Vascular bundle connection between seed stalk and seed coat of Caragana arborescens
2018-03-27ZhihuiLuanDaoweiZhouDiankunShao
Zhihui Luan·Daowei Zhou·Diankun Shao
Introduction
The seed stalk provides the connection between plant generationsand is considered as the ‘umbilical cord’orjoining point between the seed and ovary wall of a plant(Jaisankar et al.2014).The seed stalk develops from a small-headed handleoftheovule,anditistheonlypathwaythroughwhich the plant transmits photosynthates into the seed(Bouman and Schier 1979).The funiculus of the angiosperm ovule maybelongandthin,orshortandstout,oritmaybemissing completely.If missing,the ovule is sessile(Wang and Ren 2007).Generally,the ovule develops into a seed following plantfertilization,andthe funiculusdevelopstoaseed stalk;however,the funiculus fails to directly develop into a seed stalk in the ovules of some plants,such as Liliaceae plants(Bouman 1977;Tilton and Horner 1980;Matsui et al.1993;Umeda et al.1994),in which the third bead layer of the funiculus finally develops into an aril(Tilton and Horner 1980).In some Magnoliaceae plants,the appendage of the handle that protrudes from the funiculus forms a closed junction with the horseshoe-like outer integument(McNaughton and Robertson 1974),and finally,this junction develops into a goblet-shaped outer integument(Matsui et al.1993;Umeda et al.1994).
The diversion of photosynthates into the seed,by the seed stalk,increases seed yield.The cross-sectional view of the seed stalk shows a junction between the inner integument and the seed stalk at the base of the handle.At that junction are two closely connected layers of sclerenchymatous cells that form a very large depression(Wang et al.2003).The function of this depression is to attach the seed and facilitate its growth.In particular,at the hilum,fencelike cells form a small opening,which is the only channel for the outer mesocarp cells to transport photosynthates to the seed(Wang et al.2003).For example,if the funiculus of soybeans have well-developed vessel systems,the transfer of photosynthates will be very ef ficient.Subsequently,seed production will also increase(Wang et al.2004).The funiculus vessel system is considered an important anatomical structure for enhancing or improving breeding for high light ef ficiency(Wang et al.2003,2004).
Caragana arborescens,an erect shrub or small tree,commonly known as caragana or Siberian pea tree(Allen et al.1955),is used as an ornamental hedge in northeastern China(Zhang and Chen 1990)because of its tolerance to drought in cold,semiarid regions.In Inner Mongolia,millions of seedlings have been planted for medicinal purposes,such as treatment of cough and gynecological disease(Zhao 2006),and for field and green belt purposes(Milovidov 1925).C.arborescens has pod fruits that contain many seeds(Liu 1993).The caragana has a slightly bitter tasting ‘pea’,usually 3–4 in a pod,which is edible but should be cooked before eating.These ripen in July and if left,will fall from the shrub,and the seeds will grow to become a new plant next to the parent shrub.In some areas of the United States,this plant is considered an invasive species.Grasshoppers can defoliate this species in some years,but it recovers well from the attacks.However,there is no knowledge on the seed stalk of C.arborescens.
In this study,scanning electron microscopy(SEM)was employed to investigate the morphology,transmission route of photosynthetic products of the seed stalk of C.arborescens,and evaluate the role of the seed stalk in seed maturation.Our finding would be of great practical signi ficance for the breeding of C.arborescens and would provide theoretical evidence to advance breeding of C.arborescens.
Materials and methods
Specimen collection and treatment
Since 31 May 2008,30 fresh fruits were collected from the upper,middle and lower layers of the same Caragana plant after flowering at 9:00 h on the day of collection.Half of the fresh fruits were fixed in 2.5%glutaraldehyde(pH 6.7)at 4°C,and the other half was naturally dried.Each sample was numbered.
Scanning electron microscopy
The full seed of each fruit was cut with a knife along the junction between the base of the seed stalk and the endocarp layer.The fixed seeds were washed three times with 0.1 M PBS,dehydrated with a graded ethanol series,which was replaced by isoamyl acetate.Then,seeds were criticalpoint dried and sputter-coated with gold–palladium.Samples were then observed with a Hitachi S-3000N scanning electron microscope(Hitach;Tokyo,Japan)at an accelerating voltage of 15.0 kV.
Results and discussion
Morphology of the funiculus
The funiculus of the C.arborescens seed appeared a hammer-like shape,approximately 1750 μm long and 375 μm wide.Parenchymatous cells were densely arranged in regular shapes on the funiculus surface,measuring(19.05 × 38.09)–(152.38 × 171.43)μm2.In cross section,the handle was full of parenchymatous cells,and the center contained a large number of vessel cells,which have a transport function(Fig.1).
Morphology of seed stalk base

Fig.1 Cross section of the funiculus of C.arborescens seed viewed with scanning electron microscopy(×1000,lower right graph scale:dotted scale bar 50 μm)
During seed formation,the funiculus,which serves as the only bridge that connects the ovule with the external environment,has a particular role.At an early stage of seed development,the funiculus develops completely into a seed stalk.At the junction between the seed stalk and seed,there is a group of elongated cells forming a ring(Fig.2).A very large hollow is present in the center of the ring,which is composed of two layers of sclerenchymatous cells.This ring is important for sustaining the attachment during the development of seeds in the embryo sac,and a few vessels were found at the top of the ring.At a late stage of seed development,the boundary between the ring and the seed coat is very apparent.The ring is blunter than at the early stage and has a deeper,more pronounced hollow.During seed maturation,the volume of the seed stalk increased,and the boundary of the stalk with the seed coat cells became indistinct as the hollow disappeared.A small opening in the center leads to the inside of the seed.
Like the morphological structure of soybean,a ring formed by a group of elongated cells was seen at the junction between the Caragana seed stalk and seed.The opening in the center of the ring is closed during early seed development and opens as the seed matures,in agreement with the observations of the transmission tissues in the soybean funiculus(Wang et al.2004).This change is associated with the transport of the large amount of photosynthates required for seed growth.With the growth of seeds,the ring enlarges,and its boundary with seed coat is apparent.However,the boundary is indistinct after seed maturation.This change in the boundary may be explained as follows:the seed stalk cells divide rapidly as the seeds grow,and they differentiate into cells for transporting nutrients;the seed stalk falls off after the seeds mature,and the seed coat cells divide and displace the original seed stalk cells.In addition,the number of vessels on the top of the ring gradually increases as the seeds enlarge,and the lumen diameter of the vessels also enlarges.These changes likely increase transport rates.
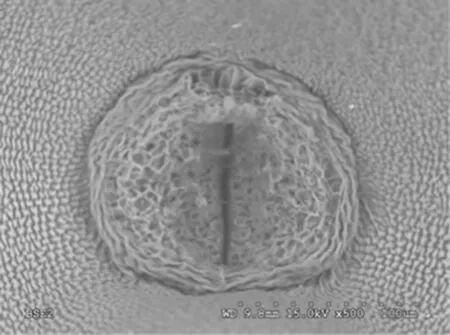
Fig.2 Scanning electron micrograph of surface of the junction between seed stalk and seed of C.arborescens as arrows pointing(×500,lower right scale:dotted scale bar 100 μm)
Transport route of photosynthates
Early in seed development,two layers of sclerenchymatous cells were densely arranged at the growth site of the seed stalk.They formed two fence-like layers around the entire growth site.These layers appeared fence-like and had a long spindle shape(Fig.3),when the opening of these fence-like cells was closed in the seed stalk.Numerous vessels extended from the seed stalk to the interior part of the seed.Later in seed development,the seed coat cells formed a large opening at the junction between the seed stalk and seed coat,thus opening the route for transferring photosynthates.In addition,the vessels extended into the seed along both sides of the seed coat cells.
Pathway for material transport
During late seed development,two potential pathways were apparent for transporting materials from the seed stalk to the seed interior.The junction between seed stalk and seed is seen by the longitudinal section of the seed.One portion of vessels extended into the seed coat cells(Fig.3),while most of the vessels went directly into the embryo(Fig.4).The vessels were arranged in a spiral pattern outside the seed coat and associated with seed coat cells.The vessels were concentrated in a net-like manner inside the seed coat cells,presumably showing the pattern of transport.
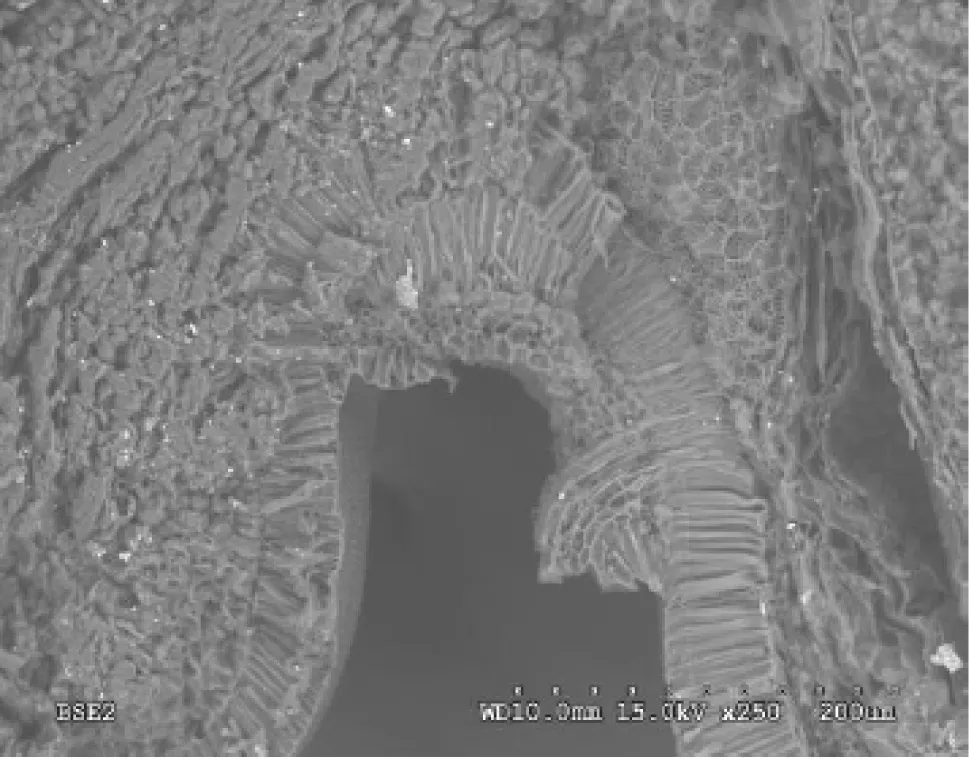
Fig.3 Scanning electron micrograph of vessel extending into the seed coat of C.arborescens as the arrow pointing and fence-like layers in the circle(×250,lower right scale:dotted scale bar 200 μm)
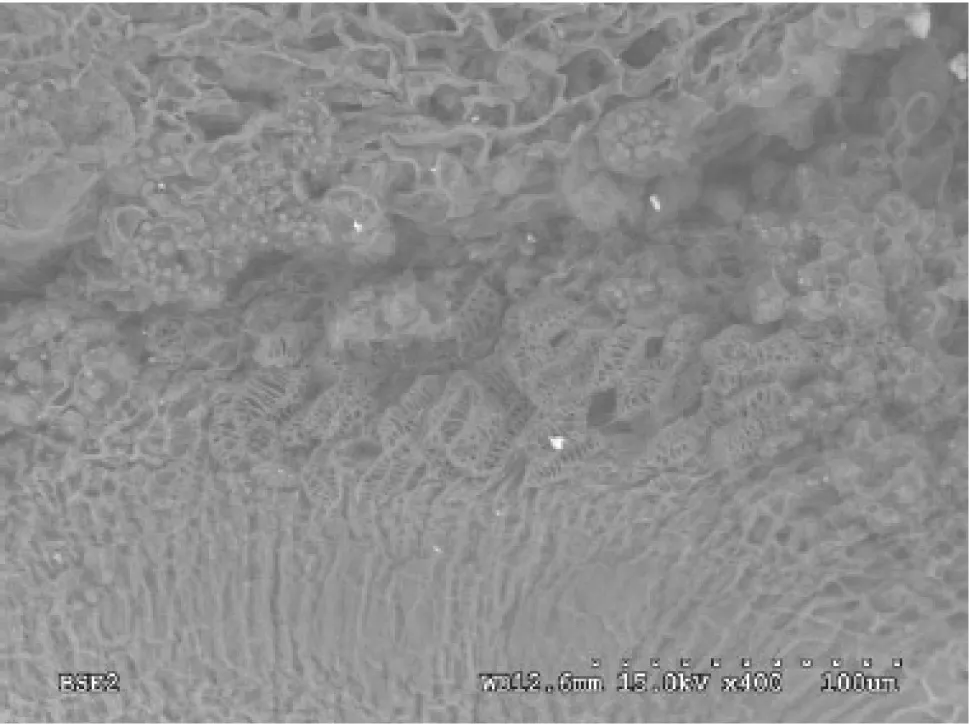
Fig.4 Scanning electron micrograph of vessel extending into the embryo as arrows pointing(×400,lower right scale:dotted scale bar 100 μm)
SEM observation of longitudinal sections of the seeds revealed two routes of transport.One route started from the seed stalk,extended through the seed coat,and reached the inside of the seed,while the other directly entered the embryo.Both pathways were associated with vessels.However,the screw thread was thickened on the vessels in the seed stalk,and densely packed vessels,characterized by thickening of the net thread,were observed inside the seed coat cells.The vessels with thickened screw threads facilitate the entrance of external substances into the seed,while the vessels with thickening of the net threads inside the seed coat cells facilitate the even distribution of external nutrients in the seed.Further studies are required to investigate the cells that constitute this route and their effects on photosynthate transport.Extensive studies on the ultrastructure of the cells in the seed stalk will help elucidate structure–function relationships.
Vessel morphology

Fig.5 Scanning electron micrograph of vessels cross seed coat into the embryo(×700,lower right scale:dotted scale bar 50 μm)

Fig.6 Scanning electron micrograph showing detail of vessels anatomy inside the seed(×900,lower right graph scale:dotted scale bar 50 μm)
Vessels were presents in the funiculus for transport.During seed maturation,a minor alteration was seen in the morphology of the vessels.At an early stage of seed development,densely packed vessels were found on the top of ring at the junction between the seed stalk and seed,with a lumen of small diameter.The vessels extended along the seed stalk into the interior part of the seed in a radial pattern,and there were a large number of granular photosynthetic assimilation products in the cells around the vessels,which demonstrated the function of vessels to transport photosynthetates.The vessels were densely packed at the seed coat.Secondary thickenings on the cell walls were mainly characterized by thickening of the net thread(Fig.5).At late stage of seed development,the vessels were overlapped.The secondary thickening of the cell wall was still characterized by thickening of the net thread;however,the arrangement was regular.This variation may be caused by the different functions of various vessels.In the seed stalk,the photosynthetic products of leaf and pod were various,and the vessels with thickening of the net thread may facilitate the transportation of the photosynthetic products.Reducing sugar are substances that transport photosynthetic products inside the seed,and the vessels with thickening of the screw thread would facilitate the rapid transportation.After the full maturation of the seed,there was large space in the middle of the vessels,which may enhance the ef ficiency of material transportation.
Distribution and morphology of vessels inside the seed
Extensive distribution of the vessels was found inside the seed.Starting from the seed stalk,the vessels extended at the lower part of the seed coat cells(Fig.6).
Conclusions
SEM observation of the seed stalk of C.arborescens after flowering reveals that the mature seed stalk is mainly consisted of vessels, sclerenchymatous cells and parenchymatous cells.The gathering elongated cells form a ring at the junction between seed stalk and seed,and there is a large hollow produced by two layers of sclerenchymatous cells in the interior seed,which functions to sustain seed growth.The opening is closed in the center of the ring at early stage of seed development,and the opening enlarges with the maturation of the seed.There are two routes of material transportation which start from the seed stalk:one from seed coat to embryo,and another directly entering the embryo.Vessels,which are abundant in the seed stalk,are the pathway for absorption of nutrients by the seeds.The vessels are densely packed in the seed stalk,and the secondary thickening of the cell wall of the vessels is characterized by net thread thickening,while the secondary thickening of the cell wall of the vessels within the seed is characterized by screw thread thickening.The morphological characteristics of vessel are adaptive to its functions.
AcknowledgementsWe thank Professor Jimin Zhao of Changchun University and Wei Zheng of JiLin Provincial Institute of Education for their comments on the manuscript.
Allen EK,Gregory KF,Allen ON(1955)Morphological development of nodules on Caragana arborescens Lam.Can J Bot 33(2):139–148
Bouman F(1977)Integumentary studies in the Polycarpicae IV.Liriodendron tulipifera L.Acta Bot Neerl 26(3):213–223
Bouman F,Schier S(1979)Ovule ontogeny and seed coat development in Gentiana with a discussion on the evolutionary origin of the single integument.Acta Bot Neerl 28(6):467–478
Jaisankar I,Sankaran M,Singh DR,Damodaran V(2014)Genetic variability and divergence studies in pod and seed traits of Pongamia pinnata(L.)Pierre.,accessions in Bay Islands.J For Res 25(2):351–358
Liu YX(1993)Caragana arborescens.Flora of China.Beijing:Science Press 42(1):13–67
Matsui M,Imaichi R,Kato M(1993)Ovular development and morphology of some magnoliaceae species.J Plant Res 106(4):297–304
McNaughton JE,Robertson BL (1974)Megasporogenesis and megagametogenesis in Aloe africana Mill.South Afr J Bot 40(1):75–79
Milovidov P(1925)Bactéries des tubercules radicaux de certaines légumineuses.Publs Fac Sci Univ Charles 49(1):3–40
Tilton VR,Horner HT Jr(1980)Stigma,style,and obturator of Ornithogalum caudatum(Liliaceae).Am J Bot 67(7):1113–1131
Umeda A,Imaichi R,Kato M(1994)Ovular development and morphology of the outer integument of Magnolia grandi flora(Magnoliaceae).Am J Bot 81(3):361–367
Wang ZF,Ren Y(2007)Advances in the study of the angiosperm ovule.Chin Bull Bot 24(1):49–59(in Chinese)
Wang XD,Cang J,Cui L,Gui MZ(2003)Scanning electron microscopic observation on seed stalk of soybean.J Chin Electr Microsc Soc 22(6):500(in Chinese)
Wang XD,Cang J,Zhang P,Cui L(2004)Scanning electron microscopic observation on seed stalk transmission tissue of soybean.J Chin Electr Microsc Soc 23(4):354(in Chinese)
Zhang ER,Chen F(1990)The rapid propagation on tissue culture of Caragana arborgce.J Northeast Agric Coll 23(1):94–97(in Chinese)
Zhao YZ(2006)The distribution pattern and ecological adaptation of Caragana sibirica Fabr.Bull Bot Res 26(1):402–404(in Chinese)
杂志排行
Journal of Forestry Research的其它文章
- Robust optimization for volume variation in timber processing
- Mini-cutting technique for Khaya anthotheca:selection of suitable IBA concentration and nutrient solution for its vegetative propagation
- Reconstructing the size of individual trees using log data from cut-to-length harvesters in Pinus radiata plantations:a case study in NSW,Australia
- Phenotypic variation in Phoebe bournei populations preserved in the primary distribution area
- Effects of soil drought stress on photosynthetic gas exchange traits and chlorophyll fluorescence in Forsythia suspensa
- Flavonoid content and radical scavenging activity in fruits of Chinese dwarf cherry(Cerasus humilis)genotypes
