Variation in seed morphometric characters,oil content and azadirachtin content of seeds,in vitro shoot cultures and callus cultures among different populations of Azadirachta indica
2018-03-27FatimaShirinAnamikaRaiNeeluSingh
Fatima Shirin·Anamika Rai·Neelu Singh
Introduction
Many higher plants are major sources of natural products used as pharmaceuticals,agrochemicals, flavours,fragrances,food additives and pesticides.Despite substantial advances in the field of synthetic organic chemistry,plants are still the major source of about 25%of all prescribed medicines and provide the raw materials used extensively by the pharmaceutical industries.Plant cell cultures have proved to be an important tool for the study of biosynthesis of secondary products(Whitaker and Hashimoto 1986).Azadirachtin is one of the most potent biopesticides so far developed from a plant source.With increasing awareness for pesticidal residues in the environment due to indiscriminate use of synthetic pesticides,biopesticides are attaining increased attention.One of the most potent biopesticides currently in use is isolated from seeds of Azadirachta indica (commonly known as neem).Azadirachtin is the most important constituent of neembased biopesticides and is a limonoid(C35H44O16,tetranortriterpenoid).It acts by showing antifeedant,growth regulatory and antifertility effects against a wide spectrum of insects(Satdive 2007).With increasing demand for this biopesticide,plant cell and organ cultures have been recognised as an alternate source for year round production of azadirachtin(Allan et al.1999).
Current supply of azadirachtin compound from neem trees will not meet the increasing demand if the extraction from seeds remains the only source.Thus there is need for the development of commercially viable alternatives for its production.Chemically,it is a complex molecule and this complexity precludesasyntheticproduction system.Although the synthetic route is established,total chemical synthesis of it is not economically viable(Prakash et al.2002;Singh and Chaturvedi 2013).Keeping in view of all these factors,plant cell culture can be seen as a potential alternative production system(Prakash et al.2002).
Azadirachtin is present in all parts of the tree but its highest concentration is present in mature seeds.All commercial formulations and products based on azadirachtin are prepared by extraction of seeds collected from naturally grown plants.However,this approach has several disadvantages,including heterogeneity in azadirachtin content and enormous heterozygosity prevalent in the genus due to cross pollination,its long reproductive cycle,recalcitrant nature and poor seed yields(Sidhu and Behl 1996;Kaushik et al.2007;Wewetzer 1998;Sidhu et al.2003;Singh and Chaturvedi 2013).High azadirachtin content(>0.50%by kernel weight)neem seeds are sold in the market at around Rs.10 per kg;otherwise the price is less than Rs.3 per kg.Due to low azadirachtin(<0.50%)content in the seeds,only around 24%of neem seeds are collected for processing to manufacture azadirachtin formulations(Gupta et al.2010).Furthermore,due to low shelf life of seeds,the azadirachtin content drops to 32%within 4 months of storage(Yakkundi et al.1995).
The present study was undertaken to quantify the variation among different populations of Azadirachta indica for azadirachtin content and other related parameters.The objective was to study the variation with respect to azadirachtin production in seeds of original in vivo populations and their in vitro cultures and to select populations of neem yielding high azadirachtin content under in vitro conditions.The in vitro approach was adopted because under controlled and uniform culture conditions,the portion of total variation attributable to environment or genotype can be quanti fied.We quanti fied variation among trees collected from 10 different populations,viz.Chhatarpur,Katni,Sihore,Khandwa,Bargi(Jabalpur),Shahdol,Chhindwara and Gwalior in M.P.and two populations in Chhattisgarh,viz.Raigarh and Bilaspur for azadirachtin content in the in vivo seeds,in vitro shoot cultures and callus cultures.
Materials and methods
Selection of trees and collection of plant material from different populations
Field surveys were carried out and twenty neem trees each were selected for sampling from 10 different populations of central India.The trees were selected from eight populations of M.P.,viz.Chhatarpur,Katni,Sihore,Khandwa,Bargi(Jabalpur),Shahdol,Chhindwara and Gwalior and two populations of Chhattisgarh,viz.Raigarh and Bilaspur.The trees selected were healthy with straight boles and well developed crowns with fruits.Height(m),gbh(inch)and GPS location of each tree was recorded.Fully ripe fruits greenish yellow to yellow in colour,were collected directly from the branches of individual trees.Fruits were brought to the lab and depulped manually using sand.The seeds were washed thoroughly with water to remove all traces of pulp from the seed coat.Depulped and washed seeds were dried at room temperature under a fan.They were stored in a cold room at 4°C.Kernels were removed from depulped seeds for analysis.We recorded 100 seed weight,kernel(%)in seed,and neem oil(%)in seeds for all 200 trees sampled.
Establishment of in vitro cultures
Aseptic in vitro cultures were established for all 10 populations using nodal segments on MS medium(Murashige and Skoog 1962).Callus cultures were also established using nodal segments for 10 populations,viz.Chhatarpur,Katni,Sihore,Khandwa,Bargi,Shahdol,Chhindwara,Gwalior,Raigarh and Bilaspur on MS medium supplementedwith5 mg L-1BA,10 mg L-1NAA and 10 mg L-1IBA(Babu et al.2006).
In vitro shoot cultures and callus cultures
Neem seeds were depulped to isolate kernels and weight of the sample was recorded at each stage.1 g of seed kernel was dried for a minimum of 8 h at 40°C until constant weight was achieved.The dried kernels were crushed and de-fatted to extract neem oil.Oil was obtained from the crushed neem kernels using soxlet apparatus by extracting in petroleum ether followed by the extraction in methanol and thereafter completely evaporating the solvent in a water bath to recover Neem oil.Neem oil percentage was then calculated on a kernel weight basis.The oil thus obtained wasdissolved in distilled waterand was fractioned first with petroleum ether to remove oil traces and then this aqueous fraction was fractioned with ethyl acetate.The ethyl acetate extract was dried,dissolved in HPLC grade methanol(Fisher Scienti fic,Qualigen India Pvt.Limited)and analyzed using Reverse Phase Analytical C18column of HPLC(Waters High Pressure Liquid Chromatograph)following the procedure of Govindachari et al.(1995).Azadirachtin was detected at 220 nm wavelength.The mobile phase consisted of a mixture of acetonitrile:water(70:30)at a flow rate of 1.0 mL min-l.The results are presented on a percentage weight basis and quanti fied by comparing with the peak of the external standard of azadirachtin(Ranbaxy India Private,Limited).Isolation of azadirachtin from shoot cultures and callus cultures was achieved using the cold extraction method.1 g Samples of in vitro shoots and calluses were dried at 40 °C for 3–4 h.The dried shoot or callus sample was kept in methanol for 24 h at room temperature followed by soaking in a water bath for solvent removal.The extract thus obtained was dissolved in distilled water and fractionation was achieved using ethyl acetate.The ethyl acetate layer was collected and the solvent was completely removed.The residue thus obtained was dissolved in HPLC grade methanol and analysis was done using the Reverse Phase C18column of HPLC.The quantity of azadirachtin obtained from the in vitro shoot,callus cultures,and their respective seed samples was compared and correlated.
Neem seeds were depulped and kernels were removed.Weight at each stage was recorded.Seed kernels(1 g samples)were dried at 40°C for 8 h until constant weight was achieved.The dried kernels were crushed and defatting was done to remove neem oil.The crushed kernels were run through petroleum ether in a Soxhlet apparatus and then methanol was extracted.Neem oil extract was obtained by drying on a water bath and neem oil percentage was calculated.The methanol extract was taken and complete drying was done.The extract thus obtained was dissolved in distilled water and then fractioned first with petroleum ether to remove oil traces and then the aqueous fraction was fractioned with ethyl acetate.The ethyl acetate extract was dried and then dissolved in HPLC methanol(Fisher Scienti fic,Qualigen India Pvt.Limited)and analyzed using the Reverse Phase Analytical C18column of HPLC following the procedure of Govindachari et al.(1995).Azadirachtin was detected at 220 nm wavelength.The mobile phase consisted of a mixture of acetonitrile–water(70:30)at flow rate of 1.0 mL min-l.The results are presented on a percentage weight basis and quanti fied by comparing with the peak of the external standard of azadirachtin(Ranbaxy India Pvt,Limited).Isolation of azadirachtin from shoot cultures and callus cultures was carried out using the cold extraction method.1 g samples of in vitro shoots and calluses were dried at 40°C for 3–4 h.The dried shoot or callus samples were kept in methanol for 24 h at room temperature.Methanol extract was kept in a water bath for solvent removal.The extract thus obtained was dissolved in distilled water.Fractionation was then done using ethyl acetate.The ethyl acetate layer was collected and complete solvent removal was done.The residue thus obtained was dissolved in HPLC methanol and analysis was done using the Reverse Phase C18column of HPLC.
Calculation of neem oil(%)and azadirachtin content(%)
We quanti fied the percentage of kernel present in neem seeds and calculated the percentage of neem oil on a kernel weight basis.Azadirachtin was estimated through HPLC Reverse Phase C18Column and compared with the chromatogram of azadirachtin standard.The quantity of azadirachtin obtained in the in vitro shoot cultures and callus cultures and their respective seed samples was compared and correlated.
Culture conditions and statistical analysis
The MS medium supplemented with 3%(w/v)sucrose,0.8%(w/v)agar(Microexpress Pvt.Ltd.,India),and 0.01%(w/v)myo-inositol was used for in vitro culture establishment and shoot multiplication.The inorganic salts used for preparation of the culture medium were obtained from SRL Chemicals and Qualigens Pvt.Ltd.,India and phytohormones and B vitamins from Sigma Chemicals Pvt.Ltd.,India.The pH of the medium was adjusted to 5.8 prior to autoclaving for 15 min at 1.06 kg cm-2(121°C).Each explant was cultured in a 2.5×15.0 cm glass tube containing 10 mL sterilized semi-solid medium for culture initiation and 150 mL conical flasks containing 35 mL semi-solid medium for in vitro shoot multiplication.The cultures were incubated at of(25 ± 2)°C under 16-h daily illumination with white fluorescent light(approximately 45 μmol m-2s-1).Data were subjected to one way analysis of variance(ANOVA)using the Systat®statistical package.
Results
The mean values for various seed parameters,viz.100 seed weight,kernel to seed ratio,and neem oil content in kernels,were calculated for all populations.Signi ficant variation was observed among the 10 populations for these three seed parameters.100 seed weight ranged from 10.71 to 39.85 g.Kernel to seed ratio(%)ranged from 18.63 to 76.25%(Table 1).Percent neem oil(kernel weight basis)range from 5 to 32(Table 2).
The maximum value for 100 seed weight was recorded for seeds of Raigarh(30.51 g),similar to that for seeds of Bargi(28.37 g)and Chhatarpur(26.36 g).The highest kernel to seed ratio was also recorded for seeds of Raigarh(60.11%),which was signi ficantly higher than the kernel to seed ratio of any other population(Table 1).
The neem oil(%)in kernels was greatest in the Chhatarpur population(24.2%),which was on par with that in Sihore(23.75%)and Katni(21.75%)(Table 2).
Highly signi ficant variation was recorded with respect to azadirachtin content in seeds,in vitro shoots,and calluses.Azadirachtin content in seeds among different populations ranged from 0.03 to 2.04%.Azadirachtin in in vitro shoots ranged from 0.001 to 0.250%.In callus cultures,the range of azadirachtin content was 0.006–0.067%(Table 3).For azadirachtin content of seeds and in vitro shoots,the variation between populations exceeded that within populations.Maximum azadirachtin content was recorded for the seeds from Gwalior(0.882%)and was comparable to that in seeds from Chhatarpur,Katni,Sihore,Shahdol and Chhindwara.Maximum azadirachtin in in vitro shoots was recorded for cultures from Gwalior(0.218%)and this was statistically similar to that for shoots from Sihore(0.176%).Azadirachtin content in callus cultures of nodal segments differed between populations but not within populations.Azadirachtin obtained from callus cultures was greatest in the Gwalior population(0.033%)and this was statistically similar to that for Sihore(0.028%)(Table 3).More azadirachtin was extracted from in vitro shoots than from callus cultures of nodal segments.
Seed weight was highly and signi ficantly positively correlated with kernel to seed ratio(Table 4).Signi ficantnegative correlation was recorded for 100 seed weight and neem oil.Signi ficant negative correlation was also recorded for 100 seed weight and azadirachtin content in seeds,azadirachtin content in in vitro shoots and azadirachtin content in callus cultures(Table 4).
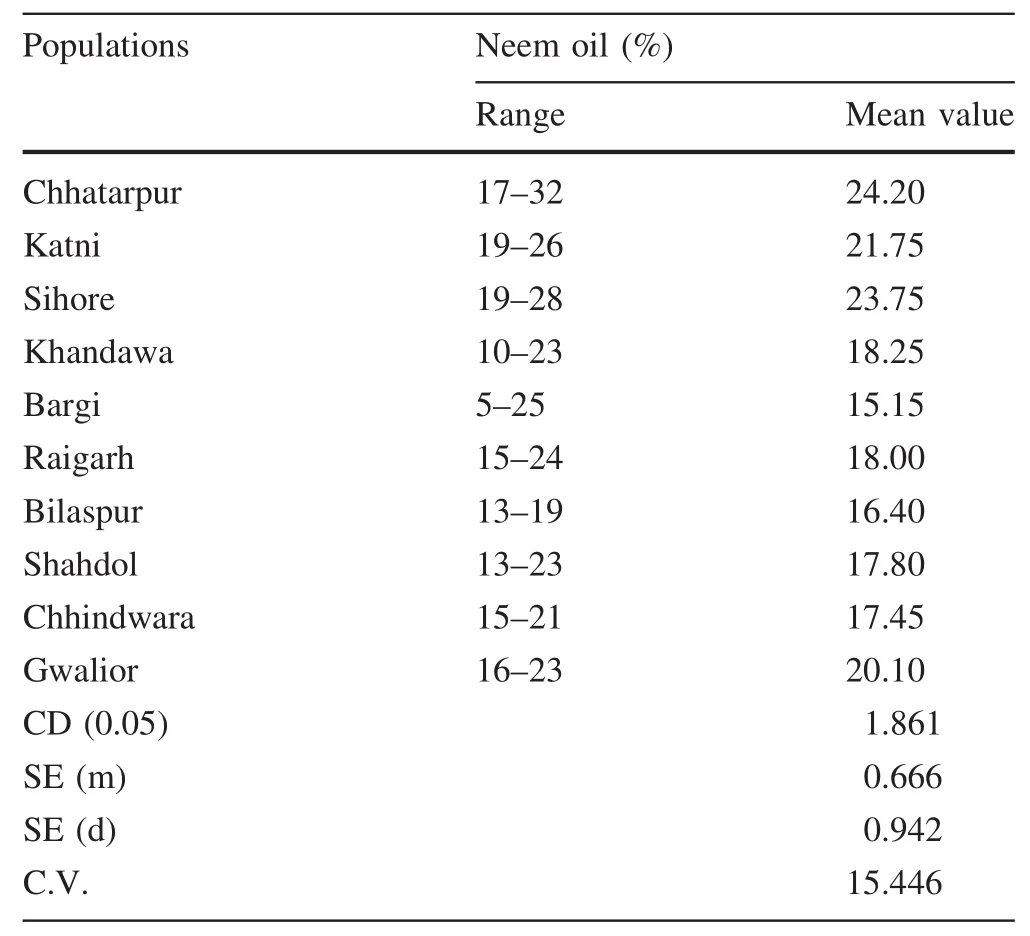
Table 2 Neem oil content in 10 populations of Azadirachta indica
Kernel to seed ratio was signi ficantly negatively correlated with neem oil percent.Signi ficant positive correlation was recorded between kernel to seed ratio and azadirachtin content in in vitro shoots,and in callus cultures.Kernel to seed ratio was unrelated to azadirachtin content in seeds.
Signi ficant positive correlation was recorded between neem oil content and azadirachtin content in seeds.Signi ficant positive correlation was recorded between neem oilcontent and azadirachtin content in in vitro shoots and callus cultures.
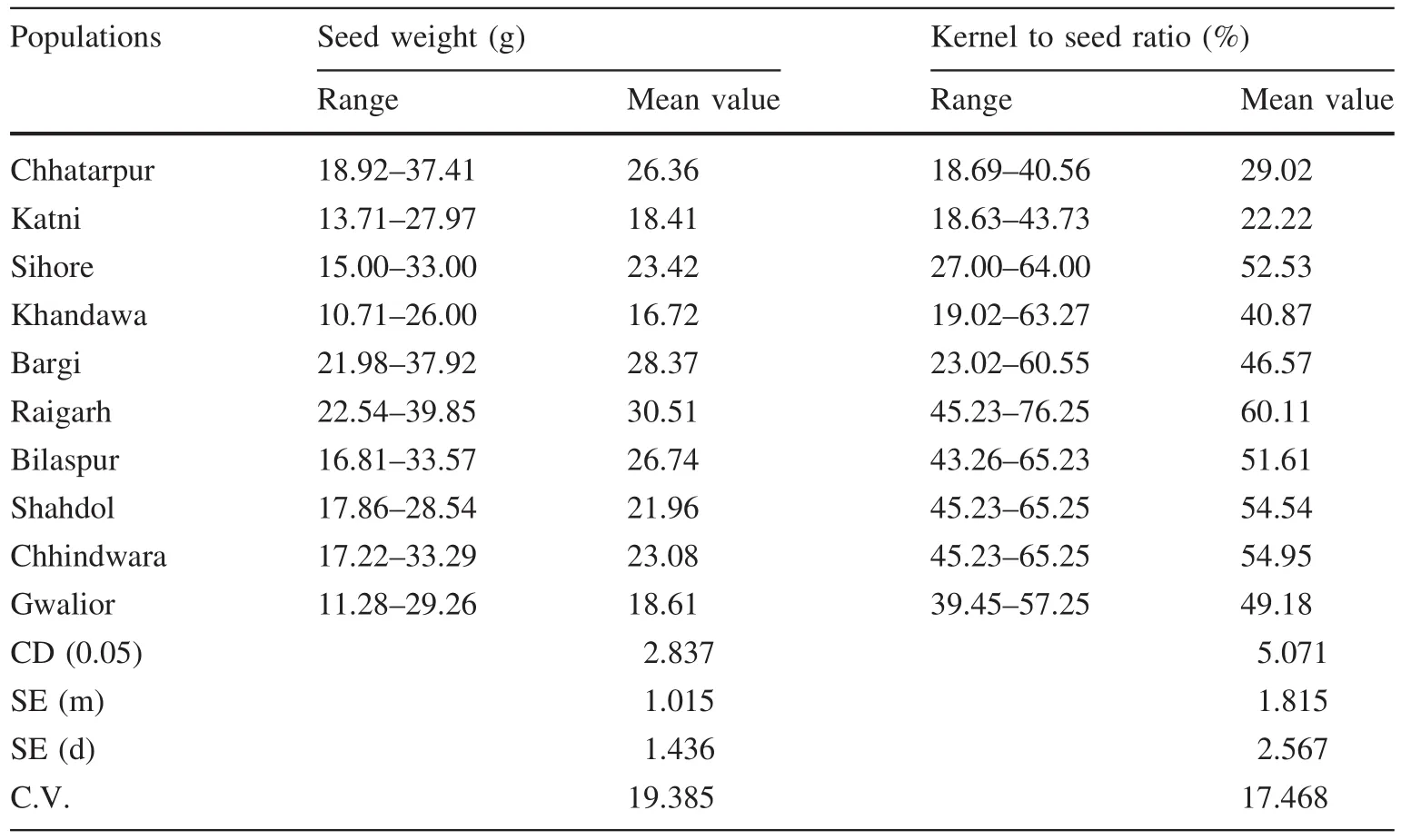
Table 1 Seed weight and kernel to seed ratio among 10 different populations of Azadirachta indica
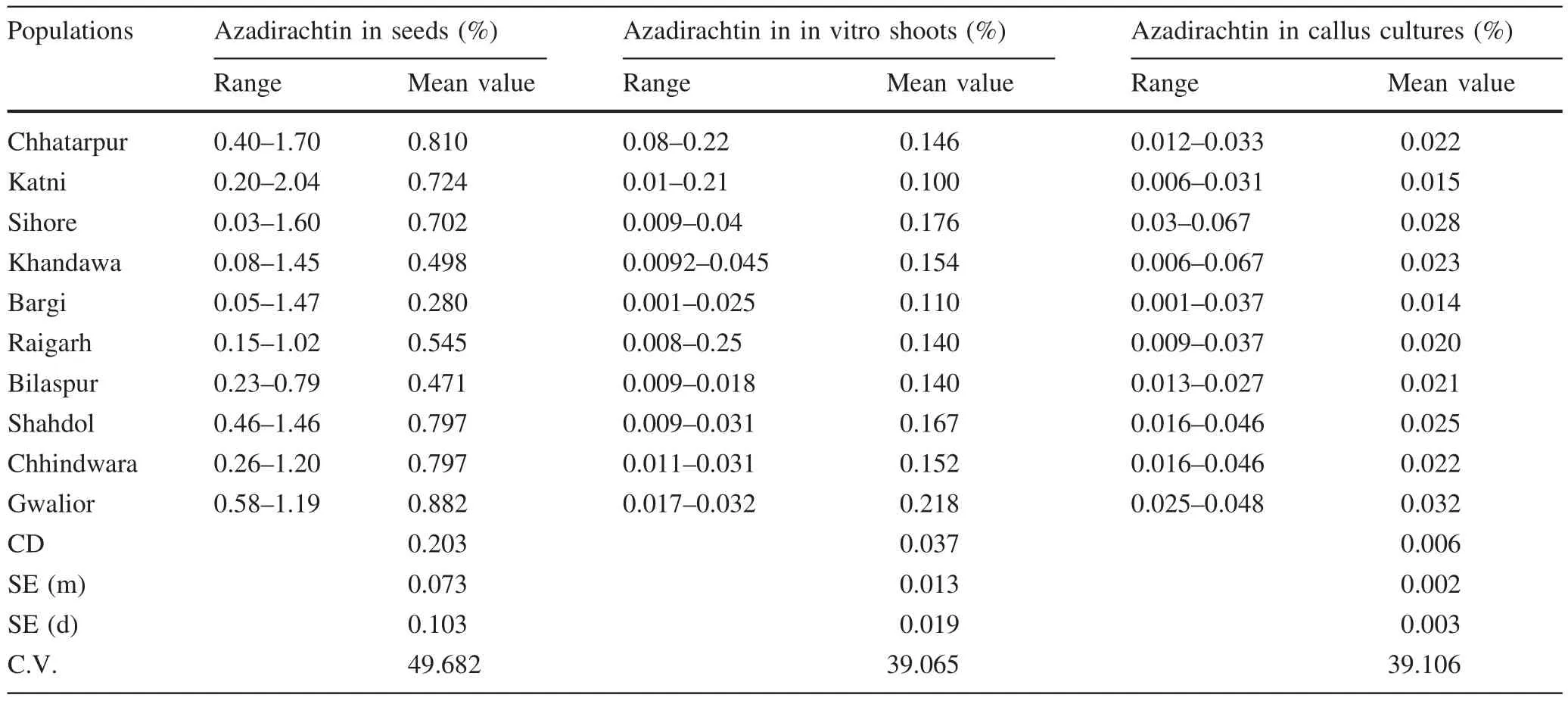
Table 3 Azadirachtin content in seeds,in vitro shoots,and callus cultures in 10 populations of Azadirachta indica

Table 4 Correlation between seed parameters and azadirachtin content in Azadirachta indica
Signi ficant positive correlation was recorded between azadirachtin content in seeds and azadirachtin content in in vitro shoots and callus cultures.
Highly signi ficant positive correlation was recorded between azadirachtin content in in vitro shoots and azadirachtin content in callus cultures.
Discussion
Variation in seed morphometric characters and azadirachtin production among different populations
In the present study,the azadirachtin content of in vivo seeds exceeded that yielded by in vitro shoot cultures and in vitro callus cultures.In contrast to these findings,Fulzele et al.(1995)reported that cultures produced higher levels of terpenoids than did parent plants of Artemisia annua.
In a study of seeds collected from different regions of India,the highest azadirachtin content in seeds was recorded in neem from south India(Kaushik et al.2007).We also documented variation in azadirachtin production by population(or region).Maximum azadirachtin content was recorded for seeds from Gwalior(0.882%)whose yield was similar to that of seeds from Chhatarpur,Katni,Sihore,Shahdol and Chhindwara.
Variation in azadirachtin and neem oil content from different countries and different regions within the country have been reported.Large variation in azadirachtin content(301.8–3161.4 mg kg-1kernel)was recorded in the studies of neem from vardious parts of India under a network project of NOVOD Board,India(Jain 2006).
Several researchers have reported variation in azadirachtin content in neem seeds due to various factors(Gupta et al.2010).Ecotypes from coastal,arid and semi-arid ecosystems yielded more azadirachtin A than did ecotypes from sub-humid regions(Rengaswamy and Parmar 1995).Similarly,Ermel et al.(1995)observed marked variation in neem seed azadirachtin content by country:Seeds from Nicaragua and Indonesia yielded more azadirachtin than did seeds from India,Burma and Mauritius.Venkateswarlu and Korwar(2005)reported marked variation between different genotype from the same location and from different locations,but there was no relationship between azadirachtin A content and rainfall, humidity or temperature.
In the present study,the amount of azadirachtin produced in the in vitro shoots exceeded that produced by callus cultures of nodal segments.
Organized cultures(like shoots)are known to produce high levels of major bioactive compounds compared to unorganized callus cultures.The content of tropane alkaloids hyoscyamine and scopolamine were found to be higher in multiple shoot cultures in comparison with callus cultures of Duboisia myoporoides(Khanum et al.2000).The flavonoid content of shoot cultures was three times higher than that in callus cultures of Plantago major and Nepeta septemcrenata(Saker and Kawashity 1998).Srividya et al.(1998)reported that root and shoot cultures produced greater amounts of azadirachtin and nimbin than did callus cultures of Azadirachta indica.Our results were similar.Similarly,Singh and Chaturvedi(2013)reported that redifferentiated shoots supported more active azadirachtin biosynthesis than did unorganized callus cultures.Similar findings have been reported by other researchers also for production of secondary metabolites under in vitro conditions(Liu et al.1997;Palazón et al.2006;Sood and Chauhan 2009;Berkov et al.2010).Under in vitro conditions,redifferentiation is generally associated with improved synthesisof secondary metabolites(Collin 2001).Singh and Chaturvedi(2013)obtained 2330 μg g-1dry weight azadirachtin in shoots from zygotic embryos.In the present study,we recorded a similar maximum yield of 2180 μg g-1of azadirachtin from shoot cultures.
Secondary products of plant origin are in great demand in global markets and therefore,it is essential to develop in vitro culture systems for continuous production of azadirachtin from neem.In the present study a maximum of 330 μg g-1of azadirachtin was yielded by callus cultures from the Gwalior population.Yield of 64 μg g-1dry wt azadirachtin was reported for callus cultures from A.indica from Nicaragua and Togo(Wewetzer 1998).Yields ranging from 4 to 189 μg g-1dry weight azadirachtin were reported from callus cultures derived from wild trees of A.indica collected from Sri Lanka(Eeswara 1998).Allan et al.(1999)reported azadirachtin yields of 7 μg g-1dry weight from callus cultures in Ghana.
Correlation coef ficient
Azadirachtin content of seeds was positively correlated with 100 seed weight,neem oil content,azadirachtin content in in vitro shoots and in calluses.It was negatively correlated with kernel to seed ratio.This means that azadirachtin content in neem seeds will be greater in trees having greater 100 seed weight and greater seed content of neem oil.Similarly,the trees producing more azadirachtin in their seeds will also produce more azadirachtin in tissue cultures,viz.in in vitro shoots and in calluses.The literature contains no earlier reports that compare and correlate azadirachtin content of in vivo seeds and in vitro cultures.Werecordedsigni ficantnegativecorrelationbetween100 seed weight and neem oil,and kernel to seed weight and neem oil.Similarly,neem seed oil content in most of the provenances was not signi ficantly correlated with morphological parameters ofseeds ina study on fiveprovenancesof northern and western India(Kaura et al.1998).
Conclusion
The variation in azadirachtin content between populations exceeded the variation between trees within a population.Thus,the wide genetic variability characteristic of neem populations with respect to azadirachtin production in seeds and in vitro cultures is expressed on large geographic scales such as regions,countries,or continents.This variability providesscopeforselectionofhighyieldingpopulationsand genetic improvement in future.Trees yielding greater amountsofazadirachtinshouldbeconservedandmultiplied.Greater numbers of neem trees should be planted in plantations and in farm fields under agroforestry systems to increase availability of seeds.This will help to meet the growing demand for neem derivatives for biopesticides in integrated pest management and organic agriculture.The trees of Gwalior and Sihore populations yielded the highest amountsofazadirachtinsothesetreesshouldbeproducedin largenumbersthroughvegetativepropagationandshouldbe grown in plantations.This will lead to production of seeds with higher azadirachtin content.
AcknowledgementsThe first author is grateful to the Indian Council of Forestry Research and Education,Dehradun for financial support to the project(No:159/TFRI/2010/Gen.-2(19)).
Allan EJ,Stuchbury T,Mordue AJ(1999)Azadirachta indica A.Juss.(Neem Tree):In vitro culture micropropagation,and the production of azadirachtin and other secondary metabolites.In:Bajaj YPS(ed)Biotechnology in agriculture and forestry.Springer,Berlin,pp 11–41
Babu VS,Narasimhan S,Nair GM (2006)Bioproduction of azadirachtin-A,nimbin and salannin in callus and cell suspension cultures of neem(Azadirachta indica A.Juss.).Curr Sci 91(1):22–24
Berkov S,Pavlov A,Georgiev V,Weber J,Bley T,Viladomat F,Bastida J,Codina C(2010)Changes in apolar metabolites during in vitro organogenesis of Pancratium maritimum.Plant Physiol Biochem 48:827–835
Collin HA(2001)Secondary product formation in plant tissue cultures.Plant Growth Regul 34(1):119–134
Eeswara JP,Stuchbury T,Allan EJ,Mordue AJ(1998)A standard procedure for micropropagation of the neem tree(Azadirachta indica A.Juss.).Plant Cell Reports 17(3):215–219
Ermel K(1995)Azadirachtin content of neem seed kernels from different regions of the world.In:Schmutterer H(ed)The Neem tree-source of unique natural products for integrated pest management,medicine and other purposes.Weinheim,New York,pp 89–92
Fulzele DP,Heble MR,Rao PS(1995)Production of terpenoids from Artemisia annua L.plantlet cultures in bioreactor.J Biotechnol 40(2):139–143
Govindachari T,Suresh G,Gopalakrishnan G(1995)A direct preparative high performance liquid chromatography procedure for the isolation of major triterpenoids and their quantitative determination in neem oil.J Liq Chromatogr 18(17):3465–3471
Gupta VK,Alhawat SP,Kumar RV,Dutta A(2010)Effect of season and year on azadirachtin A and oil content in neem(Azadirahcta indica A.Juss)seed and relationship of azadirachtin A and oil content with rainfall,temperature and humidity.Curr Sci 99(7):1953–1956
Jain A(2006)Study on morphological,chemical and biochemical parameters of neem(Azadirachta indica A.Juss)in relation to provenance variation.Ph.D thesis.Dehradun,Uttaranchal:Forest Research Institute(Deemed)University,pp 46–47
Kaura SK,Gupta SK,Chowdhury JB(1998)Morphological and oil content variation in seeds of Azadirachta indica A.Juss.(Neem)from northern and western provenances of India.Plant Foods Hum Nutr 52(4):293–298
Kaushik N,Singh GB,Tomer UK,Naik SN,Vir S,Bisla SS,Sharma SS,Banerjee KS,Thakkar P(2007)Variability in Neem(Azadirachta indica)with respect to azadirachtin content.Curr Sci 92(10):1400–1406
Khanum N,Khoo C,Khan AG(2000)Effects of cytokinins/auxin combinations on organogenesis,shoot regeneration and tropane alkaloid production in Duboisia myoporoides.Plant Cell Tissue Organ Cult 62(2):125–133
Liu CZ,Wang YC,Ouyang F,Ye HC,Li GF(1997)Production of artemisinin by hairy root cultures of Artemisia annua L.Biotech Lett 19(9):927–929
Murashige T,Skoog F(1962)A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15(3):473–497
Palazón J,Moyano E,Bon fill M,Osuna LT,CusidóRM,Piñol MT(2006)Effect of organogenesis on steroidal saponin biosynthesis in calli cultures of Ruscus aculeatus.Fitoterapia 77(3):216–220
Prakash G,Bhojwani SS,Srivastava AK(2002)Production of azadirachtin from plant tissue culture:state of the art and future prospects.Biotechnol Bioprocess Eng 7(4):185–193
Rengaswamy S,Parmar BS(1995)Azadirachtin A content of seeds of neem ecotypes in relation to the agroecological regions of India.Pestic Res J 7:140–148
Saker MM,Kawashity SA(1998)Tissue culture and flavonoids content of Nepeta and Plantago species endemic in Egypt.Fitoterapia 69(4):358–364
Satdive RK,Fulzele DP,Eapen S(2007)Enhanced production of azadirachtin by hairy root cultures of Azadirachta indica A.Juss by elicitation and media optimization. J Biotechnol 128(2):281–289
Sidhu OP,Behl HM(1996)Seasonal variations in azadirachtin in seeds of Azadirachta indica.Curr Sci 70(7):1084–1086
Sidhu OP,Kumar V,Behl HM (2003)Variability in neem(Azadirachta indica)with respect to azadirachtin content.J Agric Food Chem 51(4):910–915
Singh M,Chaturvedi R(2013)Sustainable production of azadirachtin from differentiated in vitro cell lines of Neem(Azadirachta indica A.Juss.)AoB Plants advance acces.http://aobpla.oxford journals.org/content/5/plt034.Accessed 23.11.2015
Sood H,Chauhan ERS(2009)Biosynthesis and accumulation of a medicinal compound,picroside-I,in cultures of Picrorhiza kurroa Royleex Benth.PlantCell,TissueOrgan Cult 100(1):113–117
Srividya NB,Sridevi P,Satyanaraya P(1998)Azadirachtin and nimbin content in in vitro cultured shoots and roots of Azadirachta indica A Juss.Indian J Plant Physiolo 3:128–129
Venkateswarlu B,Korwar GR(2005)Micropropagation technology for multipurpose trees:from laboratory to farmers fields.Central Research Institute for Dry land Agriculture,Hyderabad,India Research Bulletin,pp 1–30
Wewetzer A(1998)Callus culture of Azadirachta indica A.Juss.and their potential for the production of azadirachtin.Phytoparasitica 26(1):47–52
Whitaker RJ,Hashimoto T(1986)Production of secondary metabolites In:Evans DA,Sharp WR,Ammirato PV(eds)Handbook of plant cell culture.Techniques and Applications.Macmillan Publishing Company,New York,pp 264–286
Yakkundi SR,Theiavathi R,Ravindranath B(1995)Variation of azadirachtin content during growth and storage of neem(Azadirachta indica) seeds. J Agric Food Chem 43(9):2517–2519
杂志排行
Journal of Forestry Research的其它文章
- Vascular bundle connection between seed stalk and seed coat of Caragana arborescens
- Mini-cutting technique for Khaya anthotheca:selection of suitable IBA concentration and nutrient solution for its vegetative propagation
- Reconstructing the size of individual trees using log data from cut-to-length harvesters in Pinus radiata plantations:a case study in NSW,Australia
- Phenotypic variation in Phoebe bournei populations preserved in the primary distribution area
- Effects of soil drought stress on photosynthetic gas exchange traits and chlorophyll fluorescence in Forsythia suspensa
- Flavonoid content and radical scavenging activity in fruits of Chinese dwarf cherry(Cerasus humilis)genotypes
