gas and internal standard.The concentration of the CO2in the gas leaving the flask was analyzed and recorded by the online CO2analyzer.The desorption was considered complete when the CO2concentration of the outgoing gas reached 0.4%to 0.6%and kept constant for about 30 min.
The response time and the effect of the dead volume in the experiment setup were determined by running blank experiments without injection of any solvent while keeping other experimental conditions identical.Thus the initial absorption/desorption rate and absorption/desorption amount were determined by comparing the experiments to the corresponding blank ones.The amounts of CO2absorbed or desorbed were calculated by integrating the CO2concentration change with time.The total concentrations of amines in each phases were determined by standard acid–base titration with a 0.1 mol·L-1HCl,while the total CO2concentrations in solutions were analyzed by the precipitation–titration method according to[34].The CO2loading is referred to the ratio of CO2concentration to amine concentration in solutions.
The CO2absorption heat of each amine was measured by NETZSCH STA 449 F3 by the simultaneous thermal analysis method according to references[35,36].The specific heat capacity of each amine was measured by NETZSCH 214 Polyma by the differential scanning calorimetry method according to references[37,38].
2.3.Evaluation index
The absorption rate rabs(t),absorption amount Rabs(t),and CO2loading α of the absorbent were adopted to evaluate the CO2absorption performance of the TETA–DEEA–H2O system,and were calculated by Eqs.(1)–(4).At the end of the absorption experiment t=tf,the final absorption amount,Rabs(tf)is taken as the absorption capacity of the solution.

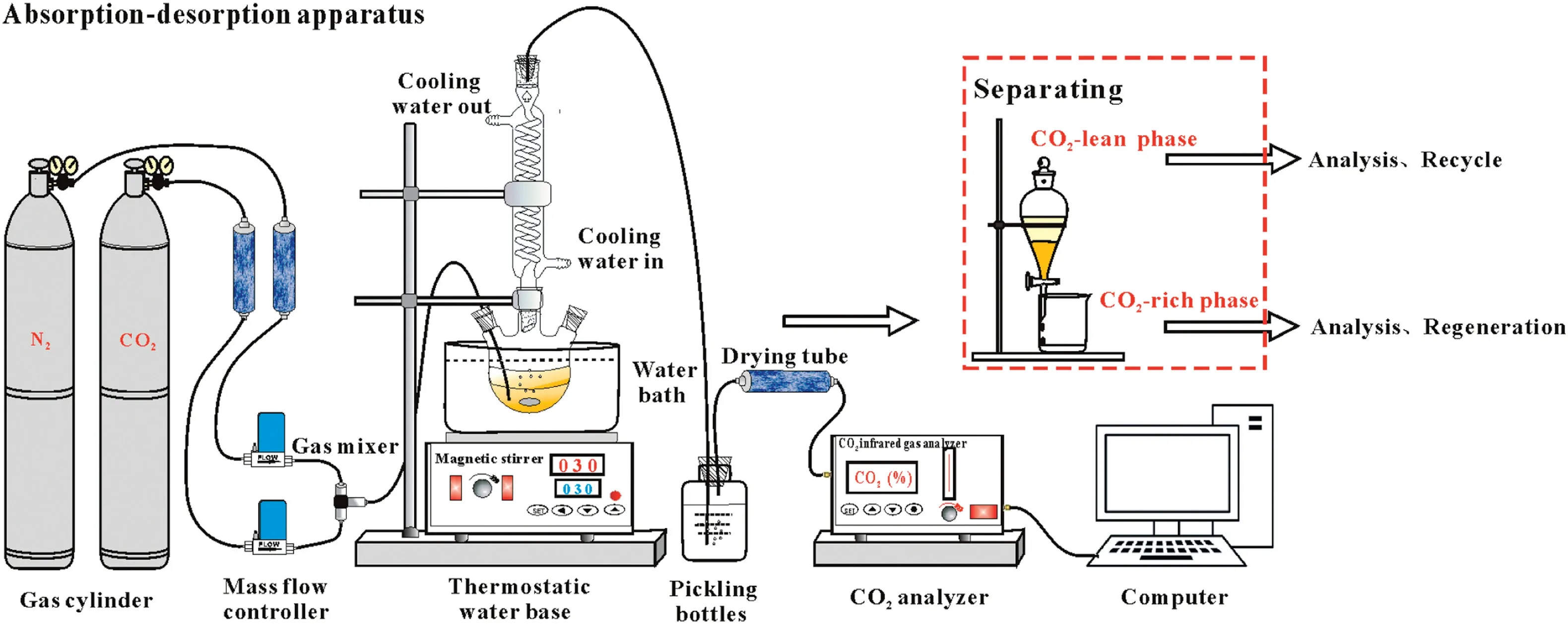
Fig.2.The CO2 absorption–desorption apparatus.
The desorption rate rdes(t),desorption amount ΔR(t),and regeneration ratio φCO2were adopted to evaluate the CO2desorption performance of the amine solutions,and were calculated by Eqs.(5)–(7).At the end of the desorption experiment t=tf,ΔR(tf)is the cyclic absorption capacity of the solution.All the variables are summarized in the Nomenclature section.
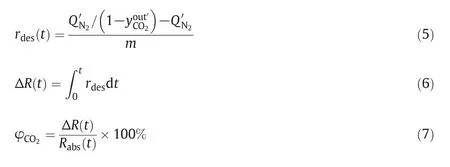
3.Results and Discussion
3.1.Reliability of the experiment and analysis methods
To achieve accurate and reliable absorption and desorption data for the TETA–DEEA–H2O system,the experimental setup(Fig.2)and method were verified by determining the CO2loading of a 5 mol·L-1MEA aqueous solution,which has been well documented[39].According to reference[40],the solubility of CO2in water is 1.23 dm3·kg-1H2O(~0.05 mol·kg-1)at 0.2 MPa and 30°C.Therefore,it was negligible in this work due to the lower solubility of CO2in water under experiment conditions.Gas phase measurements of rabs(t)and rdes(t)from 3 repetitions indicate that the absorption and desorption processes have good repeatability(maximum variation <5%)in the experimental setup.In one experiment,CO2loading of the rich and lean liquid phases was titrated to give 0.500 mol CO2·(mol MEA)-1and 0.217 mol CO2·(mol MEA)-1,respectively,and the mass balance calculation results are 0.513 mol CO2·(mol MEA)-1and 0.215 mol CO2·(mol MEA)-1,respectively.This is consistent with the literature values[39].Therefore,the experimental results are expected to be reliable for the new solvent system.In this work,each experiment was conducted for 2–3 repetitions,and the maximum relative error is less than 8%.
The experimental absorption heat of MEA was measured to be-91.30 kJ·(mol CO2)-1,which agrees well with the value of absorption heats in the literature[14,15].The specific heat capacity measurement was evaluated by that of water. The measured value of 4.12 kJ·kg-1·K-1is consistent with that in the literature[37],and the average deviation is within±2.0%.Therefore,results obtained with the current experimental setup and procedures are expected to be reliable.
3.2.Effect of absorbent compositions on the phase separation behaviors
The effect of the total amine concentration on the phase separation behavior of the CO2saturated TETA–DEEA–H2O system was studied by keeping TETA to DEEA molar ratio at 1:4(Table 2).Prior to CO2absorption,all the mixtures of total amine concentration in the range of 3 mol·L-1to 6 mol·L-1formed a homogeneous aqueous phase.The total amine concentration is found to be critical to inducing phase separation upon CO2absorption.No phase separation was observed after being saturated with CO2when the total amine concentration is less than or equal to 4 mol·L-1.This suggests that the products are water soluble and the phase separation depended on the change of the polarity of the solution,which is similar to switchable-polarity solvents[41,42].However,the viscosity of the initial solution increased with the increasing total amine concentration,which may have hindered the phase separation speed.Solid precipitation was observed in the solution of 6 mol·L-1total amines.
The effect of TETA to DEEA ratio on the phase separation behavior of the CO2saturated TETA–DEEA–H2O system was studied by keeping the total amine concentrations at 5 mol·L-1.Phase separation was observed in the TETA to DEEA ratio range of 1:9 to 3:7(Table 3).The volume ratio of the lower phase,vlower,increases with the increasing TETA to DEEA molar ratio.This suggests that CO2reacts preferentially with the TETA and the products are likely enriched in the lower phase.Thus,the carbamate of TETA should dominate the lower phase,which is proven with nuclear magnetic resonance analysis of the lower phase.The NMR results are not shown here.This is consistent with that of the DETA–PMDETA and MAPA–DEEA systems[43,44].It is worth noting that the CO2concentration,volume ratio,and viscosity of the resultant lower phase are also increased with the increasing TETA to DEEA molar ratio(Table 3).
3.3.The CO2 absorption performance of mixed amine
3.3.1.The CO2 absorption rate and absorption amount of the amine mixture
Fig.3 shows the CO2absorption rates and CO2capacities of the TETA,DEEA,and their combination.The CO2absorption rate of the 1 mol·L-1TETA is about twice that of the 4 mol·L-1DEEA in the initial 40 min.The CO2absorption rate of TETA drops rapidly as time progresses and the instantaneous TETA concentration decreases.The CO2absorption rate of the 4 mol·L-1DEEA remains relatively constant except in the initial 30 min.The instantaneous DEEA concentration may not significantly affect the CO2absorption rate,because the DEEA concentration was quite high so that the change in DEEA concentration may not have been significant.The CO2absorption rate of the mixture of 1 mol·L-1TETA and 4 mol·L-1DEEA shows the CO2absorption characteristics of both components.At the initial stage the CO2absorption rate is very high and then becomes relatively constant.However,the CO2absorption capacity of the mixed amine is lower than the sum of each amine(Fig.3(b)).This suggests that CO2reacts with TETA(Reaction(8))first,and then reacts with DEEA(Reaction(9)).This agrees well with observations in the DEEA–BDA system[24,45].
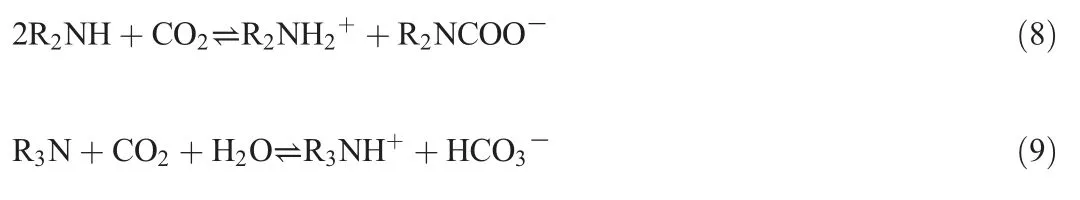
3.3.2.Effect of total amine concentration
The total amine concentration has been found to significantly affect the phase separation behavior of the system.No phase separation wasobserved for those total amine concentrations less than 4 mol·L-1(Table 2).Since amine concentration higher than 6 mol·L-1led to precipitation of solid phase,thus only the solutions with a total amine concentration of 5 and 6 mol·L-1(Fig.4)were further studied.Besides an amine concentration higher than 6 mol·L-1was found lead to higher viscosity,severe degradation,and corrosion problems,which is not suitable for industrial application[46].The molar ratio of the TETA to DEEA is maintained at 1:4.

Table 2 Effect of the total amine concentration on phase separation behavior
Generally,a higher CO2capacity was expected for the solvent with a higher amine concentration[47].However,Fig.4 shows that the CO2capacity of the 5 mol·L-1amine mixture is higher than that of the 6 mol·L-1solution.A significant increase in viscosity and solid precipitation was observed for the 6 mol·L-1amine mixture.Thus,a total amine concentration of 5 mol·L-1is preferable for phase separating CO2absorption process development.
3.3.3.Effect of the molar ratio of TETA to DEEA
The effect of the TETA to DEEA molar ratio on the CO2absorption rate and absorption amount was investigated at a total amine concentration of 5 mol·L-1(Fig.5).Fig.5(a)shows that increasing the TETA to DEEA ratio from 0.5:4.5 to 1:4 leads to a significant increase in both the CO2absorption rate and amount.However,there is no significant change in absorption rate and capacity when further increasing the ratio to 1.5:3.5.The presence of TETA significantly accelerates the CO2absorption rate.However,increasing the TETA concentration also leads to higher viscosity,which in turn negatively affects the CO2absorption process(Table 3).Thus,an optimal ratio would be expected.In addition,the CO2absorption capacity of both 1 mol·L-1TETA+4 mol·L-1DEEA and 1.5 mol·L-1TETA+3.5 mol·L-1DEEA are about 25.4%and 29.3%higher than that of the traditional 5 mol·L-1MEA solvent(Fig.5),respectively.The CO2concentration in the lower phase also increases with the increasing concentration of TETA(Table 3),which is consistent with the DETA–PMDETA system[44].
3.3.4.Effect of absorption temperature
The reaction temperature can significantly affect both the reaction kinetics and the equilibrium.Thus it is critical to achieve a desirable absorption performance.The effect of the absorption temperature was studied using the 1 mol·L-1TETA+4 mol·L-1DEEA as a typical composition(Fig.6).Fig.6 shows that both the absorption rate and CO2capacity decrease with increasing temperature.The CO2capacity decreased from 3.21 mol·L-1to 1.93 mol·L-1as the temperature is raised from 30°C to 50°C.The effect of temperature on the TETA+DEEA system is similar to that of aqueous MDEA–PZ[48]and the aqueous TETA system[49].This may be due to the decrease of the CO2solubility as the temperature rises[50],which decreases the interphase mass transfer driving force.This suggests that a lower operation temperature is favorable for CO2absorption.However,decreasing the temperature from 30°C to 20°C did not lead to better absorption performance.The reason could be complicated by stronger hydrogen bonding in the solution or lower mobility of both the reactant and productmolecules.It is worth noting that the volume ratio of the lower phase also decreases from 86.7%to 65.3%as the temperature increased from 30°C to 50°C.That is,the total amount of absorption decreased.This suggests that an appropriate absorption temperature is critical to achieve high efficiency of this solvent.

Table 3 Effect of the TETA to DEEA ratio on the phase separation behavior
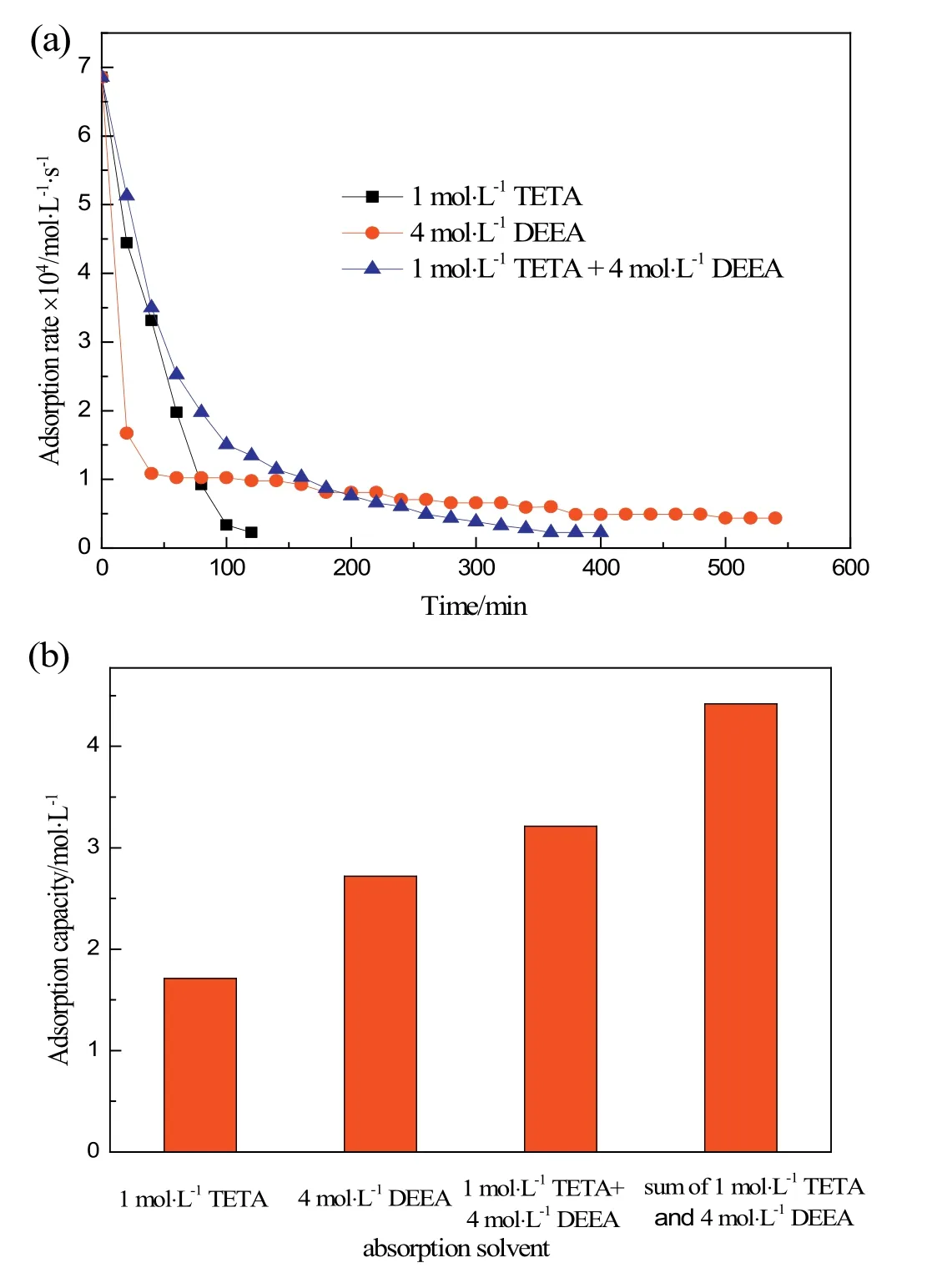
Fig.3.Comparison of(a)CO2 absorption rate and(b)CO2 absorption capacity of the TETA,DEEA,and TETA–DEEA(absorption temperature,30°C).
3.3.5.Evaluation of the CO2 absorption performance of 1 mol·L-1 TETA+4 mol·L-1 DEEA

Fig.4.Effect of total amine concentration on the CO2(a)absorption rate and(b)absorption amount(absorption temperature,30°C).
The properties of each phase after phase separation were studied using the aqueous 1 mol·L-1TETA+4 mol·L-1DEEA at 30°C(Table 4).After phase separation,the CO2saturated solution of 1 mol·L-1TETA+4 mol·L-1DEEA turned into two liquid phases,namely the upper and lower phases.The CO2concentration in the resultant upper phase is only 0.21 mol·kg-1,while the CO2concentration in the lower phase is up to 3.44 mol·kg-1which is 52.9%higher than that of MEA.Upon phase separation,up to 99.4%of the absorbed CO2is concentrated in the lower phase.Comparing with the 2 mol·L-1BDA+4 mol·L-1DEEA absorbent,the 1 mol·L-1TETA+4 mol·L-1DEEA system led to higher CO2enrichment in the lower phase[25].This suggests that 1 mol·L-1TETA+4 mol·L-1DEEA aqueous solution has a better CO2absorption performance.However,its phase separating behavior is worth further study.
3.4.The CO2 desorption performance of mixed amine
3.4.1.Effect of the desorption temperature

Fig.5.Effect of TETA to DEEA ratio on the(a)absorption rate and(b)absorption amount(absorption temperature,30°C).
It is known that a higher temperature favors desorption of gases.However,a higher desorption temperature leads to higher energy consumption of the desorption operation.Generally,desorption temperatures beyond the boiling point of the solvent are not desirable since vaporization of the solvent leads to extra consumption of energy.Thus,here only desorption temperatures below 100°C were taken into consideration to minimize the vaporization of water.Fig.7 shows that both the desorption rate and cyclic absorption capacity increase with increasing desorption temperature.The CO2cyclic absorption capacity increased from 1.83 mol·kg-1to 2.63 mol·kg-1as the desorption temperature increased from 70°C to 90°C(Fig.7(b)).The desorption process is characterized by formation of two distinct phases(Fig.7(a)).The CO2was desorbed very quickly in the initial 30 min and the desorption rate seems linearly dependent on the liquid phase CO2concentration.Then the desorption rate became slow and relatively constant.Desorption amount within the initial 30 min accounts for 70.0%of the total CO2desorbed.A CO2desorption ratio of 72%was achieved at 90°C,which corresponds to a CO2cyclic absorption capacity of 2.63 mol·kg-1.The higher desorption temperature does lead to a higher regeneration ratio of the absorbent.The regeneration ratio increased from 50%to 72%when the desorption temperature was increased from 70°C to 90°C.Thus,90°C is recommended for solvent regeneration.
3.4.2.Comparison with 5 mol·L-1 MEA
The desorption performance of the lower phase of 1 mol·L-1TETA+4 mol·L-1DEEA was compared with that of a 5 mol·L-1MEA under the same desorption conditions.Fig.8 shows that both the desorption rates and desorption amounts of 1 mol·L-1TETA+4 mol·L-1DEEA are higher than that of 5 mol·L-1MEA.The cyclic CO2absorption capacity of the lower phase of 1 mol·L-1TETA+4 mol·L-1DEEA at 90°C is 73.3%higher than that of 5 mol·L-1MEA(Fig.8(b)),which is also higher than that of 5 mol·L-1MEA desorbed at 120°C.The regeneration ratio of 1 mol·L-1TETA+4 mol·L-1DEEA reached 72%at 90°C,which is even higher than that(about 70%)of 5 mol·L-1MEA achieved at 120°C.It clearly shows that the 1 mol·L-1TETA+4 mol·L-1DEEA absorbent could achieve a higher regeneration ratio at a lower regeneration temperature than 5 mol·L-1MEA.This is very attractive for a larger scale CO2capture process.

Fig.6.Effect of the temperature on the CO2(a)absorption rate and(b)absorption amount(solvent composition,1 mol·L-1 TETA+4 mol·L-1 DEEA).
3.5.Analysis of regeneration heat duty
Energy consumption for solvent regeneration is the major concern for developing new PCC solvents and processes.Generally,the overall regeneration heat duty includes the decomposition heat,sensible heat,and vaporization heat of the solvent[13,51–53].

Fig.7.Effect of temperature on the CO2(a)desorption rate and(b)desorption amount(solvent composition,1 mol·L-1 TETA+4 mol·L-1 DEEA).
The decomposition heat was estimated by directly measuring the absorption heat since desorption is the reverse process of absorption.The absorption heat of 4 mol·L-1DEEA aqueous solution was measured to be 66.45 kJ·(mol CO2)-1by the simultaneous thermal analysis,which is much lower than that of 5 mol·L-1MEA aqueous solution(91.30 kJ·(mol CO2)-1).For 1 mol·L-1TETA aqueous solution,83.55 kJ·(mol CO2)-1was measured.The absorption heat of the aqueous solution of 1 mol·L-1TETA+4 mol·L-1DEEA was measured to be 84.38 kJ·(mol CO2)-1,which is also 8%lower than that of MEA(Table 5).The sensible heat depends on the specific heat and the temperature change. The specific heat of the lower phase of the 1 mol·L-1TETA+4 mol·L-1DEEA solvent was measured to be 2.96 kJ·kg-1·K-1by differential scanning calorimetry,which is about 20% smaller than that of 5 mol·L-1MEA (3.73 kJ·kg-1·K-1)(Table 5).Taking the difference of temperature between the absorption and desorption into consideration,the sensible heat demand to regenerate the same amount of solvent is about 55%less for the 1 mol·L-1TETA+4 mol·L-1DEEA absorbent than that required by 5 mol·L-1MEA.According to Li et al.[52],the reaction heat,sensible heat,andthe vaporization heat of a desorption process can be estimated by the absorption heat,specific heat of the rich liquid,CO2cyclic absorption capacity,the temperature increase,and the saturated vapor pressure of water.The estimated results were shown in Fig.9.It can be found that the regeneration heat duty required by the 1 mol·L-1TETA+4 mol·L-1DEEA absorbent is 25%less than that of an aqueous MEA.

Table 4 The absorption performance of 1 mol·L-1 TETA+4 mol·L-1 DEEA,2 mol·L-1 BDA+4 mol·L-1 DEEA and 5 mol·L-1 MEA

Fig.8.Comparison of 1 mol·L-1 TETA+4 mol·L-1 DEEA and 5 moSl·L-1 MEA on the CO2(a)desorption rate and(b)desorption amount.
4.Conclusions

Fig.9.Comparison of the regeneration heat duties.
The phase-changing solvent of TETA–DEEA–H2O is found to be a superior solvent system for the post-combustion CO2capture process in terms of potentially low energy demand.The CO2induced phase changing behavior of the TETA–DEEA–H2O solution is found depending largely on its composition.Low decomposition heat,smaller specific heat and lower desorption temperature,compared to the 30%aqueous MEA,can lead to 25%less energy demand for regeneration.The cyclic CO2absorption capacity of the TETA–DEEA–H2O solvent is about 74%higher than that of MEA.The suitable composition is found to be 1 mol·L-1TETA+4 mol·L-1DEEA,and the suitable absorption and desorption temperatures are determined to be 30°C and 90°C,respectively.99.4%of the absorbed CO2is found to be concentrated in the lower phase of the system.All these findings suggest that the TETA–DEEA–H2O solvent has great potential to develop a less energyconsuming PCC process.
Nomenclature
CalkaliTotal amine group(alkalinity)concentration,mol·kg-1
CCO2,lowerCO2concentration of the lower phase,mol·(kg solvent)-1
CCO2,upperCO2concentration of the upper phase,mol·(kg solvent)-1
Cp,richSpecific heat of the rich liquid,kJ·kg-1·K-1
CTETATETA concentration,mol·L-1
Ctotal,amineTotal amine concentration,mol·L-1
ΔHabsAbsorption heat,kJ·(mol CO2)-1
ΔHvap,H2OVaporization heat of water,kJ·(mol CO2)-1
m The quantity of the absorbent solution,kg nTETA:nDEEAThe molar ratio of TETA to DEEA
CO2flowrate at the outlet of the falsk,mol·s-1
Q N2N2flowrate,mol·s-1
Q′N2The flowrate of the sweeping N2,mol·s-1
Rabs(t) Accumulating CO2absorption amount,mol·(L solvent)-1
Rabs(tf) The CO2capacity of an absorbent,mol·(L solvent)-1
ΔR(t) CO2desorption amount at instant t,mol·(L solvent)-1
ΔR(tf) Cyclic absorption capacity of the absorbent solution,mol·(L solvent)-1
rabs(t) Instantaneous CO2absorption rate,mol·L-1·s-1
rdes(t) Instantaneous CO2desorption rate,mol·L-1·s-1

Table 5 Regeneration heat duty of TETA–DEEA and MEA system
ΔT The difference between the regeneration temperature and the injection temperature,K
t Absorption/desorption time,s
tfAbsorption/desorption time at the end of the experiment,s
V Total volume of the absorbent solution,L
vlowerVolume fraction of the lower phase,%
yCO2,lowerCO2mole fraction of the lower phase of the absorption,%
α CO2loading,mol CO2·(mol alkalinity)-1
μ Viscosity of the fresh absorbent at 30°C,mPa·s
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Ni/bentonite catalysts prepared by solution combustion method for CO2 methanation☆
- Modelling of a post-combustion carbon dioxide capture absorber using potassium carbonate solvent in Aspen Custom Modeller
- Mass transfer correlations for membrane gas-solvent contactors undergoing carbon dioxide desorption
- Carbon deposition and catalytic deactivation during CO2 reforming of CH4 over Co/MgO catalyst☆
- Recent developments and consideration issues in solid adsorbents for CO2 capture from flue gas☆
- High-efficiency and pollution-controlling in-situ gasification chemical looping combustion system by using CO2 instead of steam as gasification agent
