Flavonoid content and radical scavenging activity in fruits of Chinese dwarf cherry(Cerasus humilis)genotypes
2018-03-27PengfeiWangXiaopengMuJunjieDuYuGaryGaoDonghaiBaiLutingJiaJianchengZhangHaiyanRenXiaofangXue
Pengfei Wang·Xiaopeng Mu·Junjie Du,5·Yu Gary Gao·Donghai Bai·Luting Jia·Jiancheng Zhang·Haiyan Ren·Xiaofang Xue,5
Introduction
Chinese dwarf cherry(Rosaceae:Cerasus humilis(Bge.)Sok.;Yu 1979)is a small shrub endemic to China and has been recorded in 13 provinces(Du et al.1993a).This plant has considerable economic importance as a food product;commercial Chinese dwarf cherries are known as ‘calcium fruit’in China because of their high calcium content and its bene ficial effect on helping humans absorb calcium from other foods that they consume(Mu et al.2015).The fruit of Chinese dwarf cherry can be consumed fresh or used in wine,juice,jam,and several other products.Moreover,Chinese dwarf cherry fruits have high potential for use in the healthcare industry,because of the fruit’s flavonoid contents(63.8 to 155.2 mg/g FW,fresh weight),a major quality factor along with sugar,organic acid,and vitamin content(Li et al.2014).Flavonoids,mainly present as colouring pigments in plants,also function as potent natural antioxidants at various levels to prevent free radical generated damage in the human body(Hossain and Rahman 2011).Regular intake of dietary flavonoids from plants has been shown to reduce the effects of oxidative damage from cardiovasculardiseases,diabetes,and otherillnesses(Makita et al.2016).Spencer(2010)demonstrated that fruit flavonoids may exert particularly powerful actions on mammalian cognition and may reverse age-related declines in memory and learning.Other reports showed that higher intake of foods rich in flavonoids may also contribute to weight loss and maintenance in adulthood and may help to re fine dietary recommendations for the prevention of obesity and its potential consequences(Bertoia et al.2016).
Since Chinese dwarf cherry has striking white flowers in spring and colorful fruit in autumn,this plant group also has the potential to be an excellent landscape shrub outside China,particularly in North America and Europe.Chinese dwarf cherries are also characterized by high level resistance to drought and cold,and the ability to tolerate saline or alkaline soils.Plants grow vigorously in sandy wasteland soils(Mu et al.2015),therefore Chinese dwarf cherry has potential to be used as an economic plant in those areas where other food crops cannot be grown.Chinese dwarf cherries have been successfully grown as ground cover for erosion control in mixed forests(Mu et al.2015).
The agronomic importance of Chinese dwarf cherry promptedthecreationofabreedingprogramin1987atShanxi AgriculturalUniversity(Taigu,Shanxi,China).Thisprogram focused on germplasm collection and selection,fruit cultivation,and its uses(Du et al.1993b).Since 2005,a number of improvedChinesedwarfcherrycultivarshavebeendeveloped and released(Cao et al.2005;Wang et al.2013).Farmers in northernChinabegansmall-scaleproductionofthesesuperior cultivars during early 2002 and large-scale production from 2006(Du et al.2012).In 2016,the total area of cultivation in China was estimated to be approximately 7000 ha.
With rising consumer demand for plant-originated flavonoid substances and increasing interest in nutraceuticals and functional foods,plants with higher than normal flavonoid contents,such as blueberries,plums and peaches,sea buckthorns,strawberriesandappleshavebeenstudiedinthepast2 decades(Hassanpour et al.2011).Plant breeders have also initiated several selection programs to find the lesser known fruits with higher flavonoid contents such as Chinese dwarf cherry,elderberry,cornelian cherry,medlar,and bilberry(Hassanpour et al.2011;Li et al.2014).Detailed information about the health-promoting components of more Chinese dwarf cherry genotypes could lead to greater acceptance of this fruit crop by consumers and growers in China and beyond.Comparative studies of genotypes of Chinese dwarf cherry and additional breeding efforts will help us more ef ficiently develop better cultivars with health bene fits.
Despite the growing awareness of Chinese dwarf cherry and the increasing number of cultivars,no research has been conducted on genotypic differences in flavonoid content,composition,and free-radical scavenging abilities.Such studies are necessary to facilitate the uses of Chinese dwarf cherry in functional foods and as ingredients in pharmaceuticals,nutraceuticals,and medicine.
We investigated total flavonoid content and composition in fruits from 16 Chinese dwarf cherry genotypes.The free radical scavenging activity of the flavonoid extracts was also examined with a 2,2-diphenyl-1-picrylhydrazyl(DPPH)assay.To the best of our knowledge,this is the first such report on Chinese dwarf cherry fruits to provide scienti fic evidence for its nutraceutical,medicinal uses,and other applications.
Materials and methods
Plant materials and chemicals
Sixteen genotypes of Chinese dwarf cherry were grown in the Horticultural Station of Shanxi Agricultural University(37°26′N,112°32′E).Among these genotypes,Nongda 3,4,5,6,7 are five cultivars released by Shanxi Agricultural University;the rest are genotypes with excellent agronomic traits which could be used for future breeding.Fifty plants(4 years old)from each genotype were treated with the same irrigation,disease,and pest-control management procedures.One hundred fruits per genotype(two from each plant)were randomly harvested by hand when fully ripe(based on color and flavor)(Fig.1).Fruit pits were removed immediately after harvesting and the peel was separated from the flesh and stored at-40°C prior to analyses.Sampling was repeated thrice.
Methanol and HPLC-grade water were purchased from Sigma-Aldrich(St.Louis,MO,USA),as were the following flavonoid standards:cyanidin-3-O-glucoside,catechin,epicatechin,proanthocyanidins B1,proanthocyanidins B2,phloretin-2-O-xyloglucoside,phloretin-2-O-glucoside,phloretin-2,4-O-diglucoside,quercetin-3-O-glucoside,quercetin-7-O-glucoside,quercetin-7-O-acetylglucoside,quercetin-3-arabinoside,myricetin,quercetin,and quercetin-3,7-di-O-glucoside.Butylated hydroxytoluene(BHT)and 2,2-diphenyl-1-picrylhydrazyl(DPPH)were purchased from Puxi Biotech Co.,Ltd(Beijing,China).Chromatographicgrade formic acid and acetonitrile used for HPLC were purchased from Alltech Scienti fic (Beijing,China).

Fig.1 Ripe fruits of 16 Chinese dwarf cherry genotypes.Nongda 3,Nongda 4,Nongda 5,Nongda 6 and Nongda 7 are five commercially cultivated varieties;the rest is a number of fine lines with excellent agronomic traitswhich were selected from severaldifferent germplasm nurseries
Analytical-grade sodium carbonate,sodium acetate,ferric chloride,methanol,hydrochloric acid,and acetic acid were obtained from Yongli Chemical Industry Co.,Ltd(Shanxi,China).Double-distilled water was produced via a Milli-Q System(Millipore,Billerica,MA,USA)and 0.22 μm Millipore membrane filters were purchased from ANPEL Scienti fic Instrument Corporation(Shanghai,China).
Extraction and determination of total flavonoid contents
Flavonoids were extracted using the adapted ethanol- fluxing method from Van Acker et al.(2011),with modi fications.Total flavonoid content(TFC)was determined with a colorimetric method(Sabli et al.2012)with slight modi fications.The extract was dissolved in 50 mL 60%(v/v)ethanol,and 1 mL diluted extract was then mixed with 0.75 mL of 25%NaNO2in a 25-mL volumetric flask.After 6 min,0.75 mL 10%A1(NO3)3was added to the reaction mixture,which was incubated at room temperature for another 6 min before 10 mL 4%NaOH was added.The mixture was then immediately diluted to 25 mL with 60%(v/v)ethanol and thoroughly agitated.Absorbance was measured at 510 nm with a UV–Visible spectrophotometer(W-2450,Shimadzu,Japan),versus a blank containing all reagents except extracts.Rutin was used as the standard for the calibration curve.Extract and fraction TFCs were expressed as mg rutin equivalents(RE)per g fresh weight(FW)of sample(mg/g).
Determination of flavonoid composition
Flavonoid extract preparation followed Chang et al.(2011)with some modi fications.Fruits(1 g)were ground in liquid nitrogen and mixed with 5 mL methanol-formic acid–water(80:1:19,v/v/v).The mixture was then sonicated at 45°C for 45 min and centrifuged at 12,000 rpm for 10 min to collect the supernatant.These steps were repeated three more times per genotype.The collected supernatant was supplemented to 5 mL with a methanol-formic acid–water solution(80:1:19,v/v/v),and then filtered through the Millipore membrane before being subjected to HPLC.An HPLC system(Agilent Technology 1200 series,Palo Alto,CA)equipped with a diode array detector(DAD)was used for the identi fication and quanti fication of flavonoid components.A C18analytical column(250×4.6 mm inner diameter;particle size,5 μm)was used for the separation.The binary mobile phase comprised 2%acetic acid(solvent A)and acetonitrile(solvent B).The gradient program was as follows:0%B to 40%B in 60 min,40%B to 70%B in 5 min,and 70%B to 0%B in 10 min.The flow rate was set as 1.0 mL/min for a total run time of 70 min.The injection volume was 20 μL for all samples.The detector was set at 280,360,and 520 nm for simultaneous monitoring of different flavonoid compounds.Quantitative determination of each compound was carried out by comparing the retention time of chromatographic peaks in speci fic wavelength spectra with those of authentic standards.Data are reported here as mean±SD(n=3).
Determination of radical scavenging activity
A DPPH assay was used to evaluate the free-radical scavenging potential of the antioxidative compounds(Saeed et al.2012).We followed the assay procedures of Li et al.(2015)with a slight modi fication.Extract(0.5 mL of 0.5 mg/mL)was mixed with 2 mL of freshly made 0.25 mM methanolic-DPPH,and methanol was added until the final volume reached 5 mL.The final DPPH concentration was 0.1 mM.After 30 min,absorbance decrease in the reaction mixture was measured at 517 nm,with lower absorbance indicating higher free radical scavenging activity.A DPPH solution without extract was used as the control and a 0.5 mL BHT solution(0.5 mg/mL)was used as the standard antioxidant.Analyses were performed in triplicate for the control and for each extract.Activity was expressed as the radical scavenging percentage with the following equation: Percent scavenged DPPH(%)=[(A1-A2)/A1]×100.A1is control absorbance and A2is the absorbance of the DPPH solution with extract added.
Statistical analysis
The differences among means were evaluated using Tukey’s multiple comparison test,and the statistical signi ficance was set at p<0.05(Granato et al.2015)using SPSS software(SPSS Inc.,Chicago,IL,USA).Results are expressed here as mean±SD.Variability in flavonoid composition among 16 Chinese dwarf cherry genotypes was assessed by a principal component analysis(PCA)using SAS 8.0(SAS Institute,Cary,NC,USA).
Results
Total flavonoid contents
Total flavonoid contents in fruit peel and flesh of 16 genotypes of Chinese dwarf cherry varied signi ficantly(Table 1).Peel TFCs ranged from 33.5 to 72.8 mg/g RE⋅FW across genotypes,whereas the range of flesh TFCs was 4.3–16.9 mg/g RE⋅FW.On average,peel TFCs were about seven times higher than flesh TFCs in all 16 genotypes.‘DG-1’and ‘W-3’possessed the highest and lowest peel TFCs,while ’10-32’and ‘Nongda 6’had highest and lowest flesh TFCs,respectively(Table 1).Our results also showed that the peel TFCs of genotypes with deep-colored fruit peel(Red or Orange)were higher than light-colored genotypes(Yellow or Yellow–Red blush),while the flesh TFCs showed similar results that genotypes with light color fruit flesh(White or yellow)had lower TFCs.
Flavonoid composition
The HPLC–DAD analysis identi fied 14 flavonoids:cyanidin-3-O-glucoside(C3G);catechin(C);epicatechin(EC);proanthocyanidins B1(PA-B1);proanthocyanidins B2(PA-B2);phloretin-2-O-xyloglucoside(PXG);phloretin-2′-O-glucoside(PG);phloretin-2′,4′-O-diglucoside(PDG);quercetin-3-O-glucoside(Q3G);quercetin-7-O-glucoside(Q7G);quercetin-7-O-acetylglucoside(Q7AcG);quercetin-3-arabinoside(Q3A);myricetin(M);and quercetin(Q)(Table 2).Genotypes varied considerably in their flavonoid pro files.Some genotypes did not have all 14 flavonoidcomponents.Forexample, ‘Nongda5’ only contained eight flavonoid components,and lacked C3G,PXG,PG,Q3G,Q3A,and M(Table 2).‘W-3’also lacked C3G in its fruits.In contrast,‘Y04-26’had the highest C3G content(16.4%),followed by ‘Wenfenli’(12.3%)and ‘10-32’(12.2%).
In general,the most abundant flavonoids present in Chinese dwarf cherry fruits were PA-B1,PA-B2,PG,and PDG.PA-B1 ranged from 24.5 to 51.7%of the total flavonoids,followed PDG(3.1–46.5%),PA-B2(4.8–20.4%)and PG(0–16.0%).
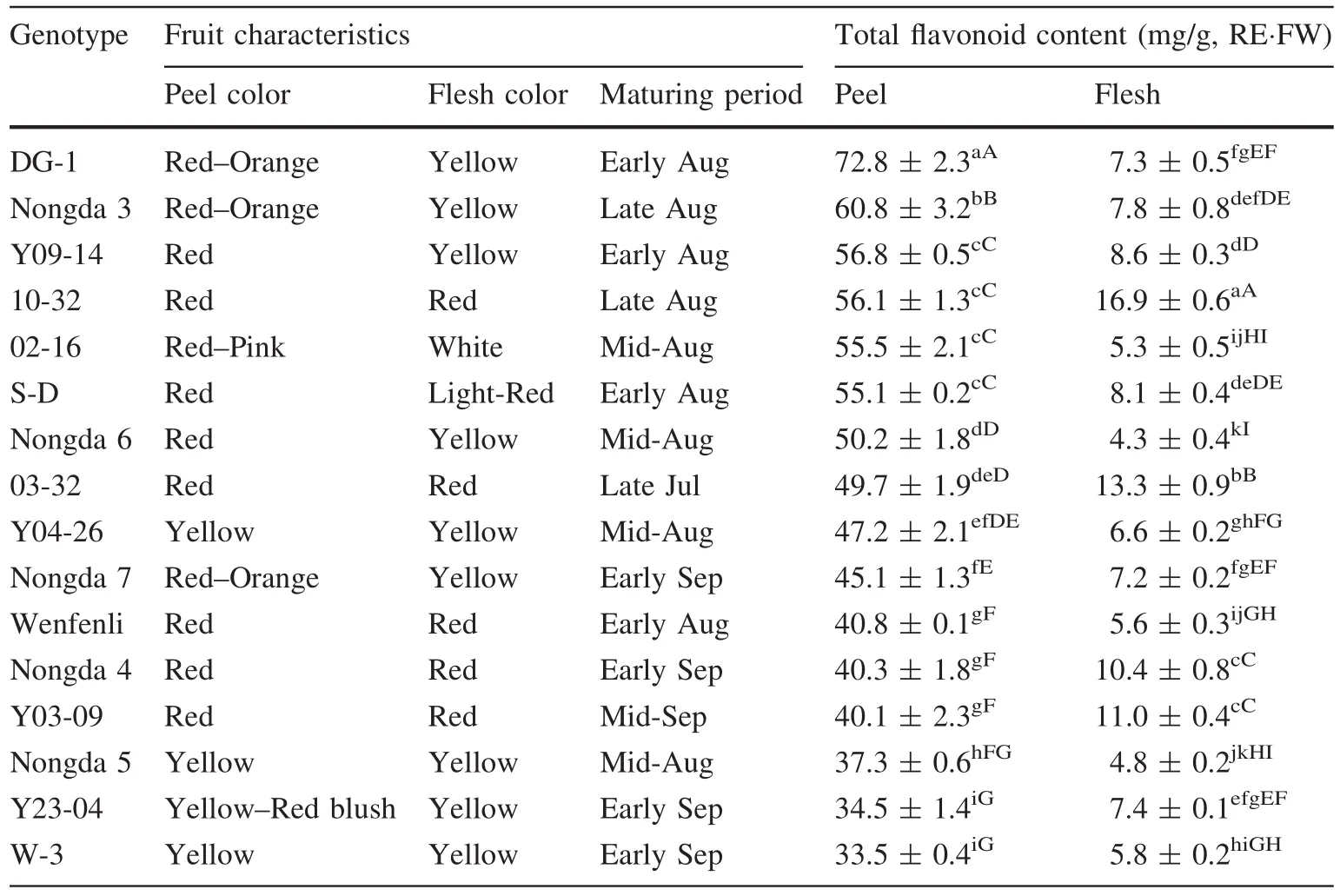
Table 1 Characteristics of 16 Chinese dwarf cherry genotypes and total flavonoid content in different fruit parts
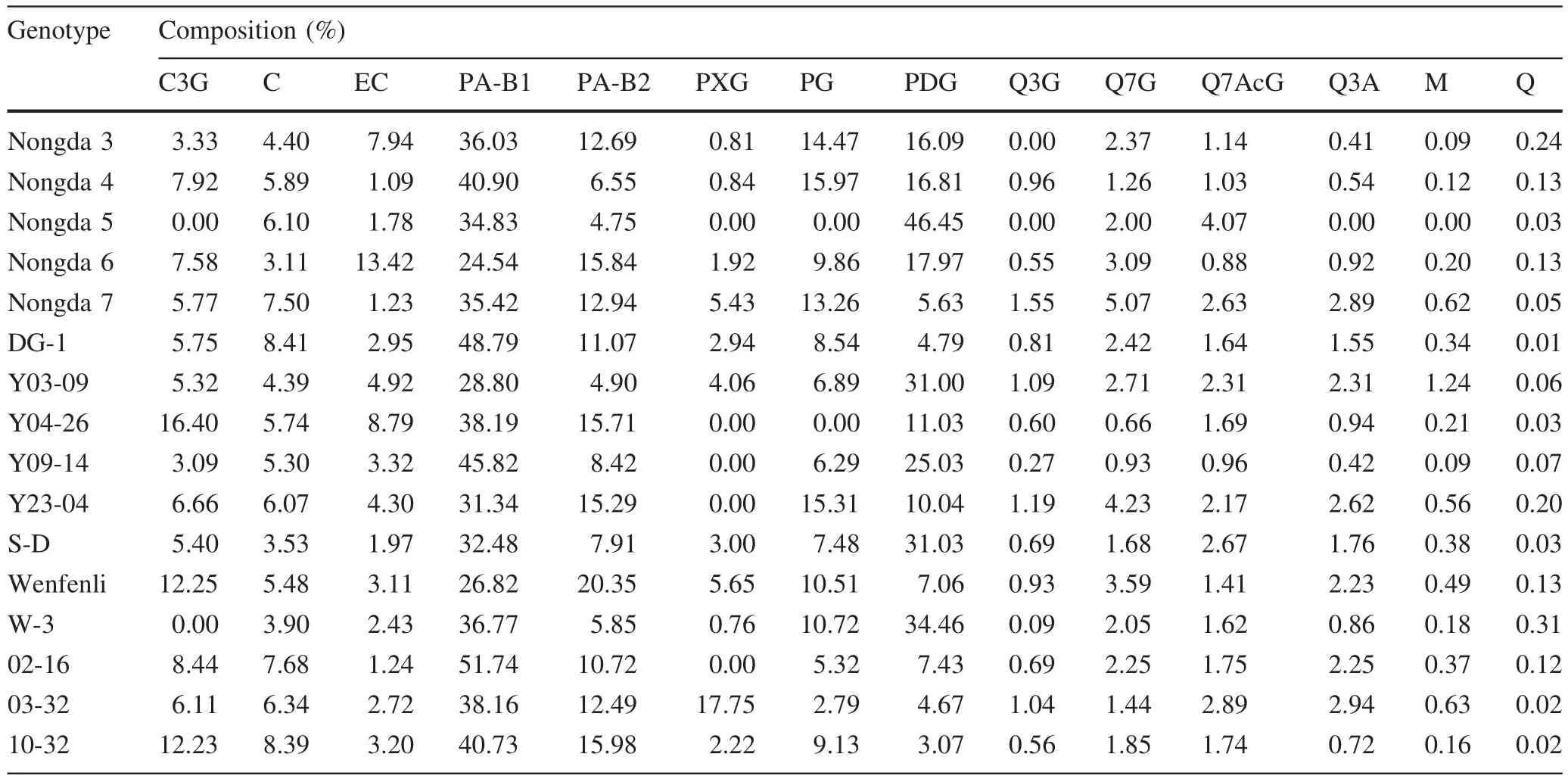
Table 2 Flavonoid composition in mature fruits of 16 Chinese dwarf cherry genotypes
Principal component analysis on variation in flavonoid composition
The PCA results identi fied four components that explained 81.72%of the total variation in flavonoid composition among the 16 Chinese dwarf cherry genotypes(Table 3).The first principal component(PC1)contributed 36.15%of the total variation,followed by PC2(21.92%),PC3(12.37%),and PC4(11.28%).Large,positive values were associated with PG,PA-B1,and PA-B2,suggesting that these three flavonoids greatly contributed to PC1.Scatterplots from the PCA based on the flavonoid concentrations in the fruit samples showed that ‘Nongda3’, ‘Nongda4’,‘Nongda6’,and ‘10-32’belonged in the first group(Fig.2),characterized by high PA-B1,PA-B2,and PG content.In PC2,the largest contributions came from PXG,Q3G,and M.Genotypes belonging to this group(characterized by low PXG levels)were ‘Nongda5’, ‘Y04-26’, ‘Y09-14’,‘Y23-04’,and ‘W-3’(Fig.2).The remaining genotypes were placed in the third group,characterized by low PA-B1 and PA-B2 content.These data suggest that crossing within groups may be more ef ficient for directed breeding of Chinese dwarf cherries possessing highly desired flavonoid compositions.
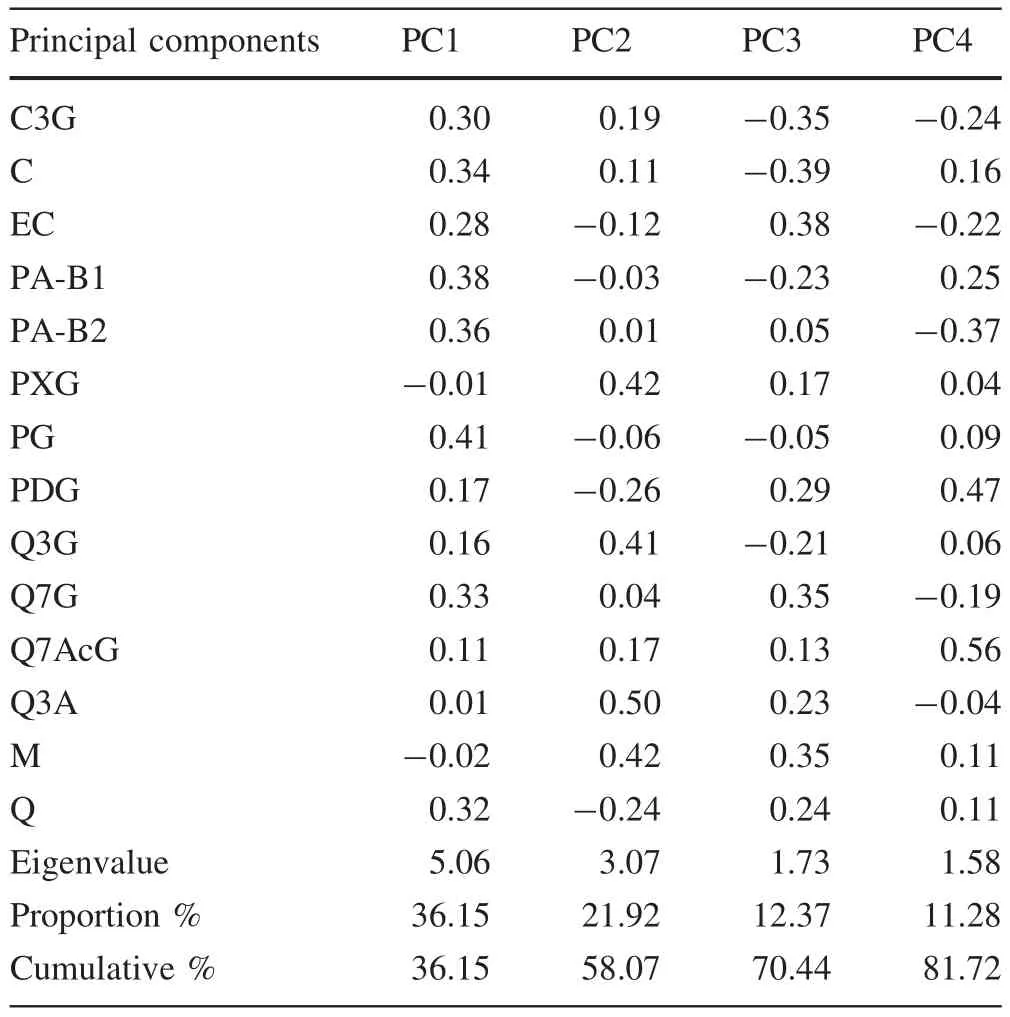
Table 3 Eigenvectors and eigenvalues of the first four principal components grouping the flavonoids in 16 Chinese dwarf cherry genotypes
DPPH radical scavenging activity
Reducing power(expressed as the percentage of scavenged DPPH)of all genotypes was compared with that of BHT,a known reducing agent(Fig.3).
All extracts had a near instantaneous response at every tested concentration,with absorbance values decreasing rapidly in the first20 min.Steady-state absorbance occurred after 20–40 min for various concentrated extracts.‘Nongda 4’and BHT(p ≤ 0.05)did not differ in scavenged DPPH,but all other genotypes had signi ficantly lower scavenging activity than BHT(p ≤ 0.05).‘Nongda 4’, ‘Nongda 3’, ‘10-32’, ‘Nongda 6’,and ‘Wenfenli’exhibited the highest reducing power of all genotypes.The variation in scavenging ability across genotypes might have been due to their different flavonoid compositions.
Discussion
Fruits have health bene fits and are good sources of natural flavonoids(Lin and Tang 2007).Liu et al.(2002)cited many studies showing that TFC variation is linked to fruit color;for example,TFC in red raspberry varieties was greater than that in yellow varieties.We hypothesized that fruit colors of Chinese dwarf cherry were also closely related to TFC.Our results showed that the genotypes with deeply colored fruit peel(red or orange)had higher peel TFCs than did light-colored genotypes in most cases and the flesh TFCs showed similar results.These findings con firm results of studies on other fruits and that genotypic differences in fruit TFCs may be attributable to geography,climate factors,and botanical origins(Li et al.2014;Mattila et al.2016).Despite being considered by-products of fruit processing,fruit peels have higher TFCs than the edible flesh and are in fact an abundant source of natural antioxidants(Balasundram et al.2006).Thus,fruit peels could have considerable economic potential for food processors.
C3G belongs to the flavonoid class and is a member of the anthocyanin family,the largest group of pigments present in many reddish fruits including grapes,cherries,blueberries,blackberries,plums,and apples(Ding et al.2006;Chang et al.2011;Qin et al.2013).C3G was previously identi fied as the major anthocyanin in Chinesedwarf cherries.Our results showed that neither‘Nongda 5’nor ‘W-3’fruits contained C3G as their peel and flesh are yellow.
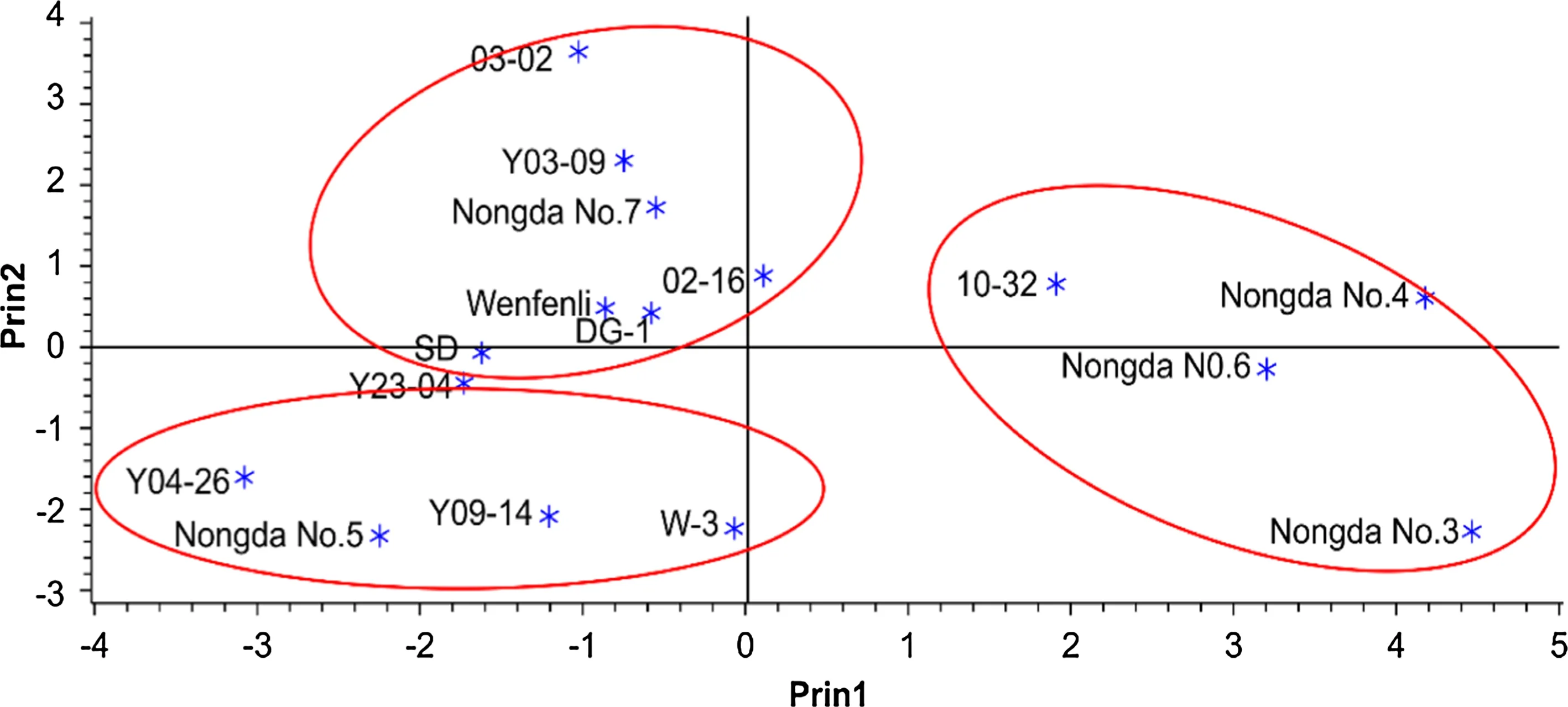
Fig.2 Scatterplot of principal component analysis based on flavonoids concentrations in fruits of 16 Chinese dwarf cherry genotypes.The three circles indicate the genotypes belonging to the top three principal components

Fig.3 Scavenged DPPH percentage of flavonoid extracts from 16 Chinese dwarf cherry genotypes compared with the Butylated hydroxytoluene(BHT)standard
Proanthocyanidins(PA),also known as condensed tannins,are oligomers of catechin,epicatechin,and their gallic acid esters,bound together with B-type and A-type linkages(Kyraleou et al.2016).The results of this study are in agreement with research demonstrating that B-type PAs are the predominant flavonoid in most fruits(Ou and Gu 2014).PAs are capable of modulating in flammatory responses that are involved in the development of cardiovascular disease and some cancers;they also have protective effects against type 2 diabetes.Moreover,they exhibit strong antioxidant activity and other potentially health-promoting qualities(Chen et al.2016).
PGs(also known as phlorizins)and PDGs are glycosylated phloretins in the chemical class of dihydrochalcones(DHCs).As major flavonoids,they are mainly found in apples and their processed products such as apple juice(Gosch et al.2010;Xu et al.2014).In this study,the highest PG and PDG contents were found in ‘Nongda 4’and ‘Nongda 5’,respectively,whereas the latter genotype and ‘Y04-26’contained no PG.PGs are frequently used in foods,beverages,food additives,pharmaceuticals,and cosmetics because of their antioxidase activities and ability to lower blood glucose levels(Kim et al.2014).Rosaceous plant species differ in their PG contents;this flavonoid has therefore been used for chemotaxonomic differentiation of the Rosaceae(Challice 1981;Vrhovsek et al.2004).Our results clearly demonstrated that Chinese dwarf cherry fruits are high in PG content,making them a good source of flavonoids for food and medical industries.
DPPH is a nitrogen-centered free radical that stabilizes easily through accepting an electron or hydrogen radical;this process can be visualized with a color change from violet to yellow(Meir et al.1995).Substances that trigger this reaction are considered antioxidants and therefore radical scavengers.Butylated hydroxy toluene(BHT)and butylated hydroxyanisole(BHA)are commonly used synthetic antioxidants,which are restricted by legislative rules because they are suspected to have some toxic effects and are potential carcinogens(Hossain and Rahman 2011).Fruit-originated flavonoids,as natural antioxidants,could be good replacements of BHT and BHA because of their strong free-radical scavenging activities.
Conclusions
We compared the total flavonoid content,composition,and DPPH scavenging activity of 16 Chinese dwarf cherry genotypes.Fourteen flavonoids were identi fied by HPLC,and flavonoid composition varied considerably across genotypes.DPPH assays revealed that flavonoid extracts exhibited signi ficant antioxidant activity.Chinese dwarf cherry fruits showed free-radical scavenging activity and proved to be flavonoid-rich.Thus they have potential to be used as sources of natural antioxidants.Further investigation to isolate and purify flavonoids from Chinese dwarf cherries may contribute to the development of nutraceutical products or new drugs aimed at preventing diseases related to oxidative stress in humans.
AcknowledgementsThis study was supported by the Major Subject of Shanxi Science and Technology Research (Grant No.20121101010),the Platform Construction of Science and Technology of Shanxi Province(Grant No.2013091004-0101),and the Doctoral Research Fund ofShanxiAgriculture University (GrantNo.2015ZZ19).
Balasundram N,Sundram K,Samman S(2006)Phenolic compounds in plants and agri-industrial by-products:antioxidant activity,occurrence,and potential uses.Food Chem 99:191–203
Bertoia ML,Rimm EB,Mukamal KJ,Hu FB,Willett WC,Cassidy A(2016)Dietary flavonoid intake and weight maintenance:three prospective cohorts of 124 086 US men and women followed for up to 24 years.BMJ 352:i17
Cao Q,Du JJ,Wang QJ,Du JM(2005)A new variety of Chinese dwarf cherry(Cerasus humilis Bunge)-‘Nongda 3’.Acta Hortic Sin 35:370
Challice J(1981)Chemotaxonomic studies in the family Rosaceae and the evolutionary origins of the subfamily Maloideae.Preslia 53:289–301
Chang H,Lan YP,Zhou JH,He HJ,Gao HJ,Feng CP(2011)Isolation and identi fication of anthocyanins in the fruits of Prunus humilis Bunge.Food Sci 32:59–63
Chen MH,McClung AM,Bergman CJ(2016)Concentrations of oligomers and polymers of proanthocyanidins in red and purple rice bran and their relationships to total phenolics, flavonoids,antioxidant capacity and whole grain color.Food Chem 208:279–287
Ding M,Feng R,Wang SY,Bowman L,Lu Y,Qian Y,Castranova V,Jiang BH,Shi X(2006)Cyanidin-3-glucoside,a natural product derived from blackberry,exhibits chemopreventive and chemotherapeutic activity.J Biol Chem 281(25):17359–17368
Du JJ,Yang HY,Chi JW(1993a)Distribution and groups of Chinese dwarf cherry(Cerasus humilis)in Shanxi province.Crop Var Resour 2:6–7
Du JJ,Yang HY,Chi JW(1993b)Preliminary studies of selective breeding in Chinese dwarf cherry.China Fruits 3:23–24
Du JJ,Wang PF,Zhang JC(2012)Industrial present situation,problems and development countermeasures of Chinese dwarf cherry(Cerasus humilis)in Shanxi province.Shanxi Fruits 2:38–40
Gosch C,Halbwirth H,Stich K(2010)Phloridzin:biosynthesis,distribution and physiological relevance in plants.Phytochemistry 71:838–843
Granato D,Karnopp AR,van Ruth SM(2015)Characterization and comparison of phenolic composition,antioxidant capacity and instrumental taste pro file of juices from different botanical origins.J Sci Food Agric 95:1997–2006
Hassanpour H,Yousef H,Jafar H,Mohammad A(2011)Antioxidant capacity and phytochemical properties of cornelian cherry(Cornus mas L.)genotypes in Iran.Sci Hortic 129(3):459–463
Hossain MA,Rahman SMM(2011)Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple.Food Res Int 44(3):672–676
Kim MS,Park SH,Han SY,Kim YH,Lee EJ,Park JHY,Kang YH(2014)Phloretin suppresses thrombin-mediated leukocyte-platelet-endothelial interactions.Mol Nutr Food Res 58(4):698–708
Kyraleou M,Kotseridis Y,Koundouras S,Chira K,Teissedre PL,Kallithraka S(2016)Effect of irrigation regime on perceived astringency and proanthocyanidin composition of skins and seeds of Vitis vinifera L.cv.Syrah grapes under semiarid conditions.Food Chem 203:292–300
Li WD,Li O,Zhang A,Li L,Hao JH,Jin SJ,Yin SJ(2014)Genotypic diversity of phenolic compounds and antioxidant capacity of Chinese dwarf cherry(Cerasus humilis(Bge.)Sok.)in China.Sci Hortic 175:208–213
Li JE,Fan ST,Qiu ZH,Li C,Nie SP(2015)Total flavonoids content,antioxidant and antimicrobial activities of extracts from Mosla chinensis Maxim.cv.Jiangxiangru.LWT Food Sci Technol 64:1022–1027
Lin J,Tang C(2007)Determination of total phenolic and flavonoid contents in selected fruits and vegetables,as well as their stimulatory effects on mouse splenocyte proliferation.Food Chem 101:140–147
Liu M,Li XQ,Weber C,Lee HY,Brown J,Liu RH(2002)Antioxidantand antiproliferative activitiesofraspberries.J Agric Food Chem 50:2926–2930
Makita C,Chimuka L,Steenkamp P,Cukrowska E,Madala E(2016)Comparative analyses of flavonoid content in Moringa oleifera and Moringa ovalifolia with the aid of UHPLC-qTOF-MS fingerprinting.South Afr J Bot 105:116–122
Mattila PH,Hellström J,Karhu S,Pihlava JM,Veteläinen M(2016)High variability in flavonoid contents and composition between different North-European currant(Ribes spp.)varieties.Food Chem 204:14–20
Meir S,Kanner J,Akiri B,Philosof-Hadas S(1995)Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves.J Agric Food Chem 43:1813–1817
Mu XP,Aryal N,Du JM,Du JJ(2015)Oil content and fatty acid composition of the kernels of 31 different cultivars of Chinese dwarf cherry[Cerasus humilis(Bge.)Sok].J Hortic Sci Biotechnol 90:525–529
Ou K,Gu LW(2014)Absorption and metabolism of proanthocyanidins.J Funct Foods 7:43–53
Qin LN,Zhang JL,Qin ML(2013)Protective effect of cyanidin 3-O-glucoside on beta-amyloid peptide-induced cognitive impairment in rats.Neurosci Lett 534:285–288
Sabli F,Mohamed M,Rahmat A,Ibrahim HA,Bakar MFA(2012)Antioxidant properties of selected Etlingera and Zingiber species(Zingiberaceae)from Borneo Island.Int J Biochem 6(1):1–9
Saeed N,Khan MR,Shabbir M(2012)Antioxidant activity,total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L.BMC Complement lternative Med 12:221
Spencer JPE(2010)The impact of fruit flavonoids on memory and cognition.Br J Nutr 104(3):40–47
Van Acker SA,Van Den DJ,Tromp MN,Bast A(2011)Structural aspects of antioxidant activity of flavonoids.Free Radic Biol Med 35:331–342
Vrhovsek U,Rigo A,Tonon D,Mattivi F(2004)Quantitation of polyphenols in different apple varieties.J Agric Food Chem 52:6532–6538
Wang PF,Cao Q,Du JJ,Zhang JC,Mu XP(2013)A new fresh-eating cultivar of Chinese dwarf cherry(Cerasus humilis Bunge)‘Nongda 7’.Acta Hortic Sin 40:181–182
Xu LJ,Gu JR,Chen QQ,Jiang BP,Zhang W(2014)Quantitation of phlorizin and phloretin using an ultra high performance liquid chromatography-electrospray ionization tandem mass spectrometric method.J Chromatogr B 960:67–72
Yu DJ(1979)Drupe fruit.In:Yu DJ(ed)China fruit taxonomy,1st edn.Agricultural Press,Beijing,p 65
杂志排行
Journal of Forestry Research的其它文章
- Vascular bundle connection between seed stalk and seed coat of Caragana arborescens
- Effects of continuous nitrogen addition on microbial properties and soil organic matter in a Larix gmelinii plantation in China
- Reconstructing the size of individual trees using log data from cut-to-length harvesters in Pinus radiata plantations:a case study in NSW,Australia
- Phenotypic variation in Phoebe bournei populations preserved in the primary distribution area
- Effects of soil drought stress on photosynthetic gas exchange traits and chlorophyll fluorescence in Forsythia suspensa
- The selection and stability analysis of stable and high Taxol-producing cell lines from Taxus cuspidata
