Mini-cutting technique for Khaya anthotheca:selection of suitable IBA concentration and nutrient solution for its vegetative propagation
2018-03-27JoamirBarbosaFilhoMariaAnglicaDiCarvalhoLeandroSilvadeOliveiraEnasRicardoKonzenGilvanoEblingBrondani
Joamir Barbosa Filho ·Maria Angélica Di Carvalho ·Leandro Silva de Oliveira ·Enéas Ricardo Konzen ·Gilvano Ebling Brondani,
Introduction
Khaya anthotheca Welw.(Meliaceae)is a valuable tropical hardwood species native to Africa.It is commonly known as African mahogany,white mahogany or grand bassam mahogany.Its natural distribution encompasses areas between 20°S and 10°N,from Sierra Leone eastwards to Uganda and Tanzania,and southwards to Angola,Zambia,Malawi,Mozambique and Zimbabwe.K.anthotheca is encountered in lowland rain forests and riverine fringe forests in areas with 600–1000 mm annual rainfall(Joker 2003;Opuni-Frimpong et al.2008).It is a large tree,reaching up to 60 m in height,with a straight bole that reaches considerable height before branching.Leaves and flowers organized in in florescences can both reach 40 cm in length(Joker 2003).
Khaya anthotheca contributes to the economies of the countries in which it naturally occurs,and is traded in international markets where it generates abundant revenues(Opuni-Frimpong et al.2008).Khaya anthotheca is used for high-class cabinet work and for the production of veneers and any application where good quality and medium weight hardwood is needed.With wood density at 12%,wood moisture is 0.62 kg m-3(Joker 2003).The importance of this species extends to medicinal properties,as limonoid extracts revealed potent antimalarial activity(Lee et al.2008).
Swietenia macrophylla is a mahogany native to Brazil and other countries from South and Central America.The tree is economically the most valuable and important mahogany.However,it has been intensively exploited,and its genetic resource base has been considerably deleted due to illegal logging and trade,especially in the northern area of its distribution(Degen et al.2013).Furthermore,its cultivation throughout South and Central America is severely impaired by the insect Hypsipyla grandella,whose larvae bore into leading shoots of young plants,causing reduction in height increment and forks on the main stem(Cornelius 2001).The introduction of African mahoganies such K.anthotheca to South American countries like Brazil has been encouraged for commercial purposes(Franc¸a et al.2015),having potential to replace S.macrophylla.Currently,however,in the case of K.anthotheca,the availability of seeds and plants for plantations are still scarce in Brazil due to their recent introduction to the country(Pinheiro 2011).Furthermore,there is limited knowledge of vegetative propagation of the species using methods such as mini-cuttings,grafting or layering.This raises a considerable challenge for its breeding.
Clonal propagation has the potential to subsidize plant production of selected genotypes,supplying the demands of the market and thereby diminishing the constant need for obtaining seeds.As regards K.anthotheca,little work has been done concerning its vegetative propagation.One of the most signi ficant contributions toward this goal was reported by Opuni-Frimpong et al.(2008),they evaluated the roles of leaves,age and auxin concentration(indole-3-butyric acid—IBA)in the vegetative propagation of K.anthotheca and K.ivorensis.Elsewhere,stem tip cuttings of K.anthotheca were also evaluated for their adventitious rooting potentialwhen inoculated with arbuscular mycorrhizae fungi(AMF),revealing that sixmonth old seedlings were able to develop roots without the application of plant growth regulators(Dugbley 2015).Some related species have also been examined for their adventitious rooting potential from cuttings,such as with K.ivorensis(Tchoundjeu and Leakey 1996;Owusu et al.2014),K.senegalensis(Ky-Dembele et al.2011)and K.grandifoliola(Owusu et al.2014).Recent studies also reported the in vitro propagation of Khaya(Hung and Trueman 2011;Okere and Adegeye 2011).
Improving propagation techniques such as cuttings and mini-cuttings for K.anthotheca and other mahoganies might lead to more attractiveness of this tree species to the market for many countries,since it would accelerate massive production with selected and desirable genotypes.
In this research,we analyzed the ef ficiency of propagation of K.anthotheca by the mini-cutting technique.First,we tested different concentrations of nutrients from a nutrient solution composition for mini-stumps in development(Brondani et al.2014).Thereafter,we evaluated the adventitious rooting potential with two auxin(i.e.,IBA)concentrations combined with three nutrient solutions.With this research,we outline prospects for future largescale clonal propagation of African mahogany,which might be used for breeding programs aimed at clonal propagation ofsuperiorgenotypes.Furthermore,we emphasize this technique as a strategy to develop a germplasm bank to conserve valuable genetic resources obtained from this species.
Materials and methods
Plant materials and location of the experiments
Seedlings of K.anthotheca were grown from germinated seeds obtained from BioSementes (http://www.biose mentes.com.br),company that imported the seeds from Ghana,West Africa.The seeds were imported according to Brazilian laws and registrations(RENASEM,BA0104/2011).Seedlings were grown in polyethylene bags containing substrate composed of 50%dark soil(A horizon)and 50%cattle manure.After planting,the seedlings were kept in a shade house(50%shading).Four-month old plants were selected for the experiments.
Two experiments were conducted at the N’tacua nursery,in Cuiabá,Mato Grosso,Brazil(15°35′56′S,56°06′05′W,165 m,a.s.l.).According to Köppen’s categorization,this area has a tropical wet and dry climate(Aw),with rainy summers(Alvares et al.2013),annual average temperature of 25.8°C,and maximum temperature reaching 43°C.A general scheme of the sequential experiments is presented in Fig.1.
Effect of nutrient solutions on mini-stumps(Experiment 1)
A seminal mini-garden was established with K.anthotheca seedlings transplanted into polyethylene bags containing 4.5 L of sand(0.10<particle size<0.25 mm),with a thin layer(3 cm)of crushed stone.Roots were pruned and leaf area reduced by 50%to avoid the effects of dehydration after transplantation.The seminal mini-garden was established in an area of 15 m2,covered with crushed stone and with 50%shading.

Fig.1 Flowchart of vegetative propagation of Khaya anthotheca through mini-cutting technique.S100—nutrient solution with 100%of the original concentration of macro and micronutrients from the reference solution(Table 1);S50—50%of the original concentration;S25—25%of the original concentration;IBA—indole-3-butyric acid
Ten days after transplanting,air layering was performed at 15 cm from the basal portion of each plant,a procedure aimed at inducing axillary shoots by breaking apical dominance.The layering strategy increased the production of new propagules for the experiment.Moreover,this strategy was adopted to reduce the stress introduced by cutting off the apical bud for the induction of axillary shoots.After 30 days,dead plants were replaced by new seedlings,thereafter,these plants were referred to as ministumps of the seminal mini-garden.
The mini-stumps were systematically divided into three plots to receive different treatments(nutrient solutions)aimed at maintaining different levels of nutritional statusand selecting the best nutrition composition for their growth and development.Allplants were spaced 10×30 cm.The nutrient solution was initially prepared according to Brondani et al.(2014)(modi fied nutrient solution),being considered the reference solution for further steps of the experiment(Table 1).
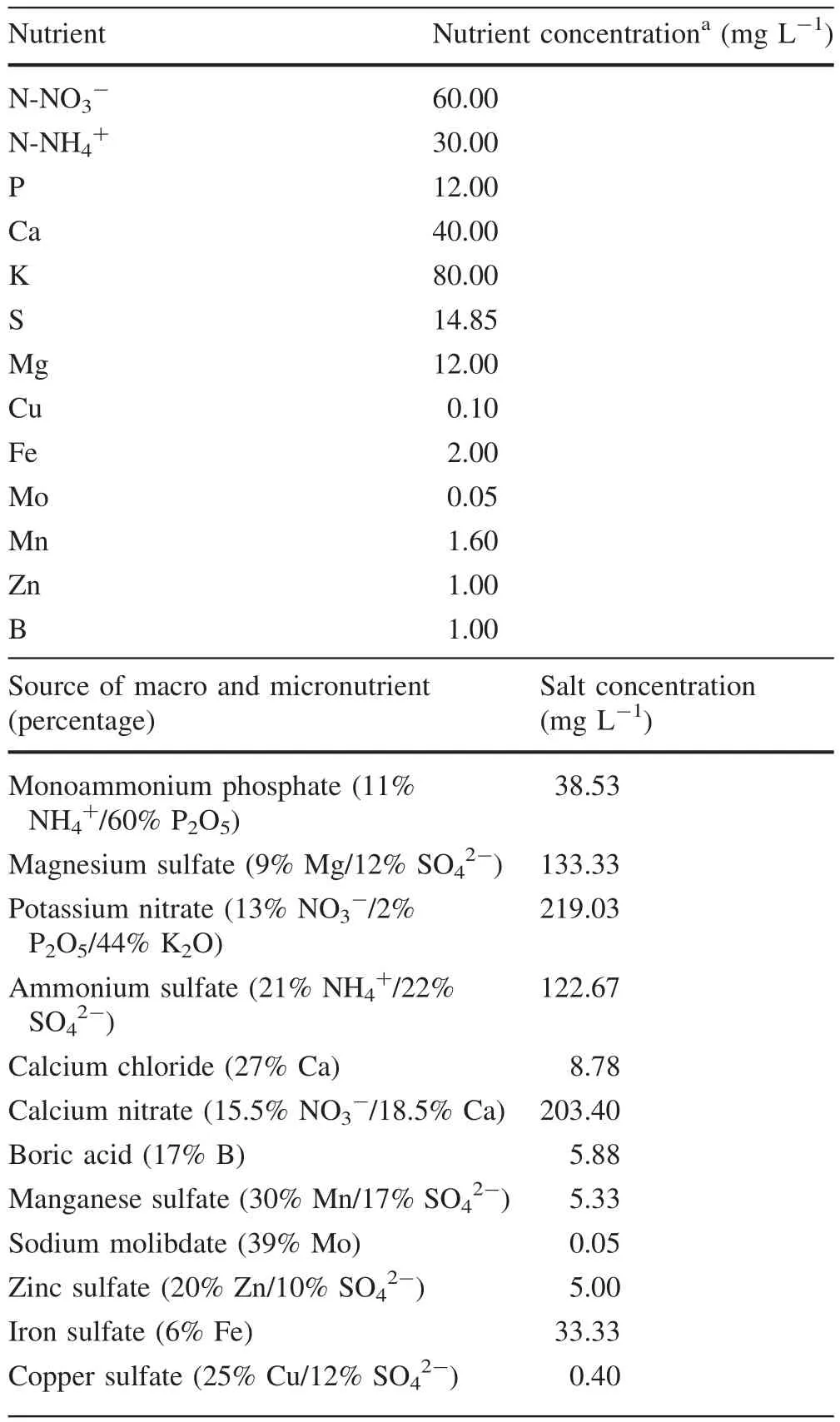
Table 1 Basic nutrient solution components for fertigation of the seminal mini-garden of Khaya anthotheca
Each of the three plots was assigned a different concentration of the nutrient solution from the original:100%(S100),50%(S50)and 25%(S25).Fertigation was always performed in the early morning,between 07:00 and 08:00 a.m.The mini-stumps were supplied with 100 mL of the assigned nutrient solution every 4 days.The nutrient solution was evenly distributed on the surface of the soil around the mini-stumps.Additionalperiodic water requirements were met with irrigation using water only.
One hundred and twenty days after fertigation,we performed the first collection of shoots(Collection 1)for producing mini-cuttings.The shoots were collected in the early morning(from 08:00 to 10:00 a.m.)to avoid dehydration episodes of both the mini-stumps and mini-cuttings.The experiment was conducted over a 12 month period(January 2014 to January 2015),with five shoot collection events.The first collection was in January;the 2nd(Collection 2)in April;the 3rd(Collection 3)in June;the 4th(Collection 4)in August;and the 5th(Collection 5)in November(see flowchart from Fig.1).The mini-cuttings were transferred to polyethylene bags for conducting the second experiment.
This first experiment was conducted in a completely randomized split-plot design with factorial arrangement(3×5),subdivided by time(1st,2nd,3rd,4th and 5th shoot collections)and three concentrations of nutrient solution(100,50 and 25%).
Effect of combined IBA and nutrient solution concentrations on mini-cuttings(Experiment 2)
The mini-cuttings(i.e.,produced from mini-stumps originated from seeds)obtained from the previous experiment were divided into two sets with equal numbers for each shoot collection.The bottom portion of each mini-cutting from a set was placed in indole-3-butyric acid(IBA)diluted in hydroalcoholic solution(2 g L-1),while the other set received no IBA treatment(0 g L-1)(Fig.1).Propagules were collected in the early morning and placed in pots containing water at room temperature to keep turgidity after detachment.The mini-cuttings were prepared with their basal cut in the shape of a bevel to facilitate their placement in the substrate.Each cutting was 6 cm(±3 cm)in length with one axillary bud.Each minicutting(containing two leaves and stem apical meristem)had its leaf area reduced by 50%.
The mini-cuttings were inserted to a depth of 2 cm in the substrate in the polyethylene bags(100 cm3)containing commercial soil(i.e.,decomposed pine bark,vermiculite,fibrous peat,initial fertilizer with N=4%,P2O5=14%and K2O=8%,and micronutrients).Prior to use,all bags were super ficially sterilized with sodium hydrochloride with 0.25%of active chlorine for 24 h.
The mini-cuttings were placed in a greenhouse covered with white plastic low density polyethylene(anti-ultraviolet 200 μm),with 50%shading.The greenhouse was equipped with a sprinkler irrigation system,which was automatically turned on every time the temperature reached 36 °C and turned off when it was 33 °C or less.Relative humidity was sustained at values of at least 80%.The bags were placed on a table with height of 1.5 m.
The experiment was conducted in completely randomized split-plot design in factorial arrangement(3×5×2)subdivided through time.The factors comprised of three nutrient solutions(100,50 and 25%), five propagule collections and two concentrations of IBA(0 and 2 g L-1).The experiment was performed with five replicates.
The mini-cuttings stayed in the greenhouse for 30–45 days.They were then transferred to a shade house,where they stayed for 30 days.Finally,they were taken from the shade house and placed in outdoor conditions for 30 days,to harden off.We evaluated the performance of the mini-cuttings in all environments.
A general view of all the steps from the seminal minigarden to the mini-cutting technique is sequentially presented in Fig.2a–j.An overall picture of the mini-stumps is shown in Fig.2a before the shoot collections,while Fig.2b,c show the mini-stumps after shoot collection events.
Response variables
In the two experiments,variables were measured.In Experiment 1,we evaluated mini-stump survival(MS)and determined the production of mini-cuttings per square meter per year(PMS):

where PMS—number of mini-cuttings per square meter per year(mini-cuttings m-2a-1);NMI—number of mini-cuttings per mini-stump;DA—total number of days per year(365 days);IC—period between each collection event(variable according to productivity);AE—effective area of each mini-stump(10×30 cm).
From Experiment 2,we evaluated the survival rate of mini-cuttings after their stay in the greenhouse(GHS)and the shade house(SHS).Moreover,the percentage of rooting and callus formation were evaluated from all treatments and mini-stumps,to verify which treatment combination(nutrient solution×IBA concentration) was more effective.
Histological analyses
Samples from the central region of some mini-cuttings were collected in order to analyze their cellular and tissue characteristics using optic microscopy.The samples were fixed in formaldehyde and modi fied glutaraldehyde(glutaraldehyde 1%;paraformaldehyde 4%in sodium phosphate buffer—NaH2PO4⋅H2O at 0.1 M; pH 7.2)(Karnovsky 1965).Subsequently,samples were submitted to two vacuum series(-600 mmHg)for 30 min each.After that,samples were stored at 4°C for 30 days.

Fig.2 Sequence illustration of the strategies adopted for producing and growing mini-stumps and mini-cuttings from Khaya anthotheca seedlings.a General view of the mini-stumps.b,c Mini-stumps after different shoot collection events.d–g Sequence of steps undertaken for layering of mini-stumps.h Example of mini-stump after layering removal.Shoots are visible.i General view of the mini-cuttings placed in polypropylene tubes.j Zoomed view of some mini-cuttings
Afterwards,samples were dehydrated with an ethyl-alcohol series with increasing concentrations for 15 min in each solution from the gradient(10,20,30,40,50,60,70,80,90 and 100%,v v-1).The samples were embedded with hydroxyethylmethacrylate resin (Historesin,Leica®,Hildeberg,Germany).The blocks containing the samples were prepared according to manufacturer’s instructions,being stored for 28 days at 24°C and thereafter analyzed.The blocks containing the samples were sectioned longitudinally to a thickness of 10 μm using an automatic rotary microtome Microm HM 355S(Thermo Scienti fic).The sections were stained with toluidine blue(0.05%,v v-1)in a phosphate buffer and citric acid(Sakai 1973)for 20 min and mounted on slides with a synthetic resin(Entellan®).The slides were analyzed and photographed with a light microscope(Opton)and images were captured in micrometric scale.
Statistical analysis
The data collected from the two experiments were submitted to Hartley’s test(P<0.05)to verify variance homogeneity among treatments and to Lilliefors’s test(P<0.05)to check fornormaldistribution.When necessary,the data were transformed through Box–Cox test.Thereafter,the data were submitted to ANOVA(P<0.05).Complimentary analyses with Tukey’s and Duncan’s tests were performed when signi ficance was detected through ANOVA.
Results
Mini-stump survival and mini-cuttings yielding
The five collection events of mini-cuttings and the nutrient solutions had no impact on mini-stump survival(MS)over a period of 365 days(Table 2).High survival rates were recorded for all treatments,averaging 97%.
Conversely,the production of mini-cuttings from ministumps(PMS)was affected by the collection period and the concentration of the nutrient solution(Table 2).Furthermore,there was interaction between the collection period and the nutrient solution,according to ANOVA analysis,so that these variables concomitantly interfered with shoot yields of the mini-stumps.Over the course of propagule collections,increased number of shoots were produced,as mini-stumps were supplied with the nutrient solution andpruned.New shoots were grown from the development of axillary buds.
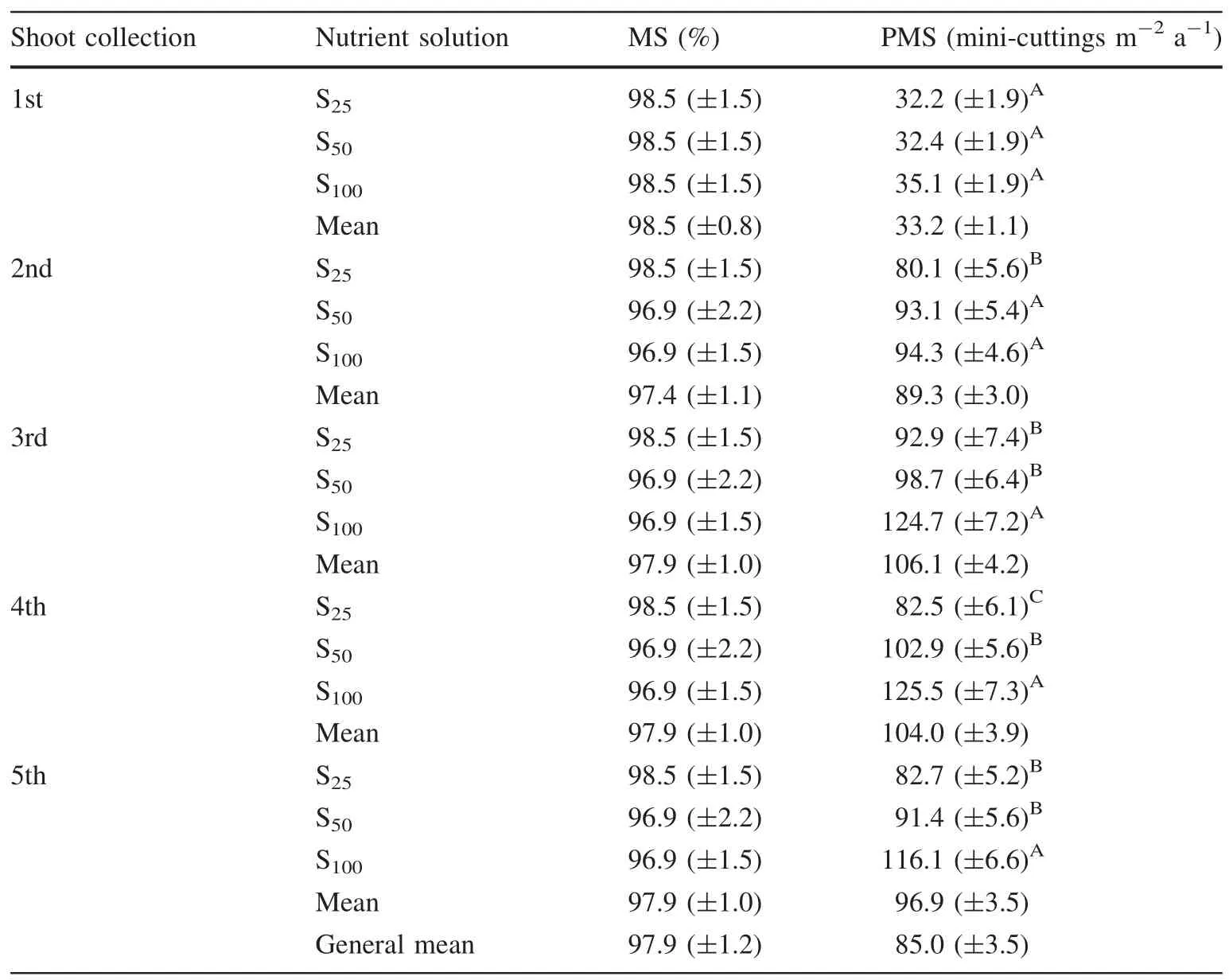
Table 2 Mini-stump survival rate(MS)and production of mini-cuttings per square meter per year(PMS)of Khaya anthotheca mini-stumps treated with different nutrient solutions
During the first shoot collection,the nutrient solution showed no signi ficant effect on the production of minicuttings(Table 2).From the second to the fifth collections,the mini-stumps treated with S50and S100solutions exhibited higher shoots yield than S25.The original concentration of macro and micronutrients(i.e.,S100)yielded the highest number of shoots,with numbers over 100 minicuttings m-2a-1.
Survival of mini-cuttings
The survival rates of mini-cuttings after their stay in the greenhouse(GHS)were similar regardless of nutrient solution or plant growth regulator applications(i.e.,IBA)during the 3rd and 4th collections(Table 3).Major differences were observed in the 1st and 2nd shoot collections,but stabilized thereafter.In the 5th collection there was signi ficant difference with the application of 2 g L-1IBA,decreasing the mini-cuttings survival in S25(Table 3).Overall,the survival rates of mini-cuttings ranged from 17 to 95%.
The percentage of mini-cutting survival from the shade house(SHS)varied with nutrient solution concentration and IBA application(Table 3).There was no variation for those collected from the 4th collection,for either the nutrient solution or IBA application.The IBA application resulted in higher values for SHS in the 1st and 2nd collections,independent of the nutrient solution.
In general,the nutrient solutions S50and S100associated with the application of IBA to the basal portion of the minicuttings resulted in the best survival rates after the shade house stay(Table 3).
Adventitious rooting
The adventitious rooting ratio of the mini-cuttings maintained in outdoor condition showed similar results when comparing the three nutrient solutions(S100,S50and S25)(Table 4).Some examples of adventitious roots are shown in Fig.3.The application of IBA was associated with greater percentages of rooting,especially during the first three collections.The treatment with S50and S100with IBA resulted in the best percentages of rooting during the five stages(ranged from 17 to 81%).Callus induction on the basal portion of the mini-cuttings showed similar results from the three nutrient concentrations tested.However,
higher percentages of callus induction were observed when no IBA was applied.

Table 4 Adventitious rooting percentage and callus incidence on Khaya anthotheca minicuttings

Fig.3 Adventitious rooting in mini-cuttings of Khaya anthotheca by 2 g L-1IBA application.a First adventitious rooting event observed,as indicated by the white arrow.b–d Examples of adventitious roots from different mini-cuttings after 105 days.Bars represent 4 cm
Histological analysis
A considerable percentage of the mini-cuttings developed adventitious roots at the end of the evaluation of the experiment.Thus,the materials are probably juvenile(i.e.,mini-stumps originated from seeds).Histological sections evidenced this aspect,which could be visualized as the transition from primary to secondary growth(Fig.4a).The transition was identi fied due to the presence of vascular bundles in a pattern of primary tissue growth concomitantly with the secondary type.The latter is evidenced from the reorganization of the vascular system(Fig.4b).
Discussion
The survival rates of the mini-stumps of K.anthotheca were not affected by the five consecutive collections and the nutrients solutions(S100,S50,S25),as low mortality rates were observed throughout the whole experimental period.This gives evidence to the suitability of the minigarden system used in this research,where mini-stumps showed good performance in yielding shoots for further propagation steps.These results are also related to the ontogenetic stage of the mini-stumps,which originated from seeds.Juvenile materials exhibit a predisposition to growth and development when submitted to different cultivation conditions in propagation systems(Wendling et al.2014a,b,2015).
The management of the mini-garden with the pruning of each mini-stump and balanced nutrition,luminosity and proper irrigation were essential for high survival and high yield of propagules.Mineral nutrition has a preponderant role in such propagation systems(Rowe et al.2002;Cunha et al.2009),since it maintains the propagule donor plant vigor,enabling the continuous production of stem cuttings(e.g.,cuttings and mini-cuttings)and the recovery when shoots are detached from the mini-stumps(Hartmann et al.2011).
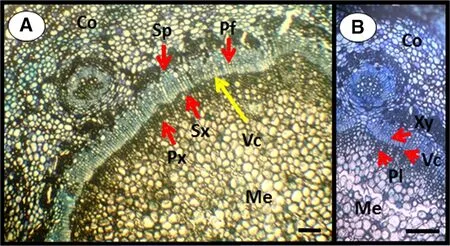
Fig.4 Transverse section of the stem of a mini-cutting of Khaya anthotheca.a Starting point of secondary growth.b Detail from the cambial region of the mini-cutting.Co Cortex,Pp primary phloem,Sp secondary phloem,Px primary xylem,Sx secondary xylem,Vc vascular cambium,Xy Xylem,Pl Phloem,Vc vascular cambium,Me medulla.Bars represent 10 μm
Our results are similar to those from other studies that analyzed the performance of mini-cuttings of forest tree species(Cunha et al.2009;Wendling et al.2010;Brondani et al.2012b;Wendling et al.2015),reporting survival rates higher than 90%.One of the studies was performed with a clonal mini-garden of Cedrela fissilis mini-stumps over 150 days,with collection of shoots every 30 days.The experiment resulted in 100%survival(Xavier et al.2003).
In this study,the average mini-cuttings productivity of K.anthotheca was superior up to 80 mini-cuttings m-2a-1,which represents approximately 1.8 mini-cuttings per mini-stump(Table 2).This number was higher than that obtained for C. fissilis,which averaged 1.3 mini-cuttings per mini-stump,with regular collection every 30 days(Xavier et al.2003).In Erythrina falcata,this variable averaged 1.3 as well(Cunha et al.2008).Similar values were also found for Eucalyptus by Wendling et al.(2000),averaging 1.7 mini-cuttings per mini-stump.Nevertheless,Eucalyptus propagules are generally collected at intervals of seven to 21 days with reduced spacing among plants(Alfenas et al.2009),which differs from our study,which used intervals of two and three months between collection events.Another detail which differs from the other propagation systems adopted for mini-cuttings of Eucalyptus spp.is the cultivation container.The mini-stumps of K.anthotheca were grown in pots with capacity of 4.5 L,spaced at 10×30 cm.Hypothetically,better performance would be obtained if the mini-stumps of K.anthotheca were grown in a semi-hydroponic with sand and controlled fertigation(i.e.,over time intervals),and with reduced spacing between plants(e.g.,8×10 cm;10×10 cm)(Brondani et al.2012a).Such a strategy could be the goal of further studies.
The production of shoots was low from the first collection.At this stage,the mini-stumps were adapting to the culturalpractices.Moreover,plants had no proper arrangement of their canopy yet,an implication of the loss of apical dominance and increasing the number of axillary buds for producing shoots.Studies with Eucalyptus and C.fissilis revealed similar results,as a reduced number of buds was observed at the first collection event(Wendling et al.2000;Xavier et al.2003).After the first collection of shoots,plants usually switch from orthotropic to plagiotropic growth,stimulating the development of dormant axillary buds,an important change for the development of a clonal mini-garden,independent from the origin of the mini-stumps(i.e.,clonal or seedling stock plants).
The best results concerning the mini-stump productivity were found when the nutrient solution was applied at 100%(S100)of the concentration of each macro and micronutrient(Table 2).Optimal levels of nutrition are essential for the success of the propagation,being important to balance nutrients according to the species demands in a mini-garden system(Cunha et al.2009;Hartmann et al.2011).Nevertheless,the ideal conditions for K.anthotheca cultivation are not yet established and require further study.
One essential goal in a protocol for the propagation of a species is to ensure proper conditions for stimulating adventitious rooting of the propagules in consonance with the characteristics of the material of interest(Assis and Ma fia 2007).In this study,the mini-cuttings survival obtained from the greenhouse(GHS)varied with the nutrient solution(50–100%)(Table 3).Considering the genetic variability of these materials(i.e.,produced from seeds),their performance was very good.Moreover,after the third collection,the survival rate was higher than 84%(Table 3).Temperature(30–40 °C in greenhouse),light and moisture were controlled to adequate levels,which positively in fluenced the rooting ofeach propagule(Wendling and Xavier 2005;Wendling et al.2014a,b).
In Eucalyptus species,studies have shown mini-cutting survival rates higher than 80%after the greenhouse,such as with E.grandis×E.urophylla(Goulart and Xavier 2008;Goulart et al.2008,2010)and E.cloeziana(Almeida et al.2007).Nevertheless,this response has no direct implication in high rooting ratios,as the mini-cuttings might survive due to favorable conditions of the environment without developing roots for a period.Losses are higher when the propagules are transferred to the shade house,where environmental conditions are altered,such as with the reduction of temperature and air humidity(Brondani et al.2012a,b),conditions in which propagules that emit no roots are not able to withstand,impairing their survival.
The survival rates of K.anthotheca mini-cuttings after the shade house(SHS)ranged from 17 to 95%(Table 3),with seasonal effects according to the periods of propagule collection.In general,the best results were obtained with the application of IBA associated with the nutrient solutions S100and S50.Comparatively,mortality of E.grandis mini-cuttings after acclimation in a shade house reached 85%,which could also be related to seasonal variation(Wendling and Xavier 2005).This was similar to that reported for Liquidambar styraci flua(Wendling et al.2010),in which environmental changes(i.e.,due to the transfer of propagules from the greenhouse to the shade house aiming at acclimation)might lead to water de ficit in the propagules,accelerating tissue apoptosis.
The percentage of adventitious rooting of K.anthotheca mini-cuttings ranged from 17 to 81%(Table 4),considering the shoot collection,IBA application and nutrient solution.In general,mini-cuttings with the application of IBA combined with S100and S50recorded higher percentages of adventitious rooting.Callus induction was more pronounced with the treatments without IBA supply(Table 4),independent of the concentration of the solution evaluated.
Our results are similar to previous studies of K.anthotheca cuttings(Opuni-Frimpong et al.2008)and K.senegalensis(Ky-Dembele et al.2011).In these studies,the plant growth regulator affected the number and length of adventitious roots per cutting,but no impact was observed on survival.Additionally,experiments performed in Indonesia with cuttings of K.anthotheca reached up to 75% of rooting,when plant growth regulators were employed(Pinheiro 2011).In the American native mahogany S.macrophylla,the application of IBA was also effective for adventitious rooting.The highest rooting of cuttings obtained from adult trees reached 62.5%with the application of a 0.4%IBA solution(Azad and Matin 2015).In our research,however,the analyses were performed with mini-cuttings,which brings the advantage of introducing higher juvenility to the tissues being propagated,although we already had consistently juvenile tissues because they originated from seeds planted one year earlier.
Adventitious rooting is a trait with quantitative inheritance,and thus,is modulated by both environmental and endogenous factors.Furthermore,an extensive line of evidence shows that auxins play essential roles in regulating adventitious root development(Overvoorde et al.2010;Pijut et al.2011;Pop et al.2011).The use of auxins stimulates rooting of different propagules,but the concentration to be used for this purpose varies with the species,its level of maturity,the environment and the way the plant growth regulator is provided to the plants(Wendling et al.2014a,b).The production of roots without the IBA application is because of the endogenous production of auxin in each plant(e.g.,endogenous auxin synthesis)(Leakey et al.1990;Hartmann et al.2011).The optimal concentration for exogenous application has been previously tested for K.anthoteca cuttings,being set at 8 g L-1(Opuni-Frimpong et al.2008).In our study,the application of 2 g L-1IBA resulted in high rooting percentages,especially when combined with nutrient solutions at 100 and 50%of the original solution(Brondani et al.2014),and considering the juvenile tissues of the mini-stumps(Pijut et al.2011;Wendling et al.2014a,b).This re flects in the application of lower concentrations of IBA to induce adventitious rooting.
The high percentages of adventitious rooting might have been favored by the juvenility of the tissues from the minicuttings.The histological pro file(Fig.4a,b)of the minicuttings evidenced this condition,since we observed cells undergoing the transition from primary to secondary growth concomitantly with typical cells from primary growth.The repeated cell division during plant development lead to changes in shoot meristems,which progressively change undifferentiated tissues to more specialized ones(Wendling et al.2014a).The use of mini-stumps from seeds provided juvenile tissues that were only at initial stages of tissue differentiation,which might result in higher cellular competence for the induction of adventitious rooting.In a practical sense,the seminal mini-garden of K.anthotheca was ef ficient for producing shoots for obtaining mini-cuttings during the whole year,and represents for growers an excellent alternative to continuously growing plants from seeds.
Our study has important implications for the propagation of K.anthotheca.A balanced nutrient solution along with the use of IBA as plant growth regulator ensured good results for adventitious rooting of mini-cuttings.The consecutive acclimation steps,from the greenhouse to outdoor conditions also need careful control of time and conditions.In general,all these conditions might be employed in further experiments and in the establishment of nurseries for large-scale production of the species.However,additional research is needed on different provenances and progenies of the species,given the potential genetic variability for adaptation,acclimation and rooting.These analyses might provide an indication of superior genotypes to be employed in breeding programs assisted by clonal propagation of K.anthotheca.
Conclusions
By testing three concentrations of a reference nutrient solution,we found that the solutions with 100 and 50%from the original concentration provided the highest shoot yields for further steps of the mini-cutting technique.Furthermore,we veri fied that those nutrient solutions combined with 2 g L-1IBA led to the highest survival rates of mini-cuttings after cultivation in greenhouse conditions and after a period in the shade house.Moreover,the combination of S100and S50with IBA led to the highest percentages of adventitious rooting.The high survival and rooting of mini-cuttings probably were favored by the presence of juvenile tissues,as revealed by the analyses of histological sections.Our results provide insights for further steps aimed at massive propagation of K.anthotheca,which might assist breeding programs of this species.
AcknowledgementsWe thank CNPq(National Council for Scienti fic and Technological Development,Brazil)and CAPES(Coordination for the Improvement of Higher Level Personnel,Brazil)for their financial support.
Alfenas AC,Zauza EAV,Ma fia RG,Assis TF(2009)Clonagem e doenc¸as do eucalipto.Federal University of Vic¸osa–UFV,Vic¸osa,p 500
Almeida FD,Xavier A,Dias JMM,Paiva HN(2007)E ficiência das auxinas(AIB e ANA)no enraizamento de miniestacas de clones de Eucalyptus cloeziana F.Muell Rev Árvore 31:455–463.doi:10.1590/S0100-67622007000300011
Alvares CA,Stape JL,Sentelhas PC,Gonc¸alves JLM,Sparovek G(2013)Köppen’s climate classi fication map for Brazil.Meteorol Z 22:711–728.doi:10.1127/0941-2948/2013/0507
Assis TF,Ma fia RG(2007)Hibridac¸a˜o e clonagem.In:Borém A(ed)Biotecnologia florestal.Suprema Grá fica e Editora,Vic¸osa,pp 93–121
Azad MS,Matin MA(2015)Effect of indole-3-butyric acid on clonal propagation of Swietenia macrophylla through branch cutting.J Bot.doi:10.1155/2015/249308(Article ID 249308)
Brondani GE,Baccarin FJB,de Wit Ondas HW,Stape JL,Gonc¸alves AN,Almeida M(2012a)Low temperature,IBA concentrations and optimal time for adventitious rooting of Eucalyptus benthamii mini-cuttings.J For Res 23:583–592.doi:10.1007/s11676-012-0298-5
Brondani GE,Wendling I,Brondani AE,Araujo MA,Silva ALL,Gonc¸alves AN(2012b)Dynamics of adventitious rooting in mini-cuttings of Eucalyptus benthamii×Eucalyptus dunnii.Acta Sci Agron 34:169–178.doi:10.4025/actasciagron.v34i2.13059
Brondani GE,Baccarin FJB,Bergonci T,Gonc¸alves AN,Almeida M(2014)Miniestaquia de Eucalyptus benthamii:efeito do genótipo,AIB,zinco,boro e coletas de brotac¸o˜es.Cerne 20:147–156.doi:10.1590/S0104-77602014000100018
Cornelius JP(2001)The effectiveness of pruning in mitigating Hypsipyla grandella attack on young mahogany(Swietenia macrophylla King)trees.For Ecol Manag 148:287–289.doi:10.1016/S0378-1127(00)00531-4
Cunha ACMCM,Wendling I,Ju´nior SL(2008)Miniestaquia em sistema de hidroponia e em tubetes de corticeira-do-mato.Ciência Florestal 18:85–92.doi:10.5902/19805098513
Cunha ACMCM,Paiva HN,Leite HG,Barros NF,Leite FP(2009)In fluência do estado nutricional de minicepas no enraizamento de miniestacas de eucalipto.Rev Árvore 33:607–615.doi:10.1590/S0100-67622009000400003
Degen B,Ward SE,Lemes MR et al(2013)Verifying the geographic origin of mahogany(Swietenia macrophylla King)with DNA-fingerprints.Forensic Sci Int Genet 7:55–62.doi:10.1016/j.fsigen.2012.06.003
Dugbley PW(2015)Susceptibility of vegetatively propagated Khaya anthoteca to arbuscular mycorrhizae fungi(AMF)soil inoculum infection.Sci Res 3:13–18.doi:10.11648/j.sr.20150301.13
Franc¸a TSFA,Arantes MDC,Paes JB et al(2015)Características anatoˆmicas e propriedades físico-mecaˆnicas das madeiras de duas espécies de mogno africano.Cerne 21:633–640.doi:10.1590/01047760201521041877
Goulart PB,Xavier A(2008)Efeito do tempo de armazenamento de miniestacas no enraizamento de clones de Eucalyptus grandis × E.urophylla.Rev Árvore 32:671–677.doi:10.1590/S0100-67622008000400008
Goulart PB,Xavier A,Cardoso NZ(2008)Efeito dos reguladores de crescimento AIB e ANA no enraizamento de miniestacas de clones de Eucalyptus grandis×Eucalyptus urophylla.Rev Árvore 32:1051–1058.doi:10.1590/S0100-67622008000600010
Goulart PB,Xavier A,Dias JMM(2010)Efeito de antioxidantes no enraizamento de miniestacas de clones de Eucalyptus grandis × E.urophylla.Rev Árvore 34:961–972.doi:10.1590/S0100-67622010000600001
Hartmann HT,Kester DE,Davies FT Jr,Geneve RL(2011)Plant propagation:principles and practices,8th edn.Prentice-Hall,Sa˜o Paulo,p 915
Hung CD,Trueman SJ(2011)In vitro propagation of the African mahogany Khaya senegalensis.New For 42:117–130.doi:10.1007/s11056-010-9241-9
Joker D(2003)Khaya anthotheca(Welw.)C.D.C.Seed Lea fl69:1–2
Karnovsky MJ(1965)A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy.J Cell Biol 27:137–138
Ky-Dembele C,Tigabu M,Bayala J et al(2011)Clonal propagation of Khaya senegalensis:the effects of stem length,leaf area,auxins,smoke solution,and stockplant age.Int J For Res.doi:10.1155/2011/281269(Article ID 281269)
Leakey RRB,Mesen JF,Tchoundjeu Z,Longman KA,Dick JM,Newton A,Matin A,Grace J,Munro RC,Muthoka PN(1990)Low-technology techniques for the vegetative propagation of tropical trees.Commonw For Rev 69:247–257
Lee SE,Kim MR,Kim JH et al(2008)Antimalarial activity of anthothecol derived from Khaya anthotheca(Meliaceae).Phytomedicine 15:533–535.doi:10.1016/j.phymed.2007.08.001
Okere AU,Adegeye A(2011)In vitro propagation of an endangered medicinal timber species Khaya grandi flora C.Dc.Afr J Biotechnol 10:3335–3339.doi:10.5897/AJB10.236
Opuni-Frimpong E,Karnosky DF,Storer AJ,Cobbinah JR(2008)Key roles of leaves,stockplant age,and auxin concentration in vegetative propagation of two African mahoganies:Khaya anthotheca Welw.and Khaya ivorensis A.Chev.New For 36:115–123.doi:10.1007/s11056-008-9087-6
Overvoorde P,Fukaki H,Beeckman T(2010)Auxin control of root development.Cold Spring Harb Perspect Biol 2:a001537.doi:10.1101/cshperspect.a001537
Owusu SA,Opuni-Frimpong E,Antwi-Boasiako C(2014)Improving regeneration of mahogany:techniques for vegetative propagation of four African mahogany species using leafy stem cuttings.New For 45:687–697.doi:10.1007/s11056-014-9431-y
Pijut PM,Woeste KE,Michler CH(2011)Promotion of adventitious root formation of dif ficult-to-root hardwood tree species.Hortic Rev 38:213–251.doi:10.1002/9780470872376.ch6
Pinheiro AL(2011)Ecologia,silvicultura e tecnologia de utilizac¸a˜o dos mognos–africanos(Khaya spp.).Sociedade Brasileira de Agrossilvicultura–SBAG,Vic¸osa,p 102
Pop TI,Pam fil D,Bellini C(2011)Auxin control in the formation of adventitiousroots.NotBotHortiAgrobotCluj-Napoca 39:307–316
Rowe DB,Blazich FA,Raper CD(2002)Nitrogen nutrition of hedged stock plants of Loblolly Pine.I.Tissue nitrogen concentrations and carbohydrate status.New For 24:39–51.doi:10.1023/A:1020551029894
Sakai WS(1973)Simple method for differential staining of para film embedded plant material using toluidine blue.Stain Technol 48:247–249.doi:10.3109/10520297309116632
Tchoundjeu Z,Leakey RRB(1996)Vegetative propagation of African Mahogany:effects of auxin,node position,leaf area and cutting length.New For 11:125–136.doi:10.1007/BF00033408
Wendling I,Xavier A(2005)In fluência da miniestaquia seriada no vigor radicular de clones de Eucalyptus grandis.Rev Árvore 29:681–689.doi:10.1590/S0100-67622005000500003
Wendling I,Xavier A,Gomes JM,Pires IE,Andrade HB(2000)Propagac¸a˜o clonal de híbridos de Eucalyptus spp.por miniestaquia.Ver Árvore 24:181–186
Wendling I,Brondani GE,Dutra LF,Hansel FA(2010)Mini-cuttings technique:a new ex vitro method for clonal propagation of sweetgum.New For 39:343–353.doi:10.1007/s11056-009-9175-2
Wendling I,Trueman SJ,Xavier A(2014a)Maturation and related aspects in clonal forestry-Part I:concepts,regulation and consequences of phase change.New For 45:449–471.doi:10.1007/s11056-014-9421-0
Wendling I,Trueman SJ,Xavier A(2014b)Maturation and related aspects in clonal forestry-part II:reinvigoration,rejuvenation and juvenility maintenance.New For 45:473–486.doi:10.1007/s11056-014-9415-y
Wendling I,Warburton PM,Trueman SJ(2015)Maturation in Corymbia torelliana×C.citriodora stock plants:effects of pruning height on shoot production,adventitious rooting capacity,stem anatomy,and auxin and abscisic acid concentrations.Forests 6:3763–3778.doi:10.3390/f6103763
Xavier A,Dos Santos GA,Wendling I,De Oliveira ML(2003)Propagac¸a˜o vegetativa de cedro-rosa por miniestaquia.Rev Árvore 27:139–143.doi:10.1590/S0100-67622003000200003
杂志排行
Journal of Forestry Research的其它文章
- Vascular bundle connection between seed stalk and seed coat of Caragana arborescens
- Effects of continuous nitrogen addition on microbial properties and soil organic matter in a Larix gmelinii plantation in China
- Reconstructing the size of individual trees using log data from cut-to-length harvesters in Pinus radiata plantations:a case study in NSW,Australia
- Phenotypic variation in Phoebe bournei populations preserved in the primary distribution area
- Effects of soil drought stress on photosynthetic gas exchange traits and chlorophyll fluorescence in Forsythia suspensa
- Flavonoid content and radical scavenging activity in fruits of Chinese dwarf cherry(Cerasus humilis)genotypes
