A hiddenly high hepatitis C virus related liver disease burden among Chinese patients with non-liver disease complaints: A hospital based study from 2013 to 2017
2018-03-08HuaPingHuangGuoShengYuanYuChenZhouChengGuangHuJunWeiLiuShuaiYuanYuRongQiuYiPingLiYongYuanZhangYuanPingZhou
Hua-Ping Huang, Guo-Sheng Yuan, Yu-Chen Zhou, Cheng-Guang Hu, Jun-Wei Liu,Shuai Yuan, Yu-Rong Qiu, Yi-Ping Li , Yong-Yuan Zhang, Yuan-Ping Zhou✉
1Department of Infectious Diseases and Hepatology Unit, Nanfang Hospital, Southern Medical University, Guangzhou, China
2Department of Respiratory Diseases, The First Affiliated Hospital of Hainan Medical University, Haikou, Hainan, China
3Department of Hepatobiliary Surgery, Nanfang Hospital, Southern Medical University, Guangzhou, China
4Laboratory Medicine Center, Nanfang Hospital, Southern Medical University, Guangzhou, China
5Institute of Human Virology and Key Laboratory of Tropical Disease Control of Ministry of Education, Zhongshan School of Medicine, Sun Yat-sen University,Guangzhou, China
6HBVtech, Germantown, Maryland, MD 20874, USA
1. Introduction
Globally, the morbidity and mortality attributable to hepatitis C virus (HCV) infection continue to rise. About 700 000 persons die each year from HCV related complications, including cirrhosis,hepatocellular carcinoma (HCC) and liver failure[1,2]. Recent study shows that there are a minimal 25 million people or 1.8%-3.7% of the total Chinese population infected with HCV in China, resulting in substantial economic and health burden for both patients and society[3].
Since no vaccine is available for prevention of HCV infection,the primary goal of managing HCV infected patients is to cure the infection, which is generally associated with resolution of liver necroinflammation[4-6]. With the introduction of directly antiviral agents (DAAs), they have shown to be well-tolerated and potent in shortening therapy duration and increasing the rate of sustained virologic response (SVR), and they have been used for treating patients in US, EU and Japan where DAAs received the early approvals to the market[7-11]. On 28 April, 2017, the first DAA has been approved in mainland China, and is expected to gradually replace currently Peg-interferon-a/ribavirin (Peg-IFN/RBV, P/R)based treatment.
In 2016, the World Health Organization (WHO) calls on to eliminate viral hepatitis by 2030 at the World Health Assembly.Currently adopted prevention and treatment strategies would increase the treatment rate to 80% and reduce the number of annual deaths by 65%, saving about 7.1 million lives globally by 2030[12].However, public awareness of HCV is rather low in China[13,14].A public survey released by the Chinese Foundation for Hepatitis Prevention and Control in 2007, showed that less than 1% of the surveyed participants were reported to have knowledge about HCV transmission and prevention, and only 5% had been screened for HCV infection[15]. In China, non-hepatologist physicians often order anti-HCV antibody test before an invasive examination or surgical procedure. Nevertheless, the test results are often not delivered to the patients and their primary physicians, and the anti-HCV antibody positive patients may not be referred to hepatologists for further assessments. Additionally, there are limited data about clinical characteristic and treatment status among patients who were tested for anti-HCV positive during the visits for non-liver disease complaints in China. In this study, we aimed to determine frequency of anti-HCV antibody positivity among patients visiting our hospital for non-liver disease complaints, survey whether anti-HCV positive patients had been properly advised and visited hepatologists for further assessments, and investigate their clinical characteristics as well as the HCV treatment status.
2. Materials and methods
2.1. Patients
This study was a single center based retrospective research conducted in Nanfang Hospital, Southern Medical University,Guangzhou, Guangdong, China. We searched and pooled the patients who were tested for anti-HCV antibody as routine screening between 1 January 2013 and 7 June 2017 at all Departments but the Department of Infectious Diseases and Hepatology Unit through electric medical records. We determined if the tested patients had received further assessments and proper medical care, and whether they were referred to a hepatologist. This study was approved by the ethical Committee of Nanfang Hospital, Southern Medical University. The experiments were carried out in accordance with the approved guidelines and the “informed” consent was obtained from all subjects.
The “HCV related cirrhosis” was diagnosed based on clinical,biochemical, ultrasonic, histological, radiological, and endoscopic findings, but excluded if concurrent HCC was detected. HCV related HCC patients included those with and without cirrhosis,The diagnosis of HCC was based on the criteria recommended by American Association for the Study of Liver Diseases and liver imaging results, alpha-fetoprotein serology, and/or biopsy.
2.2. HCV assays
Anti-HCV antibodies were determined using the Architect HCV assay (Abbott Japan, Tokyo), and HCV-RNA was measured using the COBAS TaqMan HCV Test v. 2.0 (Roche Diagnostics K.K.).
2.3. Questionnaires designed to study factors influencing management of HCV infection
The survey questions were made to the local clinical practice setting in China using the template of a previous international survey of barriers to HCV therapy[13]. All survey questions were reviewed, revised and finalized by an expert panel. Eligible patients were asked a series of open-ended single or multiple responses and Likert-scale questions. A pilot study was also conducted to examine the questionnaire. The stability of the questions was tested through soliciting two times of answers to the same questionnaire by the same physicians at an interval of 1-3 weeks, which were measured by calculating inter class correlation values. A value over 0.75 suggests good stability. Questions with poor quality or stability were revised and re-tested. All physicians were interviewed for about 20 min in a face-to-face format.
2.4. Survey questions
2.4.1. Assessing HCV knowledge among anti-HCV positive patients with non-liver disease complaints
A 24-item questionnaire that consists of 3 sections was used for eligible respondents. The first section focused on the demographic and clinical information of patients. The second section included the questions on management of HCV infection. The last section included 16 questions descripting HCV transmission risk, lifestyles,diagnosis, disease progression, current HCV treatment strategies and treatment outcomes. Each response was rated on a 10-point Likert scale, with 0 representing “not a barrier to treatment,” 5 representing“somewhat of a barrier,” and 10 representing “large barrier.”
2.4.2. Assessing HCV knowledge level possessed by nonhepatologist physicians
HCV knowledge among non-hepatologist physicians was determined by the level of agreement with the following statements:(i) was nucleic acid testing (NAT) of HCV RNA suggested to establish the diagnosis of chronic HCV infection immediately following a positive HCV serological test? (ii) did the Levels of HCV RNA correlate with severity of liver disease? (iii) is duration of Peg-IFN + RBV treatment the same among different viral genotypes? (iv)is it true or false that treatment should be discontinued if the treated patients remain HCV RNA positive at week 4 of the treatment? (v)does longer treatment duration earn better efficacy (regardless of virological response during therapy)? (vi) do fibrosis patients with stage 1 likely have worse treatment outcomes than patients with stage 4; (vii) does a maintained optimal dose of RBV is necessary to achieve SVR during Peg-IFN + RBV therapy? and (viii) is Peg-IFN+ RBV treatment the only standard treatment to cure HCV?
2.5. Statistical analysis
The SPSS 20 software (SPSS Inc., Chicago, IL, USA) package was used for statistical analyses. Continuous variables were analyzed by average and standard deviation. Categorical variable were expressed as frequency and rate. Independentttest and χ2test were used to compare differences between two groups. Differences withPvalues<0.05 were considered statistically significant.
3. Results
3.1. Clinical characteristics and treatment status of patients with anti-HCV antibody positive
A total of 295 294 patients received anti-HCV antibody tests,and 2 778 were found to be positive (0.94%). A majority of anti-HCV positive patients were from obstetrics, gastroenterology, and emergency clinics. The highest positivity of anti-HCV antibody was detected in patients with renal transplant [4.29% (107/2 494)] (Table 1).
However, only 45.10% (1 253/2 778) of the anti-HCV antibody(+) patients were referred to hepatologists and accepted HCV RNA test. Of these, 915 (73.03%) patients were confirmed to be positive for HCV RNA, 838 (91.58%) initiated antiviral treatment, 745 with interferon-based treatment and 93 with DAA treatment. Four patients took non-antiviral drugs, and the remaining 73 patients declined any treatment. Clinical evaluation showed that 34.10% (312/915)and 1.42% (13/915) of them had progressed to cirrhosis and HCC,respectively, from chronic hepatitis C.
Totally, 1 525 patients were informed of the positive results, but not referred to hepatologists. Among these, 976 patients did not receive proper medical care, and 549 declined further assessment.Justifications for declining further HCV infection assessment among 549 patients, included possible severe side effects by anti-viral treatment (88 patients) and old age, but with stable liver function(166 patients). No justification was known in the remaining 295 patients.
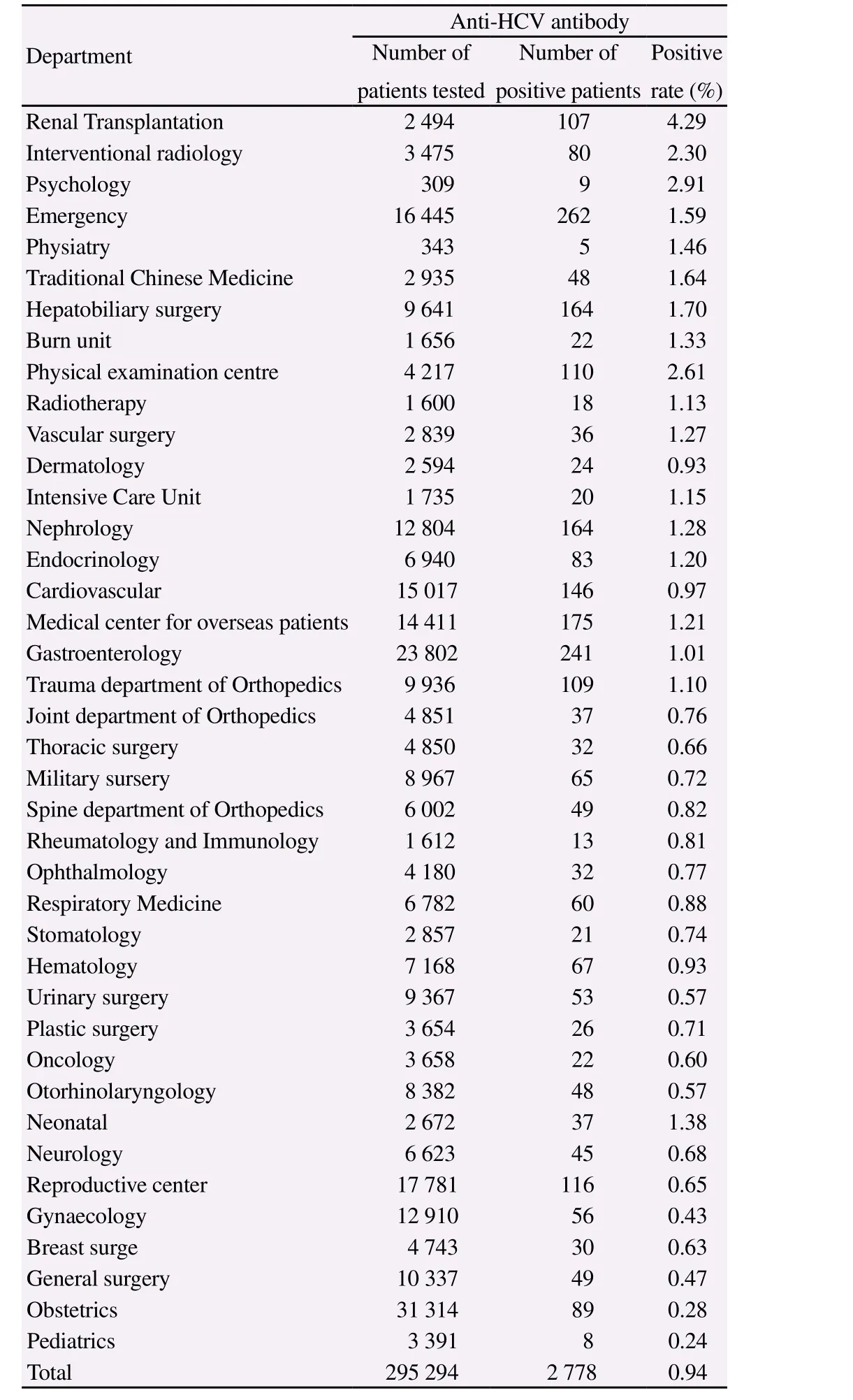
Table 1Distribution of patients tested for anti-HCV antibody at departments other than the Department of Infectious Diseases and Hepatology Unit.
3.2. Barriers to care
3.2.1. Patient-related barriers to care
We sent 360 questionnaires to both patients who received or did not receive antiviral treatment (180 for each group). All questionnaires were returned. Among them 76 subjects were excluded from the analysis as a result of missing some key data, such as the diagnosis of liver disease. The demographic and clinical variables of the total enrolled study population (n=284) are summarized in Table 2.Overall, a higher proportion of patients without receiving antiviral therapy were older, had more severe liver diseases, lower-education and lower-income compared with the patients receiving treatments(Table 2).

Table 2Differences in baseline demographics and disease characteristics [n(%)].
Compared with patients who accepted the treatments, patients who declined the treatments, had poorer recognition of HCV infection(6.2vs.5.1,P=0.003), and more likely misperceived anti-HCV treatment (Table 3). Patients in both groups concerned about the low success rate of P/R treatment (8.1vs.6.6,P<0.001) though varied to different extents and wanted to wait for new drugs (7.9vs.6.4,P<0.001) which received more than 5 points from both groups.In addition, the patients who declined treatment were more likely worried about the intolerance to interferon (5.4vs.2.9,P<0.001),and were more inclined to accept traditional treatment (traditional Chinese medicine or other alternative therapies) (6.4vs.2.5,P<0.001), compared with the counterpart within the treatment group.The access to local hepatologists or medical resources appeared to be more difficult for the patients with declined treatments compared with patients treated (5.9vs.4.8,P=0.003).
3.2.2. Physician-related barriers to care
A total of 167 physicians returned the surveys and included in analysis (Table 4). Overall, 60% of the physicians answered incorrectly to 50% questions, indicating poor knowledge with the current standard of HCV care. A majority of the respondents (92%)knew the Peg-IFN + RBV treatment, that the addition of RBV to peg-IFN could improve the likelihood of SVR, additionally, the optimal dose is required. Many physicians (65%) did not adhere to genotype-guided treatment duration suggested by the guidelines.More than 50% of the surveyed physicians incorrectly believed that longer treatment duration resulted in better SVR rates (66%),regardless of changes in HCV RNA level during therapy. There were 22% of them who did not know the standard assays for the diagnosis of HCV infection.
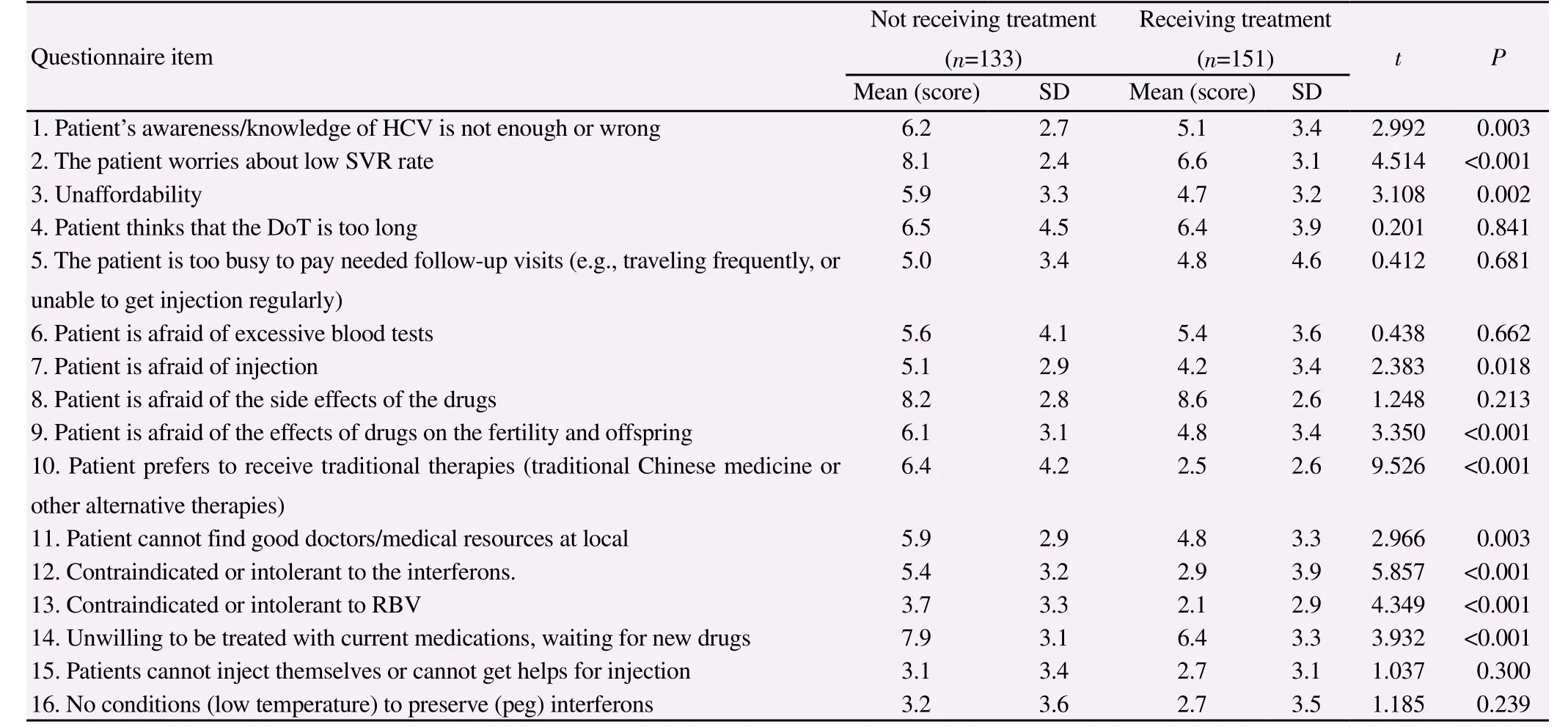
Table 3Differences in concerns, perception with anti-HCV treatment between not and receiving treatment patients.

Table 4Physicians’ opinions regarding the current status of HCV diagnosis and treatment in China (n=167)
4. Discussion
In this study, we analyzed the data of anti-HCV antibody and HCV RNA positivity and liver disease among the patients with nonliver disease complaints, who visited our hospital and received anti-HCV antibody test between 1 January 2013 and 7 June 2017; we also surveyed a portion of patients in this cohort including both referred and non-referred ones after positive anti-HCV antibody finding, as well as the non-hepatologist physicians who ordered anti-HCV antibody tests. There are several interesting findings: 1.The frequency of anti-HCV antibody positivity among the patients with non-liver disease complaints was 0.94%, higher than 0.43%in general Chinese population; 2. More than 73% of anti-HCV antibody positive patients were also HCV RNA positive. In the further evaluation of liver disease, we found that 34.1% and 1.42%of them were already at stage of cirrhosis and HCC, respectively;3. Our survey showed that there was a break in the HCV infection management chain in our care system, only 45.1% of the HCV antibody positive patients were referred to hepatologist for further assessments. Clearly, non-hepatologist physicians often lacked an appreciation of potential severity of liver disease with anti-HCV antibody positivity or/and there was not an established referral protocol for anti-HCV antibody positive patients. In addition, a significant number of HCV antibody positive patients did not take this finding seriously and nor they motivated to seek for further assessments.
The overall prevalence of anti-HCV antibody positivity detected by the third generation anti-HCV assay was 0.43% among Chinese population of ages ranging from 1 to 59 using blood samples previously collected for the 2006 National Hepatitis B Sero-survey indicating that prevalence of HCV infection in China was low[15].However, anti-HCV antibody prevalence among patients with nonliver disease complaints in this study was 0.94%, which was much higher than 0.43%. Our results suggest higher frequency of anti-HCV antibody positivity among diseased population despite their non-liver disease complaints comparing the general population. This finding also suggests that HCV infection prevalence differs among different populations, and it more frequently occurs in ill individuals comparing healthy ones. This is probably because that a diseased subject may frequently visit hospitals, engages medical procedures and increases the contacts to HCV infected patients, all of which may lead to increased exposures to HCV infectious sources[16,17].As expected, the highest HCV infection is found among the patients with liver disease complaints. For instance, nearly 10% of patients who visited Departments of Infectious Diseases in all the 3rd tier hospitals (the highest in China hospital ranking) in Guangzhou metropolitan area are HCV infected. Our data implies that it is essential to include samples from three populations of healthy, nonliver-diseased and liver diseased subjects to accurately determine HCV infection in China.
In this study, active HCV infection as marked by HCV RNA positivity among anti-HCV antibody positive patients was 73%. In liver disease evaluation, more than one third already advanced to cirrhosis and 1.42% to HCC. Given the fact that those patients did not have liver disease complaints because of the silent feature in clinical manifestations of chronic hepatitis C, a significant portion of chronic hepatitis C patients are not self-aware or diagnosed, which may have led to irreversible severe consequences among them,as shown by this study[18-23]. Thus, we have the reason to believe that chronic HCV infection among patients with non-liver disease complaints has been overlooked and it represents an enormous disease burden by hidden HCV infection in China. Our findings suggest that a routine detection of anti-HCV antibody should be included into lab tests for all the patients with non-liver disease complaints who visit their primary physicians or non-hepatology specialists. This is the first step to establish HCV infection database among the patients with no-liver disease complaints.
However, this step is not sufficient. As our survey showed,after anti-HCV antibody test there was a break between the nonhepatologist physicians and HCV management chain in our hospital,to a broad extent, in China. In this study, the finding of anti-HCV antibody positivity was delivered to the patients. Only 45% of anti-HCV positive patients were referred to hepatologists by nonhepatologist physicians, resulting in no referral in nearly 55%. There were three important problems that led to the break in managing HCV infection: 1. The non-hepatologist physicians often lacked the appreciation of the weight of an anti-HCV antibody positivity in term of potential severity of chronic liver disease of patients and did not fully recognize a need for further assessment[24]. 2. A lack of the standard referral criteria and procedure for HCV infection under which eligible patients must be referred. 3. There is a need to establish primary physician based care system in China. Under the primary physician based care system, all lab findings and diagnosis ordered by specialists will also deliver to the primary physicians,who are in charge of referrals based on the findings and established management protocols.
There were a few limitations in this study. First, this was a single center based study conducted in a 3rd tertiary hospital. The patients enrolled in this study may not be representative for patient population in the primary and secondary hospitals. Second, the questionnaire was designed for a cross-sectional investigation and customized for our hospital. Thus, the resultant survey data may not exactly reflect the status of managing HCV infection among patients with non-liver disease complaints in a broad setting.
In conclusion, we found in this study that anti-HCV antibody positivity among patients with non-liver disease complaints was 0.94%, significantly higher than 0.43% in general population in China. More than 73% of anti-HCV antibody positive patients had active HCV infection, and 34% and 1.42% of them already advanced to cirrhosis and HCC, respectively. In addition, there was a break in HCV infection care between the non-hepatologist physicians and hepatologists as only 45% of anti-HCV antibody positive patients were referred to hepatologists for further assessments. Our data suggest a hidden HCV related liver disease burden among the patients with no liver disease complaints in China. We call a routine detection of anti-HCV antibody for patients with non-liver disease complaints and an early establishment of the primary physician based referral system in China.
Conflict of interest statement
The authors declare no conflicts of interest.
This study was partly supported by the grants from the National Natural Science Foundation of China (81470856, 81772923,31470263 and 81360001). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[1] World Health Organization.Guidelines for the screening care and treatment of persons with chronic hepatitis C infection: updated version. Geneva: World Health Organization; 2016.
[2] Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence.Hepatology2013; 57(4): 1333-1342.
[3] Duan Z, Jia JD, Hou J, Lou L, Tobias H, Xu XY, et al. Current challenges and the management of chronic hepatitis C in mainland China.J Clin Gastroenterol2014; 48(8): 679-686.
[4] Mabrouk M, Esmat G, Yosry A, El-Serafy M, Doss W, Zayed N,et al. Health-related quality of life in Egyptian patients after liver transplantation.Ann Hepatol2012; 11(6): 882-890.
[5] George SL, Bacon BR, Brunt EM, Mihindukulasuriya KL, Hoffmann J, Di Bisceglie AM. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients.Hepatology2009; 49(3): 729-738.
[6] Bini EJ, Mehandru S. Sustained virological response rates and healthrelated quality of life after interferon and ribavirin therapy in patients with chronic hepatitis C virus infection and persistently normal alanine aminotransferase levels.Aliment Pharmacol Ther2006; 23(6): 777-785.
[7] Feld JJ, Foster GR. Second generation direct-acting antivirals - Do we expect major improvements?J Hepatol2016; 65(1 Suppl): S130-S142.
[8] Pawlotsky JM, Feld JJ, Zeuzem S, Hoofnagle JH. From non-A, non-B hepatitis to hepatitis C virus cure.J Hepatol2015; 62(1 Suppl): S87-S99.
[9] Lawitz E, Poordad F, Wells J, Hyland RH, Yang Y, Dvory-Sobol H, et al.Sofosbuvir-velpatasvir-voxilaprevir with or without ribavirin in directacting antiviral-experienced patients with genotype 1 hepatitis C virus.Hepatology2017; 65(6): 1803-1809.
[10] Curry MP, O’Leary JG, Bzowej N, Muir AJ, Korenblat KM, Fenkel JM,et al. Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis.N Engl J Med2015; 373(27): 2618-2628.
[11] Poordad F, Pol S, Asatryan A, Buti M, Shaw D, Hezode C, et al.Glecaprevir/pibrentasvir in patients with HCV genotype 1 or 4 and prior direct-acting antiviral treatment failure.Hepatology2017. doi: 10.1002/hep.29671.
[12] WHO.Combating hepatitis B and C to reach elimination by 2030. [Online].Available from: http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf/ [Accessed 7th Noevember 2017].
[13] Wei L, Li J, Yang X, Wang G, Feng B, Hou J, et al. Nationwide survey of specialist knowledge on current standard of care (Peg-IFN/RBV) and barriers of care in chronic hepatitis C patients in China.J Gastroenterol Hepatol2016; 31(12): 1995-2003.
[14] Wu E, Chen X, Guan Z, Cao C, Rao H, Feng B, et al. A comparative study of patients’ knowledge about hepatitis C in the United States and in urban and rural China.Hepatol Int2015; 9(1): 58-66.
[15] Xinhua News Agency. About 38 mil Chinese carry hepatitis C virus.[Online] November 23 2007. Available from: http://news.xinhuanet.com/english/2007-11/22/content_7129221.html [Accessed 17th August 2013].
[16] Chen YS, Li L, Cui FQ, Xing WG, Wang L, Jia ZY, et al. [A seroepidemiological study on hepatitis C in China].Zhonghua Liu Xing Bing Xue Za Zhi2011; 32(9): 888-891.
[17] Atsbaha AH, Asmelash Dejen T, Belodu R, Getachew K, Saravanan M,Wasihun AG. Sero-prevalence and associated risk factors for hepatitis C virus infection among voluntary counseling testing and anti retroviral treatment clinic attendants in Adwa hospital, northern Ethiopia.BMC Res Notes2016; 9: 121.
[18] Thorburn D, Roy K, Cameron SO, Johnston J, Hutchinson S, McCruden EA, et al. Risk of hepatitis C virus transmission from patients to surgeons: model based on an unlinked anonymous study of hepatitis C virus prevalence in hospital patients in Glasgow.Gut2003; 52(9): 1333-1338.
[19] Cheong JY, Um SH, Seo YS, Shin SS, Park RW, Kim DJ, et al. A practical scoring system for predicting cirrhosis in patients with chronic viral hepatitis.Hepatogastroenterology2012; 59(120): 2592-2597.
[20] Di Marco V, Calvaruso V, Ferraro D, Bavetta MG, Cabibbo G, Conte E, et al. Effects of eradicating hepatitis c virus infection in patients with cirrhosis differ with stage of portal hypertension.Gastroenterology2016;151(1): 130-139.
[21] Farid K, Omran MM, Farag RE, Arafa MM, Emran TM. Development and evaluation of a novel score for prediction of large oesophageal varices in patients with hepatitis C virus-induced liver cirrhosis.Br J Biomed Sci2017; 74(3): 138-143.
[22] Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection.Gastroenterology2011; 140(4): 1182-1188.
[23] Lee JJ, Lin MY, Chang JS, Hung CC, Chang JM, Chen HC, et al.Hepatitis C virus infection increases risk of developing end-stage renal disease using competing risk analysis.PLoS One2014; 9(6): e100790.
[24] Feng B, Zhang J, Wei L. Inadequate awareness of hepatitis C among nonspecialist physicians in China.Adv Med Educ Pract2011; 2: 209-214.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Clinical efficacy of two different tricuspid annuloplasty techniques in left cardiac valve surgery
- Visceral leishmaniasis: An immunological viewpoint on asymptomatic infections and post kala azar dermal leishmaniasis
- In vitro fungistatic activity of 36 traditional oriental medicines and their synergistic effect against Trichophyton rubrume
- Antidiabetic effects of galactomannans from Adenanthera pavonina L. in streptozotocin-induced diabetic mice
- Syzygium aromaticum ethanol extract reduces AlCl3-induced neurotoxicity in mice brain through regulation of amyloid precursor protein and oxidative stress gene expression
- Phlebotomus (Adlerius) kabulensis (Diptera: Psychodidae) a new record sand fly species from Iran: Morphological and molecular aspects
