In vitro fungistatic activity of 36 traditional oriental medicines and their synergistic effect against Trichophyton rubrume
2018-03-08YoungSooKimJinYeulMa
Young Soo Kim, Jin Yeul Ma
Korean Medicine Application Center, Korea Institute of Oriental Medicine, 70 Cheomdan-ro, Dong-gu, Daegu, 41062, Republic of Korea
1. Introduction
Fungal infections cause serious problems in immunocompromised populations, such as children and the elderly, patients infected with human immunodeficiency virus, those who have undergone transplantation, those undergoing chemotherapy, and those using long-term immunosuppressants. In recent years, the prevalence and mortality of opportunistic infections caused byCandida albicans,Aspergillusspp., andCryptococcus neoformansin immunocompromised populations has been steadily increasing worldwide[1]. Certain fungal infections caused by the invasion of fungi into the skin are not considered severe; however, these can be chronic conditions. Dermatophytosis is the most common fungal infection caused by the superficial infection of dermatophytes,which subsist upon digested keratin in the skin, nails, and hair. A large portion of chronic dermatophyte infections are considered to be caused byTrichophyton rubrum(T. rubrum), which induces tinea pedis, tinea unguium, tinea cruris, and tinea corporis[2]. Since the 1950s, various types of fungicides have been developed for effective inhibition and treatment of fungal infections via chemical synthesis.Since the development of the polyene amphotericin B (Amp B),which binds to ergosterol in fungal cell membranes to disrupt their integrity, in the 1950s, various classes of antifungal agents have been developed. These include azoles and allylamines, which inhibit ergosterol synthesis, echinocandins that block β-1,3-glucan formation in fungal cell walls, and flucytosine or griseofulvin to inhibit DNA synthesis or mitosis. However, despite these efforts,few effective antifungal agents are currently available as many have shown limitations because of their resistance and toxicity to the human body. Although antifungal resistance is generally less of a global issue than antibacterial resistance, resistance to antifungal agents, such as fluconazole and flucytosine by Candida, first reported in patients infected with human immunodeficiency virus in the 1990s, continues to be a concern[3]. Although, azole antifungals,in particular, have a mechanism to inhibit the synthesis of ergosterol in the fungal cell membrane, the resistance ofCandida albicansto azole antifungals by the efflux of antifungals and mutation of ERG11, causing a decrease in the affinity to the azoles antifungals was reported[4]. In addition, the administration of Amp B is limited to clinical treatment because of severe infusion-related toxicity resulting from the production of pro-inflammatory cytokines and nephrotoxicity[5], but several lipid-based formulations of Amp B have been developed to decrease this toxicity by limiting the exposure of human cells to Amp B[6,7]. Combination therapy of antifungal agents with different mechanisms has also been used to counteract issues of growing resistance and toxicity. Flucytosine is often used in combination with other antifungal agents primarily due to its narrow antifungal spectrum and emerging resistance.
Despite efforts to develop chemical antifungal agents, natural products, including various traditional oriental medicines (TOMs),and their constituents have attracted public attention as an alternative therapy to complement the treatment of diseases associated with fungal infections due to the emerging multidrug resistance of synthetic fungicides, which reduce the resistance and toxicity[8].In this study, we evaluatein vitrofungistatic activities 36 TOMs againstT. rubrum, which is a causative fungus for various types of tineas. In addition, because there have been few TOM compatibility studies on fungal infections, we evaluated the synergistic effects of TOM combinations as well as the interactions of TOM candidates with the conventional antifungal drugs terbinafine, which has been widely prescribed to treat various fungal diseases due to its relatively low toxicity. We hypothesized that dermatophytic growth may be more effectively suppressed using TOM combinations or TOMs in combinations with conventional antifungals and this may result in alleviation of toxicity by reducing the overall dose required.
2. Materials and methods
2.1. Chemicals and reagents
Sabouraud dextrose broth for fungal culture was purchased from BD DifcoTM (Sparks, MD, USA). Sodium chloride, terbinafine hydrochloride, gallic acid, methyl gallate, and DMSO were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Plant materials and preparation of plant extracts
A total of 36 TOMs (Table 1) were purchased from Yeongcheon Oriental Herbal Market (Yeongcheon, Republic of Korea) and identified by Professor Ki Hwan Bae, Chungnam National University, Republic of Korea. TOMs were deposited in the herb bank of the Korean Institute of Oriental Medicine. The extracts were prepared by extracting 50 g of TOMs in 1 000 mL of distilled water at 115 ℃ for 3 h (Gyeongseo Extractor Cosmos-600, Gyeongseo,Republic of Korea) or in 70% ethanol at 40 ℃ for 24 h. After filtering through testing sieves (150 μm; Retsch, Germany), the extracts were freeze-dried and placed in a desiccator at 4 ℃. The dried extract powders were stored at 20 ℃ until use. Samples for antifungal assays were prepared by dissolving the extract powders in 50% DMSO.
2.3. Fungal strain and inoculum preparation
T. rubrumATCC 62345 was grown in Sabouraud dextrose medium for 7 d at 25 ℃, and cells were resuspended in 0.85% sterile saline.After filtering the cell resuspension through Whatman filter paper no.40 (pore size: 8 μm) to collect microconidia with removal of hyphal fragments, the inoculum was diluted to (1×104)-(5×104) conidia/mL in Sabouraud dextrose broth for antifungal assay as suggested in previous reports[9,10].
2.4. Antifungal susceptibility testing
Fungistatic activity was evaluated by minimum inhibitory concentration (MIC) values, which were determined by a 2-fold dilution method using Sabouraud dextrose broth. Fungistatic activities of TOMs were measured in the range of 0-8 mg/mL, and terbinafine, tested as a positive control, ranged from 0-32 μg/mL.All tested samples contained a final concentration of 4% DMSO in the Sabouraud dextrose broth. MIC was determined as the lowest concentration that showed no visible fungal growth after incubation at 25 ℃ for 7 d. The experiments were performed in triplicate.

Table 1Traditional oriental medicines and parts used to determine fungistatic activity.
2.5. Synergy study of TOM in combination with terbinafine
Based on the results of antifungal assays, four TOMs were selected to determine if their effects were synergistic. The fractional inhibitory concentration index (FICI) of all combinations at 7 mg/mL was determined through serial dilution. In addition, 0.1×MIC TOMs in combination with terbinafine were also investigated.
The FICI was calculated using the formula:
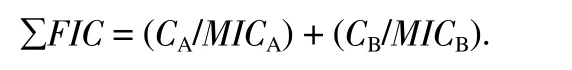
Where,MICAandMICBare the MIC values of antifungal agents A and B alone, respectively, andCAandCBare the concentrations of antifungal agents in combination, respectively. The FICI was defined as ∑FIC ≤ 0.50, synergistic; 0.50 < ∑FIC ≤ 0.75, partially synergistic; 0.75 < ∑FIC ≤ 1.00, additive; 1.00 < ∑FIC ≤ 4.00,indifferent; and ∑FIC > 4.00, antagonistic[11,12].
3. Results
3.1. In vitro antifungal assays of TOMs and comparative analysis based on the extraction solvent
To investigate the ability of TOMs to suppress fungal propagation,fungistatic activities of 36 TOMs were assessed by measuring their MICs (Table 2). MIC of the extracts ranged from 1 to 8 mg/mL. When TOM showed no fungistatic activity at 8 mg/mL,it was indicated as > 8 mg/mL. Although all 36 TOMs exhibited fungistatic activities againstT. rubrum, Table 2 shows the differences in fungistatic efficacy based on extraction solvents, which have different physicochemical properties. Ethanol extracts were more active in inhibitingT. rubrumgrowth than hot-water extracts in 25 of the 36 TOMs, and there were no TOMs showing better fungistatic activity in hot-water extracts. This property was particularly apparent in six ethanol extracts, namely, Aucklandiae radix (AR), Gentianae macrophyllae radix (GMR), Scutellariae radix (SR), Echinopsis radix, Belamcandae rhizoma, and Cassiae semen, in which the MIC values of the ethanol extracts were >2-fold lower than those of the corresponding hot-water extracts. Among them, the ethanol extracts of AR, GMR, and SR showed relatively low MIC values, indicating that they are effective in inhibitingT. rubrumgrowth. However,TOMs with MIC ratio of hot-water extract to ethanol extract (MICW/MICE) ≤ 2 other than Cantharides and Galla rhois (GR) showed weak fungistatic activities with MIC values more than 4 mg/mL in both hot-water and ethanol extracts.
The ethanol extracts of SR and Cantharides inhibitedT. rubrumgrowth most effectively with an MIC value of 1 mg/mL, and the hot-water extract of Cantharides also exhibited inhibitory effect onT. rubrumgrowth with an MIC value of 2 mg/mL. In addition,the ethanol extracts of AR, GMR, and both extracts of GR also effectively suppressed the proliferation ofT. rubrumwith MIC values of 2 mg/mL.
3.2. Synergistic effects of selected TOMs and combinations with terbinafine
Four selected ethanol extracts of TOMs, including AR, GMR, SR,and GR exhibiting high fungistatic effects, were further investigated for a synergistic effect againstT. rubrum(Table 3). FICI values for the various combinations ranged from 0.66 to 1.50, and partially synergistic (18.2%), additive (45.4%), and indifferent (36.4%) effects were observed. In particular, partial synergistic effects were observed in combinations of two TOMs: AR-GR and GMR-GR. Even though the combinations of three TOMs that included AR-GR or GMR-GR showed no synergistic effect, their FICI values were 0.79 and 0.83,close to the FICI value of 0.75 corresponding to partial synergy.Nevertheless, the combinations of AR-GMR showed the highest FICI value of 1.50, and FICI values of combinations between SR and other three TOMs (AR, GMR and GR) were 1.00, 1.10 and 1.20,respectively, indicating that the TOMs of each combinations interact indifferently for the inhibition ofT. rubrumgrowth.
We also investigated the interaction of four TOMs with terbinafine,which inhibits ergosterol synthesis by suppressing squalene epoxidase activity for the synthesis of fungal cell membrane (Table 4). The results in Table 4 indicated that GR has a synergistic effect with terbinafine. The growth ofT. rubrumwas inhibited using only 40% MIC of terbinafine by the combination with 0.1×MIC of GR. However, terbinafine interacted with each AR, GMR and SR indifferently for fungistatic actions; the FICI values in these cases indicated more than 1.50.
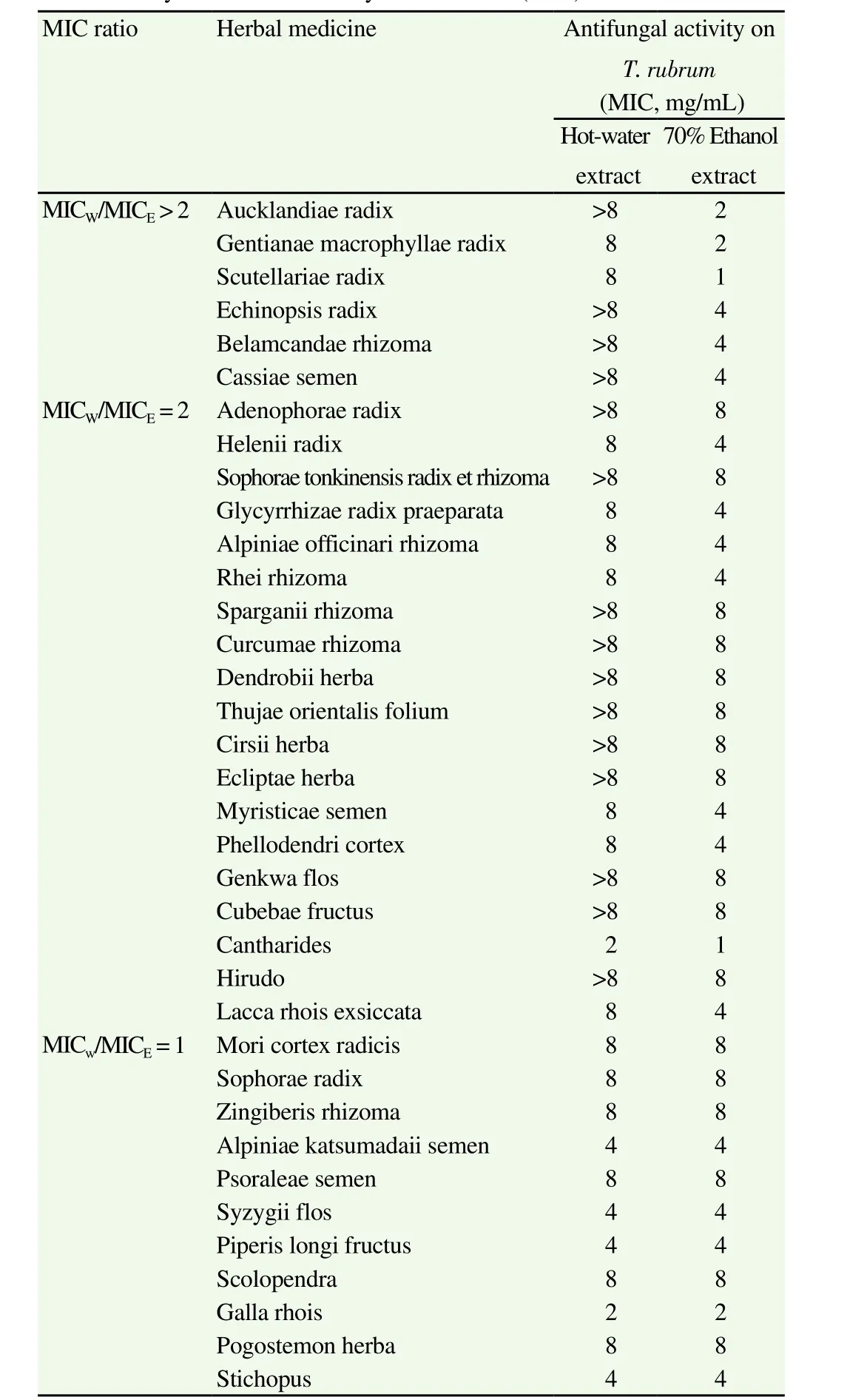
Table 2Fungistatic activities of traditional oriental medicines against T. rubrum determined by minimum inhibitory concentration (MIC).
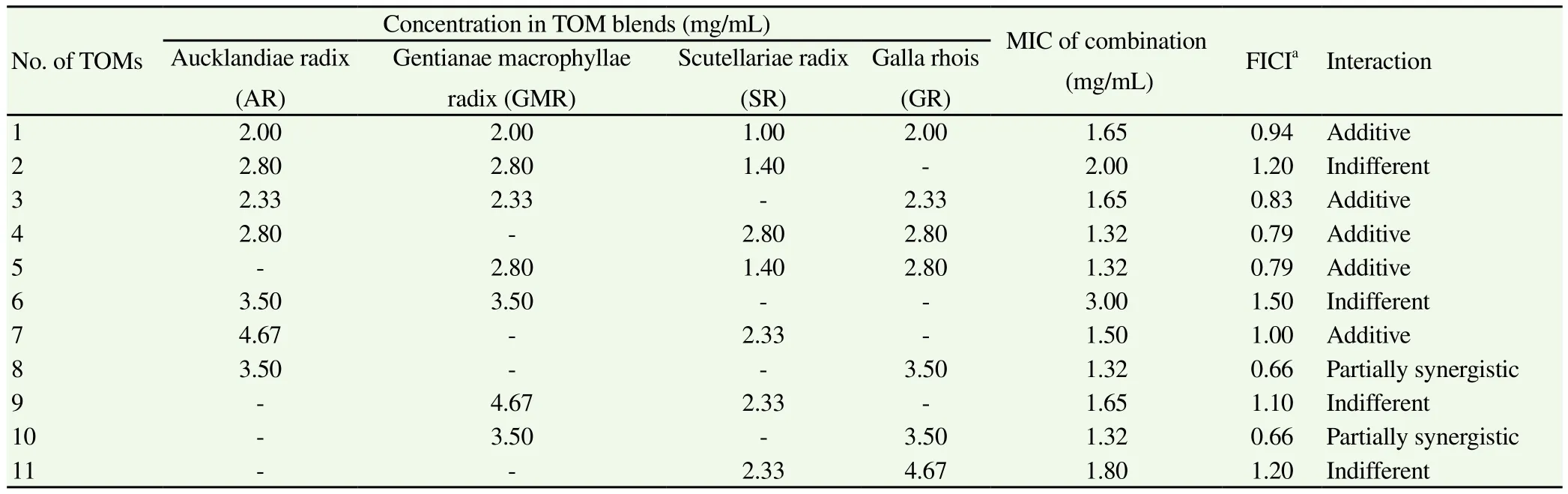
Table 3Synergistic effect of traditional oriental medicine (TOM) combinations for fungistatic activity.

Table 4Synergistic effect of traditional oriental medicines (TOMs) in combination with terbinafine for fungistatic activity.
4. Discussion
Previous studies have investigated fungistatic effects of various TOMs derived from herbs and animals to discover novel antifungal compounds for use against various fungi such as dermatophytes.The fungistatic activity of antifungal substances including TOMs and conventional drugs varies depending on the type of fungi, even in the same species. Therefore, in this study, we focused onT.rubrum, which is a major causative fungus for various types of tinea infection, to investigate the fungistatic activity of 36 TOMs. Based on the result of susceptibility test, we chose four TOMs with high fungistatic activities and examined their synergistic effects of TOM combinations as well as the interactions of TOM candidates with the conventional antifungal drug terbinafine.
In general, TOMs contain a variety of bioactive constituents,such as plant-derived secondary metabolites, which have several pharmacological effects on the human body, including antimicrobial effects. The result of fungistatic assay indicated that ethanol extracts inhibitedT. rubrumgrowth more effectively than than hotwater extracts in 25 of the 36 TOMs; in particular, AR, GMR, SR,Echinopsis radix, Belamcandae rhizoma, and Cassiae semen. Many phenolic compounds in TOMs containing single or multiple ring structures are known to have fungistatic effects. Ring structures tend to make the phenolic compounds more hydrophobic and they dissolve better in organic solvents than hydrophilic solutions[13].Based on the fungistatic activity assay, we selected top four TOMs,including AR, GMR, SR, and GR, with the lowest MIC values to analyze the synergistic effect of TOM blends and TOM-terbinafine combination. Despite the effective inhibition ofT. rubrumgrowth in both hot-water and ethanol extracts of Cantharides, it was excluded from TOM candidate for the combination studies, because its use in various tineas including athlete's foot may be contraindicated due to induction of serious blisters and its inability to be applied to mucous membranes. For further analysis of fungistatic activities and synergistic effects of four TOMs, we scrutinized the previous reports on phytochemical findings of natural product based on the constituents in TOMs.
SR, an extract from the root ofScutellaria baicalensis, contains flavonoids, including baicalin, baicalein, wogonoside, and wogonin,that have demonstrated various pharmacological effects such as fungistatic activity[14-16]. The MIC value of ethanol extract, which was 8-fold lower than that of hot-water extract, indicates that SR contains some ethanol soluble constituents with high fungistatic acitivity onT. rubrum, which is presumably due to bacalein, being soluble in organic solvents but low in aqueous solution solubility and known to have antimicrobial property[17,18]. Baicalein and SR extract exhibited the suppression of fungal growth by inducing apoptosis and inhibiting (1,3)-β-D-glucan synthesis, leading to the destruction of fungal cell walls[19,20].
Major compounds of AR and GMR are terpenoids, which are believed to disrupt fungal cell membranes and cause mitochondrial dysfunction[21]. AR, a root ofSaussurea costus(Falc.) Lipsch.,contains sesquiterpene lactones, including costunolide,dehydrocostus lactone, and alantolactone. The polarity of sesquiterpene lactones appears to be an important factor in their antifungal activity; sesquiterpene lactones with low polarity are more effective in suppressing fungal growth than those with more polarity[22]. In this aspect, higher fungistatic activity of AR in ethanol extract rather than hot-water extract may result from higher solubility of sesquiterpene lactones in organic solvents, such as ethanol, due to the low polarity of sesquiterpene lactones for antifungal activity.
GMR, a dried root ofGentiana macrophyllaPall.,Gentiana stramineaMaxim.,Gentiana crassicaulisDuthie ex Burkill,andGentiana dahuricaFisch., contains various secoiridoid monoterpene derivatives as its active constituents, which inhibit ergosterol synthesis in fungi[21]. The main secoiridoid in GMR is gentiopicroside. Although limited reports exist on gentiopicroside,other secoiridoids in GMR have been reported to inhibit fungal growth[23]. Based on the results, we speculate that GMR contains the constituents with higher extraction efficiency in ethanol than in hot water, including secoiridoids, which demonstrated fungistatic effects.
GR is a gall formed by a leaf defense mechanism ofRhus javanicaL. against wounds caused by the parasitic aphidSchlechtendalia chinensisand contains self-defensive compounds, primarily tannins,against external invasion. Although there are several previous reports that tannin-derived components in GR, including methyl gallate and gallic acid, promote growth inhibition of harmful intestinal bacteria[11,24] and phytopathogenic fungi by affecting a cAMP-related signaling pathway[25], both compounds did not exhibit any growth inhibitory effect of dermatophyteT. rubrumup to 2 mg/mL (data not shown). Our results that both hot-water and ethanol extracts of GR restricted fungal growth at 2 mg/mL, indicating that the constituents in both extracts, other than methyl gallate and gallic acid, are strongly involved in fungistatic action againstT. rubrum.
Multi-herbal therapies include herbal blends of various ingredients that have different mechanism of actions. These blends can display synergistic or complementary pharmacological effects, thereby achieving a dose reduction in the overall combination or reducing the toxicity of a single herb. In this study, four selected TOMs with high fungistatic activity were further investigated for a synergistic effect againstT. rubrum. The result showed that partial synergistic effects were observed with AR-GR and GMR-GR combinations, indicating that the constituents in AR and GMR, including terpenoids, and GR, containing tannin/tannin-derived components, may act onT.rubrumwith different mechanisms leading to synergistic effects.On the other hand, the combinations of AR and GMR showed the highest FICI value of 1.50, suggesting that sesquiterpene lactones and secoiridoids act with different antifungal mechanisms but do not result in a synergistic effect. In addition, the combinations between SR and other three TOMs with FICI value more than 1.00 indicated that fungistatic mechanism of SR does not cause any synergistic effect with the action of other three TOMs.
We also investigated the interaction of four TOMs with conventional drug terbinafine which blocks the synthesis of fungal cell membrane by suppressing biosynthesis of ergosterol, and synergistic effect was observed in the combinatino of GR and terbinafine. This effect reduced the concentration of terbinafine needed in combinations with 0.1×MIC of GR compared with that required for treatment with terbinafine alone. Since terbinafine acts as an fungistatic mechanism to inhibit the synthetic pathway of ergosterol, a component of the fungal cell wall, the synergistic effect of GR and terbinafine seems to be due to the components in the GR with different mechanisms not related to ergosterol synthesis, excluding gallic acid and methyl gallate that exhibited no fungistatic activity againstT. rubrum.Further study on fungistatic mechanism and synergistic interactions of TOMs, in particular GR, based on their constituents is warranted.Collectively, our findings indicate that multi-TOM therapy and TOMs in combination with conventional drugs may present an effective clinical treatment for superficial fungal infections such as tineas.
In summary, the present study focused on effective treatment of chronic and recurrent dermatophytosis using TOMs with high fungistatic activity. Our results demonstrated that majority of TOM ethanol extracts had higher fungistatic activities than the corresponding hot-water extracts, suggesting that fungistatic compounds in TOMs have hydrophobic properties. AR or GMR in combination with GR exhibited improved fungistatic activities indicative of a synergistic effect. Synergistic effects of GR was also observed in combination with terbinafine, suggesting that conventional drugs could be given at a reduced dose when administered concurrently with TOMs. Further investigation on changes in the fungal phenotype and metabolism and how these may impact clinical practice as well as thein vivomechanisms underlying synergistic effects is warranted.
Conflicts of interest statement
The authors declare that they have no conflict of interests.
Acknowledgment
This work was supported by the Grant K17281 from Korea Institute of Oriental Medicine (KIOM), provided by the Ministry of Science,ICT and Future Planning (MISP), Republic of Korea.
[1] Jain A, Jain S, Rawat S. Emerging fungal infections among children: A review on its clinical manifestations, diagnosis, and prevention.J Pharm Bioallied Sci2010; 2: 314-320.
[2] Graser Y, Scott J, Summerbell R. The new species concept in dermatophytes-a polyphasic approach.Mycopathologia2008; 166: 239-256.
[3] Law D, Moore CB, Wardle HM, Ganguli LA, Keaney MG, Denning DW.High prevalence of antifungal resistance inCandidaspp. from patients with AIDS.J Antimicrob Chemother1994; 34: 659-668.
[4] MacCallum DM, Coste A, Ischer F, Jacobsen MD, Odds FC, Sanglard D. Genetic dissection of azole resistance mechanisms inCandida albicansand their validation in a mouse model of disseminated infection.Antimicrob Agents Chemother2010; 54: 1476-1483.
[5] Laniado-Laborin R, Cabrales-Vargas MN. Amphotericin B: Side effects and toxicity.Rev Iberoam Micol2009; 26: 223-227.
[6] Hamill RJ. Amphotericin B formulations: A comparative review of efficacy and toxicity.Drugs2013; 73: 919-934.
[7] Mistro S, Maciel Ide M, de Menezes RG, Maia ZP, Schooley RT,Badaro R. Does lipid emulsion reduce amphotericin B nephrotoxicity?A systematic review and meta-analysis.Clin Infect Dis2012; 54: 1774-1777.
[8] Barrett D. From natural products to clinically useful antifungals.Biochim Biophys Acta2002; 1587: 224-233.
[9] Santos DA, Barros ME, Hamdan JS. Establishing a method of inoculum preparation for susceptibility testing ofTrichophyton rubrumandTrichophyton mentagrophytes.J Clin Microbiol2006; 44: 98-101.
[10] Santos DA, Hamdan JS. Evaluation of broth microdilution antifungal susceptibility testing conditions forTrichophyton rubrum.J Clin Microbiol2005; 43: 1917-1920.
[11] Choi JG, Kang OH, Lee YS, Oh YC, Chae HS, Jang HJ, et al.In vitroactivity of methyl gallate isolated from galla rhois alone and in combination with ciprofloxacin against clinical isolates ofSalmonella.J Microbiol Biotechnol2008; 18: 1848-1852.
[12] Mun SH, Kang OH, Joung DK, Kim SB, Seo YS, Choi JG, et al.Combination therapy of sophoraflavanone B against MRSA:In vitrosynergy testing.Evid Based Complement Alternat Med2013; 2013:823794.
[13] Takayama C, Fujinami A, Kirino O, Hisada Y. Quantitative structureactivity relationships of antifungal l-(3,5-dichlorophenyl)-2,5-pyrrolidinediones and 3-(3,5-dichlorophenyl)-2,4-oxazolidinediones.Agri Biol Chem1982; 46: 2755-2758.
[14] Chen H, Xu Y, Wang J, Zhao W, Ruan H. Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS,inflammation and oxidative stress in rat.Int J Clin Exp Pathol2015; 8:10139-10147.
[15] Yan WJ, Ma XC, Gao XY, Xue XH, Zhang SQ. Latest research progress in the correlation between baicalein and breast cancer invasion and metastasis.Mol Clin Oncol2016; 4: 472-476.
[16] Zhou YJ, Wang H, Sui HH, Li L, Zhou CL, Huang JJ. Inhibitory effect of baicalin on allergic response in ovalbumin-induced allergic rhinitis guinea pigs and lipopolysaccharide-stimulated human mast cells.Inflamm Res2016; 65: 603-612.
[17] Wu J, Gu L, Wang H, Tao L, Wang X. Solubility of baicalein in different solvents from (287 to 323) K.Int J Thermophys2014; 35: 1465-1475.
[18] Yang D, Hu H, Huang S, Chaumont JP, Millet J. Study on the inhibitory activity,in vitro, of baicalein and baicalin against skin fungi and bacteria.Zhong Yao Cai2000; 23: 272-274.
[19] Dai BD, Cao YY, Huang S, Xu YG, Gao PH, Wang Y, et al. Baicalein induces programmed cell death inCandida albicans.J Microbiol Biotechnol2009; 19: 803-809.
[20] Kim Y. Antibiofilm activity ofScutellaria baicalensisthrough the inhibition of synthesis of the cell wall (1,3)-b-D-glucan polymer.Korean J Microbiol Biotechnol2013; 41: 88-95.
[21] Freiesleben SH, Jäger AK. Correlation between plant secondary metabolites and their antifungal mechanisms-A review.Med Aromat Plants2014; 3: 154.
[22] Barrero AF, Oltra JE, Alvarez M, Raslan DS, Saude DA, Akssira M. New sources and antifungal activity of sesquiterpene lactones.Fitoterapia2000; 71: 60-64.
[23] Tan RX, Wolfender JL, Zhang LX, Ma WG, Fuzzati N, Marston A, et al.Acyl secoiridoids and antifungal constituents fromGentiana macrophylla.Phytochemistry1996; 42: 1305-1313.
[24] Ahn YJ, Lee CO, Kweon JH, Ahn JW, Park JH. Growth-inhibitory effects of galla rhois-derived tannins on intestinal bacteria.J Appl Microbiol1998; 84: 439-443.
[25] Ahn YJ, Lee HS, Oh HS, Kim HT, Lee YH. Antifungal activity and mode of action of galla rhois-derived phenolics against phytopathogenic fungi.Pest Biochemi Physiol2005; 81: 105-112.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- A hiddenly high hepatitis C virus related liver disease burden among Chinese patients with non-liver disease complaints: A hospital based study from 2013 to 2017
- Visceral leishmaniasis: An immunological viewpoint on asymptomatic infections and post kala azar dermal leishmaniasis
- Antidiabetic effects of galactomannans from Adenanthera pavonina L. in streptozotocin-induced diabetic mice
- Syzygium aromaticum ethanol extract reduces AlCl3-induced neurotoxicity in mice brain through regulation of amyloid precursor protein and oxidative stress gene expression
- Phlebotomus (Adlerius) kabulensis (Diptera: Psychodidae) a new record sand fly species from Iran: Morphological and molecular aspects
- Effect of RNA interference on WD101 gene of Schistosoma japonicum
