Clinical efficacy of two different tricuspid annuloplasty techniques in left cardiac valve surgery
2018-03-08ZhenWeiGeZhaoYunChengBaoCaiWangJunLongHuJianChaoLiZiNiuZhaoGangQiaoXiaoQiangQuanGuoBaoZhang
Zhen-Wei Ge, Zhao-Yun Cheng, Bao-Cai Wang, Jun-Long Hu, Jian-Chao Li, Zi-Niu Zhao, Gang Qiao, Xiao-Qiang Quan, Guo-Bao Zhang
Department of Cardiovascular Surgery, Henan Provincial People's Hospital, Zhengzhou, 450003, China
1. Introduction
Rheumatic heart disease is often associated with functional tricuspid regurgitation, which is also known as secondary tricuspid regurgitation. Tricuspid valve diseases frequently coexist with mitral and aortic valve diseases caused by many reasons, in which the incidence of tricuspid regurgitation is far higher than tricuspid stenosis. Functional tricuspid regurgitation is common, and mostly occurs secondarily in mitral valve disease due to pulmonary hypertension and right ventricular dilation[1,2]. When performing left cardiac valve replacement, rational treatment of tricuspid valve disease is extremely important for the postoperative recovery of heart functions. Inappropriate intraoperative treatment techniques could cause recurrence and progressive aggravation of tricuspid regurgitation in a short term, which would ultimately seriously affect the patients’ perioperative restoration and long-term quality of life, and may even result in death[3].
Currently, relevant reports about the intraoperative treatment principles and methods toward functional tricuspid regurgitation are divergent[4]. Some authors believe that if no serious organic valvular damage occurs, simple suture annuloplasty to narrow the annulus could achieve satisfactory result[5,6], because the tricuspid valve lies in the right low-pressure chamber. Meanwhile, others believe that no matter what degree of functional tricuspid regurgitation is present, as long as left cardiac valve surgery is performed, tricuspid annuloplasty should be performed simultaneously[7], and an artificial plastic ring(s) should be implanted to improve the long-term results[8,9]. Currently, the Chinese socio-economic level still has a wide gap compared to the developed countries. Artificial plastic ring annuloplasty performed for all functional tricuspid regurgitation might cause the controversy of ‘over treatment’ and increase the patients’ economic burdens in vain. On the other hand, a simple strategy might reduce the maintenance of long-term functions. So,clinical studies on the comparison of these two methods are urgently needed.
We retrospectively reviewed 677 patients with left cardiac valve disease and tricuspid regurgitation from March 2005 to September 2015. These patients underwent left cardiac valve surgery and one of two different tricuspid annuloplasty techniques. By comparing and summarizing the patients’ mid- and long-term follow-up results, we aimed to obtain the strategy and method of tricuspid annuloplasty that is suitable for Chinese patients with left cardiac valve diseases.
2. Materials and methods
2.1. General information
From March 2005 to September 2015, a total of 677 patients with left cardiac valve diseases and functional tricuspid regurgitation were surgically treated in our hospital. Tricuspid valvuloplasty and left cardiac valve surgery were simultaneously performed. Left cardiac valve diseases were mainly rheumatic (503 cases), accounting for 74.3%; the remainder included mitral valve degeneration (73 cases),coronary heart disease mediated mitral regurgitation (42 cases),infective endocarditis and trauma mediated mitral valve injury (53 cases), and cardiac tumors, which had affected the mitral valve(6 cases). The mean age of these patients (46±20), ranging from 14 to 76 years old, and a total of 376 males and 301 females were included.
According to NYHA, 287 patients were categorized as functional class II, 302 as functional class III, and 88 as functional class IV.Preoperatively, 371 cases had atrial fibrillation, and 306 cases were in sinus rhythm. Twenty-two patients had ‘cerebrovascular embolism’ due to preoperative left atrial thrombus shedding, the time interval between embolism treatment and heart surgery was more than three months, during which the cardiotonic and diuretic treatments were strengthened to maintain the heart functions. The preoperative tricuspid regurgitation grading was measured and evaluated by echocardiography: mild (the area of the regurgitant current was less than 20% of the right atrial area) in 164 cases;moderate (20%-40% regurgitant current area) in 332 cases; and severe (over 40% regurgitant current area) in 181 cases.
Before the operation, left ventricular systolic functions were as follows: mean ejection fraction was (55%±17%) and long axial fractional shortening was (30%±8%). The concomitant left cardiac procedures included 356 cases of mitral valve replacement (MVR),98 cases of mitral valvuloplasty (MVP), 181 cases of MVR plus aortic valve replacement (MVR+AVR), 15 cases of coronary artery bypass graft (CABG) with MVR, and 27 cases of CABG with MVP.This study was conducted in accordance with the declaration of Helsinki, and with approval from the Ethics Committee of Henan Provincial People’s Hospital. Informed consents were obtained from the participants or the family members.
2.2. Surgical treatment
After the left heart procedures, the tricuspid valvuloplasty was performed under aortic cross-clamping and cardiac arrest. A total of 353 cases were subjected to the simple suture annuloplasty (group A) while the other 324 cases were subjected to artificial plastic ring annuloplasty (group B). The selection of the tricuspid surgery techniques was mainly based on the degree of tricuspid regurgitation judged by the preoperative cardiac ultrasound, as well as by intraoperative direct observation of tricuspid valve disease.
The patients preoperatively evaluated as mild (with tricuspid annular dilation) and moderate degree of tricuspid regurgitation were more likely to undergo simple suture annuloplasty using one of the following methods:
(1) The Kay annuloplasty (the two-valve method): this method was performed to suture and close the posterior annulus with two figureof-8 sutures, and then followed by a closure of the anterior leaflet to eliminate the regurgitation.
(2) The De Vega annuloplasty: a 3-0 polypropylene pledgeted suture with bipitch needle was used. The first suture was started from the anterior antero-septal commissure, running in a clockwise direction along the anterior annulus and the posterior annulus until the posterior antero-septal commissure, and the second suture was located parallel and outside of the first suture, passing the pledget.The suture was then ligated after suturing twice to narrow the annulus, which was attached to the right ventricular free wall. In addition, the segmental De Vega annuloplasty was performed to selectively shorten the annulus at the antero-septal commissure and near the postero-septal commissure, while maintaining the intermediate portion of the anterior annulus; the modified De Vega annuloplasty was performed by suturing along the annulus while adding one gasket into each suture to reduce the shear stress on the suture toward the tissues.
(3) The ‘edge to edge’ method with/without the De Vega constrictor ring: the valve free edge in the regurgitantregion was marked with the suture. Mattress apposition technique using 5-0 polypropylene sutures and the pericardial gasket to form two or three holes to eliminate the regurgitation were performed; as for the patients with obvious annular dilatation, the constrictor ring should be added to reduce the valve tension.
Artificial annuloplasty ring implantation was performed using one of the three rings: 1) the domestic BalMed C-shaped elastic ring(3D ring), 2) the Edwards MC3 elastic ring, or 3) the S-J Tailor semi-rigid ring. The last two were both 3D structure. The suturing technique was similar, regardless of the ring used: starting from the anterior antero-septal commissure, suturing was performed along the antero-posterior valve until the posterior antero-septal commissure.Interrupted mattress technique or annulus-parallel non-pledgeted interrupted suture technique using pledgeted 2-0 suture was performed to suture the tricuspid annulus along the aforementioned range to ensure that the suture pitches on the tissues were wider than those on the artificial ring so as to effectively constrict the ring and fix the tricuspid annulus.
Intraoperative examination was performed last. Prior to unclamp the aorta, the pulmonary artery was temporarily occluded, and the right ventricular cavity was injected with water for observation. After the aorta has been unclamped and the heart resuscitated, the tricuspid valve should be able to open and close under direct vision, and the right atrial incision was sutured only if the tricuspid valve exhibited good closure.
2.3. Follow-up
The follow-up approaches included telephone follow-ups and regular clinic visits. All cases completed the two-year follow-up,and a total of 600 patients (88.6%) completed the mid-term (2 years)to long-term follow-up (5 years). The data of these patients during follow-up were obtained from the final color Doppler ultrasound images. This method was also adapted for the postoperative cardiac function determination. The postoperative cardiac surgery-related complications were not included in this study, and they did not affect the results. All data were recorded into one computer for the final statistical analysis. Follow-up periods were 2.0-10.5 years, with an average of (6.5±3.9) years. All patients alive underwent echo and clinical examination at the two-year and five-year time points.
2.4. Statistical analysis
SPSS18.0 statistical software was used for data processing. The data were expressed as mean±standard deviation (mean±SD). The counting data were expressed as percentage. Analysis of variance was used for the intra-group comparison; theChi-square test or the correctedChi-square test was used for the intergroup comparison.P<0.05 was considered to indicate a significant difference.
3. Results
3.1. Demographic results
There were no significant differences in the general demographic patient data between these two groups, including age, gender,concomitant left heart procedure, cardiac function, inner diameter of right ventricle (RV), and tricuspid regurgitation grading. The general demographic data of the patients were shown in Table 1.
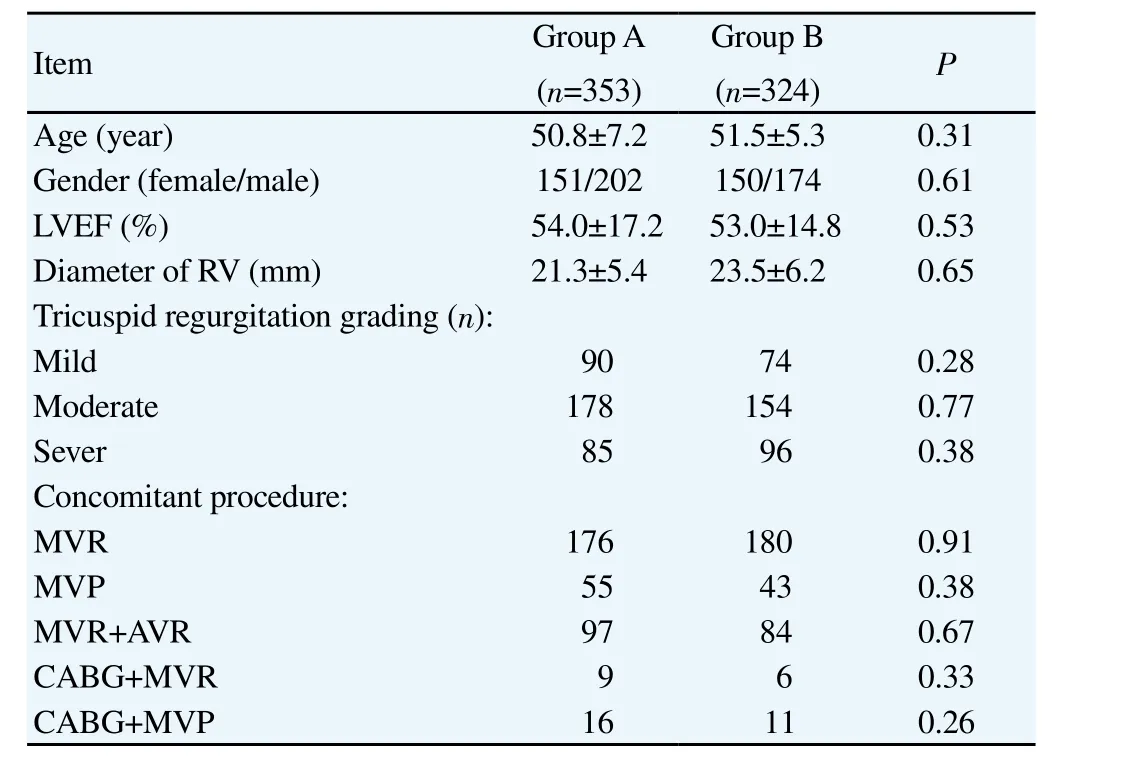
Table 1General demographic data of the patients.
3.2. Perioperative results
Five cases (1.5%) died in group A during the perioperative period,and seven cases (2.2%) died in group B. There was no significant difference between the two groups (P=0.062). On the day of surgery, all the patients were diagnosed as mild or slight tricuspid regurgitation by transthoracic or intraoperative transesophageal ultrasound in the intensive care unit (ICU).
3.3. Results of mid-term follow-up
During the two-year follow-up, no death occured. The tricuspid valve functions of the two-year follow-up patients were shown in Table 2. There was no significant difference in the incidence of mild tricuspid regurgitation between the two groups (P=0.37), but the cases that developed into moderate to severe tricuspid regurgitation in group A were significantly more than those in group B (P=0.031).
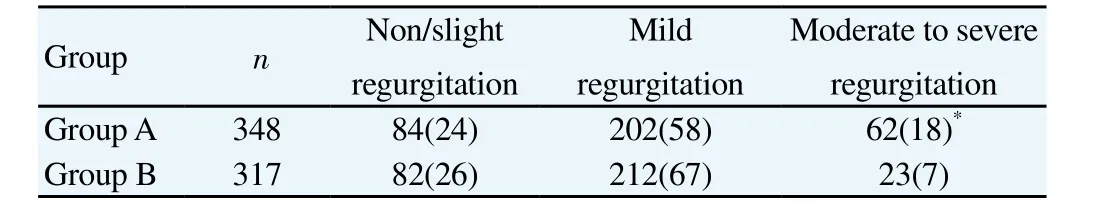
Table 2Tricuspid valve functions in the 2-year follow-up [n(%)].
3.4. Results of long-term follow-up
Five-year follow-up revealed that group A had five cases of re-hospitalization due to intractable heart failure, and ultrasonocardiograph revealed that they were with severe tricuspid regurgitation. Among these, three cases died of heart failure(mortality, 0.97%), and the other two cases underwent reoperation(tricuspid valve replacement) and were discharged after treatment.The rest of the cases in these two groups all survived with grade II-III heart function (NYHA), and the patients with poor cardiac function were usually associated with severe tricuspid regurgitation.The tricuspid valve functions in the two groups were shown in Table 3: group B exhibited significantly better tricuspid regurgitation improvement than group A (P=0.029), and the ratio of developing into moderate to severe tricuspid regurgitation in group A was significantly higher than cases in group B (P=0.007). Furthermore,the long-term follow-up revealed that compared with the two-year follow-up results, the recurrence rate of moderate to severe tricuspid regurgitation in group A was significantly increased (P=0.022),while the increase in group B has no statistic difference (P=0.52).
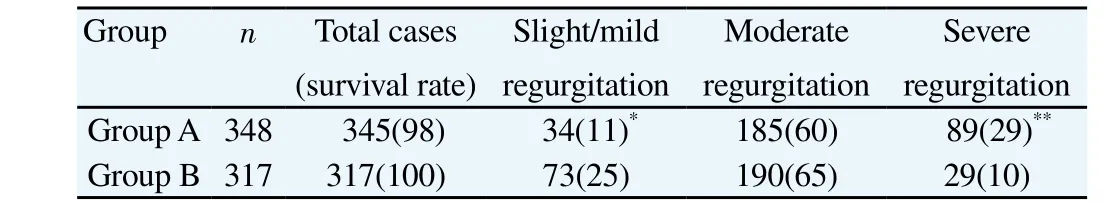
Table 3Tricuspid valve functions and survival rate according to the 5-year follow-up[n(%)].
4. Discussion
Rheumatic heart disease is often associated with functional tricuspid regurgitation, which is also known as secondary tricuspid regurgitation. Many studies have shown that[1,2] the main reason for functional tricuspid regurgitation is left heart valve disease mediated pulmonary hypertension and right ventricular enlargement, in which the tricuspid annulus dilates, leading to a change of the mechanical direction of the tendons’ tractions toward the valve. This change results in a dislocation from the normal tricuspid valve’s identical point[10]. The tricuspid valve itself and the subvalvular structures do not have organic disease, hence, it is also known as secondary tricuspid regurgitation. Many other studies found that when mitral valve disease was effectively treated and the pulmonary artery pressure was maintained at normal level or slightly higher than normal level, tricuspid regurgitation would still occur and continue to develop[11-13]. The exact mechanism should be further clarified.
The tricuspid annuloplasties, such as the De Vega method, the Kay method, or the artificial ring implantation method, aim to constrict the valve annular and leaflets. The Kay annuloplasty is mainly suitable for patients with significant valve expansion, but this surgery would change the natural anatomic relationship of the tricuspid valve orifice, leading to a risk of valve stenosis. Because the expansion of the right ventricular free wall is not dealt with, the rest of the dilated tricuspid annulus might still result in residual tricuspid regurgitation or persistent tricuspid annular dilatation. The disadvantage of the De Vega method is the annular suture may not be strong due to exclusion of the septum, resulting in the ‘guitar string’ phenomenon,namely, the sutures might be torn from the annulus, leading to one or two strings stretching over the center of the annulus. In these cases,the postoperative residual tricuspid regurgitation and the long-term tricuspid regurgitation recurrence rate would be higher. Our study also showed that the suture annuloplasty was a type of temporary support, and its long-term recurrence rate of tricuspid regurgitation was higher.
Artificial ring plasty could not only reduce the tricuspid annulus expansion, but also be more structurally suitable for the repair of the expanded tricuspid annulus, especially with the use of elastic rings with different 3D shapes in the recent years, such as Edwards MC3 ring. These rings could be better to retain the physiological shape of the tricuspid annulus and to maintain the normal morphological changes generated with heart constriction, and could also correct the deformation of the tricuspid valve[14]. Filsoufi[15] observed a group of 75 cases who underwent Edwards MC3 annuloplasty and found good early results. The 3D structure was in line with the physiological structure of the tricuspid valve, therefore, the likelihood of postoperative annulus breaking was low and the possibility of long-term recurrence of tricuspid regurgitation was also decreased. However, that study was not able to provide longterm follow-up results. In this study, the 2- to 5-year follow-up also showed good results of ring implantation. Compared with the simple suture annuloplasty, artificial ring annuloplasty not only reduced the recurrence of tricuspid regurgitation, but also significantly delayed the progression of the recurrent tricuspid regurgitation.
Meanwhile, it should be noted that due to lack of early recognition and high attention toward tricuspid valvuloplasty, the clinical patients undergoing artificial ring annuloplasty in this study mostly had worse preoperative regurgitation. However, the mid- and long-term prognoses of the patients, as well as the progression of the recurrent tricuspid regurgitation, in group I were still significantly better than those in group A.
The annular expansion might recur due to the suture-cutting,especially in patients without ring implantation. The strengthening of the annulus is significantly improved with the ring, which could block the feedback chain of annulus dilatation-tricuspid regurgitation-right atrioventricular expansion-annulus dilatation to some extent to protect the right heart functions. In a related study, Tanget al[16]found that tricuspid ring implantation could indeed significantly reduce the recurrence of tricuspid regurgitation via their multivariate regression analysis. Furthermore, this method was the only independent preventive factor for improving the long-term survival without complications.
As mentioned above, simple tricuspid valvuloplasty could not eliminate functional tricuspid regurgitation permanently. Meanwhile,improper intraoperative processing of mild tricuspid regurgitation would cause short-term recurrence and progressive aggravation of tricuspid regurgitation, eventually decreasing the quality of the patient’s life, as well as causing right heart failure and death.Therefore, some scholars have suggested that when performing left cardiac valve surgery, a synchronous tricuspid annuloplasty should be performed regardless of whether the tricuspid regurgitation exists or not and the degree of tricuspid regurgitation, especially for patients with high-risk factors and annular dilatation. Simultaneous performance of the two surgeries does not increase the perioperative mortality[17]. Taking into account the good short- and long-term results of the artificial ring annuloplasty, it has been recommended that this method should be used as the routine procedure for tricuspid valvuloplasty[18].
In summary, our study demonstrated that artificial ring annuloplasty results in significantly less tricuspid regurgitation than simple suture annuloplasty. Thus, artificial ring annuloplasty would be used as good substitute of simple suture annuloplasty for tricuspid annulus constriction.
Conflict of interest statement
All authors declare that we have no conflict of interest.
[1] Amat-Santos IJ, Castrodeza J, Nombela-Franco L, Munoz-Garcia AJ,Gutierrez-Ibanes E, de la Torre Hernandez JM, et al. Tricuspid but not mitral regurgitation determines mortality after TAVI in patients with nonsevere mitral regurgitation.Rev Esp Cardiol (Engl Ed)2017;DOI:10.1016/j.rec.2017.08.019.
[2] HH QT, Vinh PN. Progression of tricuspid regurgitation after mitral valve replacement for rheumatic heart disease.J Heart Valve Dis2017; 26(3):290-294.
[3] De Meester P, De Cock D, Van De Bruaene A, Gabriels C, Buys R,Helsen F, et al. Additional tricuspid annuloplasty in mitral valve surgery results in better clinical outcome.Heart2015; 101(9): 720-726.
[4] Fender EA, Zack CJ, Nishimura RA. Isolated tricuspid regurgitation:outcomes and therapeutic interventions.Heart2017; DOI: 10.1136/heartjnl-2017-311586.
[5] Tchantchaleishvili V, Rajab TK, Cohn LH. Posterior suture annuloplasty for functional tricuspid regurgitation.Ann Cardiothorac Surg2017; 6(3):262-265.
[6] Hata H, Fujita T, Miura S, Shimahara Y, Kume Y, Matsumoto Y, et al. Long-term outcomes of suturevs.ring tricuspid annuloplasty for functional tricuspid regurgitation.Circ J2017; 81(10): 1432-1438.
[7] Azarnoush K, Nadeemy AS, Pereira B, Leesar MA, Lambert C, Azhari A,et al. Clinical outcomes of tricuspid valve repair accompanying left-sided heart disease.World J Cardiol2017; 9(10): 787-793.
[8] Choi JW, Kim KH, Chang HW, Jang MJ, Kim SH, Yeom SY, et al.Long-term results of annuloplasty in trivial-to-mild functional tricuspid regurgitation during mitral valve replacement: should we perform annuloplasty on the tricuspid valve or leave it alone?Eur J Cardiothorac Surg2017; DOI: 10.1093/ejcts/ezx395.
[9] Wang N, Phan S, Tian DH, Yan TD, Phan K. Flexible band versus rigid ring annuloplasty for tricuspid regurgitation: a systematic review and meta-analysis.Ann Cardiothorac Surg2017; 6(3): 194-203.
[10] Pant AD, Thomas VS, Black AL, Verba T, Lesicko JG, Amini R.Pressure-induced microstructural changes in porcine tricuspid valve leaflets.Acta Biomater2017; DOI: 10.1016/j.actbio.2017.11.040.
[11] Hahn RT. The challenge of preoperative quantification of functional tricuspid regurgitation and of right ventricle function: what information is clinically relevant?Minerva Cardioangiol2017; 65(5): 480-490.
[12] Arsalan M, Walther T, Smith RL 2nd, Grayburn PA. Tricuspid regurgitation diagnosis and treatment.Eur Heart J2017; 38(9): 634-638.
[13] Chen L, Shen J, Shan X, Wang F, Kan T, Tang X, et al. Improvement of tricuspid regurgitation after transcatheter ASD closure in older patients.Herz2017; DOI: 10.1007/s00059-017-4594-x.
[14] Lee SI, Kim HJ, Kim JB, Jung SH, Choo SJ, Chung CH, et al. Medtronic Duran AnCore versus Edwards MC3 rings for tricuspid annuloplasty.Interact Cardiovasc Thorac Surg2017; 24(6): 848-854.
[15] Filsoufi F, Chikwe J, Carpentier A. Rationale for remodelling annuloplasty to address functional tricuspid regurgitation during leftsided valve surgery.Eur J Cardiothorac Surg2015; 47(1): 1-3.
[16] Tang GH, David TE, Singh SK, Maganti MD, Armstrong S, Borger MA.Tricuspid valve repair with an annuloplasty ring results in improved longterm outcomes.Circulation2006; 114(1 Suppl): I577-I581.
[17] Zhu TY, Wang JG, Meng X. Does concomitant tricuspid annuloplasty increase perioperative mortality and morbidity when correcting left-sided valve disease?Interact Cardiovasc Thorac Surg2015; 20(1): 114-118.
[18] Ro SK, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Mild-tomoderate functional tricuspid regurgitation in patients undergoing mitral valve surgery.J Thorac Cardiovasc Surg2013; 146(5): 1092-1097.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- A hiddenly high hepatitis C virus related liver disease burden among Chinese patients with non-liver disease complaints: A hospital based study from 2013 to 2017
- Visceral leishmaniasis: An immunological viewpoint on asymptomatic infections and post kala azar dermal leishmaniasis
- In vitro fungistatic activity of 36 traditional oriental medicines and their synergistic effect against Trichophyton rubrume
- Antidiabetic effects of galactomannans from Adenanthera pavonina L. in streptozotocin-induced diabetic mice
- Syzygium aromaticum ethanol extract reduces AlCl3-induced neurotoxicity in mice brain through regulation of amyloid precursor protein and oxidative stress gene expression
- Phlebotomus (Adlerius) kabulensis (Diptera: Psychodidae) a new record sand fly species from Iran: Morphological and molecular aspects
