Syzygium aromaticum ethanol extract reduces AlCl3-induced neurotoxicity in mice brain through regulation of amyloid precursor protein and oxidative stress gene expression
2018-03-08SanilaAmberSyedAdnanAliShahTouqeerAhmedSaadiaZahid
Sanila Amber, Syed Adnan Ali Shah, Touqeer Ahmed, Saadia Zahid✉
1Neurobiology Research Laboratory, Department of Healthcare Biotechnology, Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology, Islamabad, Pakistan
2Atta-ur-Rahman Institute for Natural Product Discovery, (AuRIns), Universiti Teknologi MARA Puncak Alam Campus, 42300 Bandar Puncak Alam, Selangor D. E., Malaysia
3Faculty of Pharmacy, Universiti Teknologi MARA Puncak Alam Campus, 42300 Bandar Puncak Alam, Selangor D. E., Malaysia
1. Introduction
Continuous exposure to heavy metals results in several deleterious effects on human health involving oxidative stress which is the root cause of various neurological disorders like Alzheimer’s disease (AD) and related neurological disorders[1]. Aluminum (Al),a potent neurotoxin is widely used in utensils, foils, medicines,food additives, tooth paste and for purification of drinking water[2]. Accumulation of Al in the central nervous system causes mitochondrial functional decline which increases the formation of reactive oxygen species (ROS)[3] and ultimately leads to lipid peroxidation and oxidative damage to DNA and proteins[4].
Al also decreases antioxidant enzyme activity [e.g., catalase (CAT),superoxide dismutase (SOD) and glutathione peroxidase (GSHPx)][5,6]. During oxidative stress free radicals exceed the capacity of antioxidant defense and cause cellular dysfunction, cell membrane degradation and ultimately apoptosis[7]. Protein oxidation by free radicals may cause protein structural and functional disruptions and thereby an age-related functional brain decline[8]. Oxidative stress also precedes the deposition of β-amyloid (Aβ) plaques in AD[9]. The soluble Aβ-protein is constantly expressed in neurons and other cell types and plays a cytoprotective role. However, its over expression leads to plaque formation, cell cycle arrest and cell death[10]. It also interacts with SOD1 (potent ROS scavenger)and decreases its enzymatic activity[11]. The Aβ deposition and plaque formation are associated with elevated levels of amyloid precursor protein isoforms APP751 and APP770 as observed in the AD brain[12]. Increased protein oxidation is observed in the brain regions rich in Aβ[13]. Due to these pathological consequences there is a growing interest in search of potent antioxidants to treat and prevent heavy metals induced oxidative stress and toxicity.Potential of enzymatic and non-enzymatic anti-oxidants is limited by unsolicited effects such as toxicity profile and unwanted interaction with other cellular components. The aforementioned problem emphasizes the prerequisite for elucidation of anti-oxidant potential of natural compounds presenting minimal side effects and minimum to negligible toxicity profile.Syzygium aromaticum[S. aromaticum(Family: Myrtaceae)], commonly known as clove, is an aromatic flower bud used as a spice in Africa, Asia and many other parts of the world[14].S. aromaticumoil contains a variety of chemical constituents with eugenol (79.2%) as its major chemical component.Other important components with significant biological activity include eugenol, caryophyllene, caryophyllene oxide, tanene and humulene[15]. Traditionally, it has been used for culinary purposes and to relieve abdominal discomfort. It possesses antiviral, antiinflammatory, antidiabetic and pain relieving properties[14]. The antinociceptive potential ofS. aromaticumis augmented when administered in combination with ketarol[15]. The pain relieving/antinociceptive effects ofS. aromaticumare possibly mediated via modulation of γ-aminobutyric acid receptors[16].
In addition to its antinociceptive potential, antioxidant and antibacterial properties ofS. aromaticumare also reported[17]. The antifungal activities againstFusariumgraminearumandBipolaris oryzae(commonly infect rice) are reported for clove essential oil that also makes it a potential food preservative[18].
Antiedematogenic properties ofS. aromaticumare also evaluated using carrageenan-generated paw edema mice model.S. aromaticumsubstantially reduced the paw inflammation in carrageenan administered mice; however, exact underlying mechanism for this effect remains elucidative[19].S. aromaticumextract is also rich in flavonoids, which possess anticancer potential assessed in human ovarian cancer cells (A2780)[20]. In addition, it also has the potential to reverse learning and memory deficits[21]. Based on these potential therapeutic activities associated withS. aromaticumextract, the present study aimed at investigating its antioxidant and neuroprotective effects on an AlCl3-induced mouse model of oxidative stress and neurotoxicity.
2. Materials and methods
2.1. Chemicals and reagents
The chemicals used in the experiments include aluminum chloride hexahydrate (AlCl3·6H2O), 2,2-diphenyl-1-picrylhydrazyl (DPPH;Product code: 101087701), electrophoresis chemicals (Sigma Aldrich, Germany), trizol (Invitrogen, USA), reverse transcriptase,deoxynucleotide triphosphate,Taqpolymerase (Fermentas, Thermo Scientific, USA), deuterated chloroform (CDCl3), deuterated methanol (CD3OD) and deuterated dimethyl sulfoxide (DMSO-d6).
2.2. Plant extract preparation
Fresh seeds ofS. aromaticum, which are commonly available, were procured during spring 2014 from local spice market of Rawalpindi,Pakistan and were verified by taxonomist, Dr. Muhammad Qasim Hayat from ASAB, NUST, Islamabad. The plant material specimen was stored in the neurobiology lab for further use and record. The specimen was also submitted to and available at Pakistan Natural History Museum Islamabad (Pakistan) with a specimen voucher no.42569. The extract was prepared usingS. aromaticumseeds (500 g)grinded into fine powder and sieved with 80 mesh sieve. The fine powder (10 g) was loaded into the main chamber of the Soxhlet extractor with distillation flasks containing extraction solvent (100%ethanol). After 24 h, the filtrate was collected and concentrated in rotary evaporator (R200 rotavapour Buchii) with reduced pressure at 68 ˚C to afford a crude extract. The remaining solvent extract if any,was removed by incubation at 37 ˚C and later stored at 4 ˚C until further used.
2.3. In-vitro assay for DPPH free radical scavenging activity
The experimental procedure for DPPH scavenging activity was performed according to the protocol described by [22] with minor modifications. Briefly, samples ofS. aromaticumextract and standard(ascorbic acid) were prepared in different concentrations, ranging from 100 to 0.01 μg/mL respectively. A DPPH solution (0.1 mmol/L)was added to each sample and standard. After incubation at 37 ˚C for 30 min, the absorbance was measured at 517 nm using OPTIMA SP-300 spectrophotometer. The percent antioxidant activity was calculated according to the following equation:
Percent DPPH scavenging activity = (Control absorbance - Sample absorbance)/(Control absorbance)×100.
Half maximal effective concentration for DPPH was calculated by plotting the graph using Microsoft Excel®(Microsoft Inc, USA).Concentration was plotted onx-axis whereas percent inhibition was plotted ony-axis. After that half way value of the inhibition (y) was calculated using following formula.
Half way value (y) = [Maximum inhibtion - (Maximum inhibtion-Minimum inhibtion)]/2.
Then half maximal effective concentration value was calculated using the formula:y=mx+c.
Where,yis half way inhibition, andmandcare constants.
2.4. Proton nuclear magnetic resonance (1H-NMR)spectroscopic analysis
1H-NMR spectrum ofS. aromaticumcrude extract was recorded on Bruker Avance III 500 MHz spectrometer. The chemical shifts(δ) were obtained in ppm. Tetramethylsilane was used as an internal reference standard during analysis of some samples.
2.5. Experimental animals
BALB/c mice were purchased from National Institute of Health(NIH) Islamabad, Pakistan and housed in animal house of Atta ur Rahman School of Applied Biosciences, National University of Sciences and Technology. After acclimatization period of 2 weeks animals were bred and kept in metal cages at constant temperature of(25±2) ˚C and natural light-dark cycle (12/12 h). Animals were given distilled waterad libitumand fed with standard diet consisting of 30% crude protein, 9% crude fat, 4% crude fiber and 10% moisture.Fifty male mice (35-45 g and 10-12 weeks of age) were used in experiments. All experiments performed were in compliance with the rulings of the Institute of Laboratory Animal Research, Division on Earth and Life Sciences, National Institute of Health, USA (NRC,2011). The protocol was approved from the Internal Review Board,Atta-ur-Rahman School of Applied Biosciences, National University of Sciences and Technology.
2.6. Animal treatment
Fifty male BALB/c mice (35-45 g; 10-12 weeks of age) were divided into five groups (n=10). The first group (negative control)was on normal feed and distilled water while the second group(positive control) received AlCl3·6H2O (300 mg/kg p.o) in distilled water with normal feed. Group three, classified as a self-recovery group, received AlCl3·6H2O (300 mg/kg p.o) in distilled water with normal feed for 15 d and later left untreated for 21 d. Group four received AlCl3·6H2O (300 mg/kg p.o) for 15 d followed byS.aromaticumextract (500 mg/kg) mixed in the normal feed for next 21 d. Group five receivedS. aromaticumextract (500 mg/kg) mixed in the normal feed for 21 d.
2.7. Gene expression studies
Animals in all groups were sacrificed after 24 h of the completion of treatment. All mice were anesthetized and euthanized by cervical dislocation. Brain was removed from the skull and placed on an ice cold plate. Initially, cerebellum and olfactory bulbs were removed.Brain was divided into two hemispheres and whole cortex was micro dissected from each hemisphere via forceps subsequently,hippocampus was also dissected out immediately and tissues were snap frozen in liquid nitrogen. Total RNA was isolated according to the manufacturer’s protocol using Tri-reagent (Invitrogen).Qualitative assessment was performed by running RNA samples on 2% agarose gel and visualized by Dolphin-Doc imaging system(Wealtec Corp, USA). Extracted RNA was quantified using BioPhotometer plus (Eppendorf, Germany) and equal quantity of RNA was used to synthesize the cDNA (60 μL). PCR reactions were done using a Swift Maxi Thermal Cycler Block ESCO, Singapore.The thermo cycling conditions were; initial denaturation at 95 ˚C for 5 min, followed by denaturation at 94 ˚C for 30 s. The annealing temperature was set differently for each gene (β-actin, 55 ˚C;APPCommon, 55 ˚C;APP695, 60 ˚C;APP770, 60 ˚C;SOD1, 58 ˚C and Peroxiredoxin 6, 60 ˚C) and then PCR was allowed to complete 35 cycles (30 s/cycle) at 72 ˚C. After that, extension was carried out at 72 ˚C for 10 min. β-actin gene was used as an internal control and was amplified with a specific primer pair (5'-GCC TTC CTT CTT GGG TAT GG-3'/5'-CAG CTC AGT AAC AGTC CGC-3').The specific primer sequences ofAPPCommon,APP695,APP770,SOD1andPrdx6are listed in Table 1. PCR products were separated on 2% agarose gel and visualized by Dolphin-Doc imaging system(Wealtec Corp, USA). The expression of the PCR products was densitometrically analyzed by ImageJ®analysis software.
2.8. Histology: Cresyl violet staining
Cresyl violet staining was performed to study the structural features of neurons. It is used to highlight imperative morphological features of neurons i.e. Nissl bodies or rough endoplasmic reticulum. Briefly,heart perfusion was performed in accordance with the protocol of[23]. Brain was isolated and placed in 4% paraformaldehyde for 24 h followed by paraffin embedding. After that, cortical tissue sections(4 μm) were deparaffinized in xylene for 10 min before being rehydrated by 70% isopropanol (10 min), and washed with ddH2O (5 min). Cresyl violet stain was poured over the tissues sections and left for 4 min. The sections were then washed with ddH2O and 70% acid alcohol (2 min) and later dried for 2 h and mounted with cover slips.The sections were visualized using an inverted microscope at 10 ×and 40 × resolutions; images were captured by Pixel Pro™image analysis software (Labomed, USA). The quantitative analysis for cell count was carried out in an area of 10 000 μm2(100 μm × 100 μm box) from three randomly selected sites. Following that, average values were calculated and plotted[24].
2.9. Statistical analysis
Statistical analysis of data was performed using one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test using GraphPad Prism 5.0. The data are represented as mean±SEM with a 95% confidence interval. AP-value<0.05 was considered statistically significant.

Table 1Primer sequences used for expression analysis of SOD1, Prdx6, APP Common, APP770 and APP695.
3. Results
3.1. DPPH assay for free radical scavenging activity
DPPH assay was performed to assess the free radical quenching activity ofS. aromaticumextract. Results ofin-vitroassay indicated thatS. aromaticumextract has strong potential to inhibit DPPH free radicals in dose dependent manner. The results were consistent with standard antioxidant (ascorbic acid) (Figure 1).

Figure 1. In-vitro DPPH assay of S. aromaticum extract.
3.2. Phytochemical analysis of S. aromaticum extract
The high-resolution1H-NMR spectra of ethanolic extract ofS.aromaticumseeds were recorded in DMSO-d6, revealed several polar compounds such as flavonoids (quercetin), tannins and saponins(Figure 2). Saponins consist of glucose moiety associated with triterpenoid ring at C-3 position.
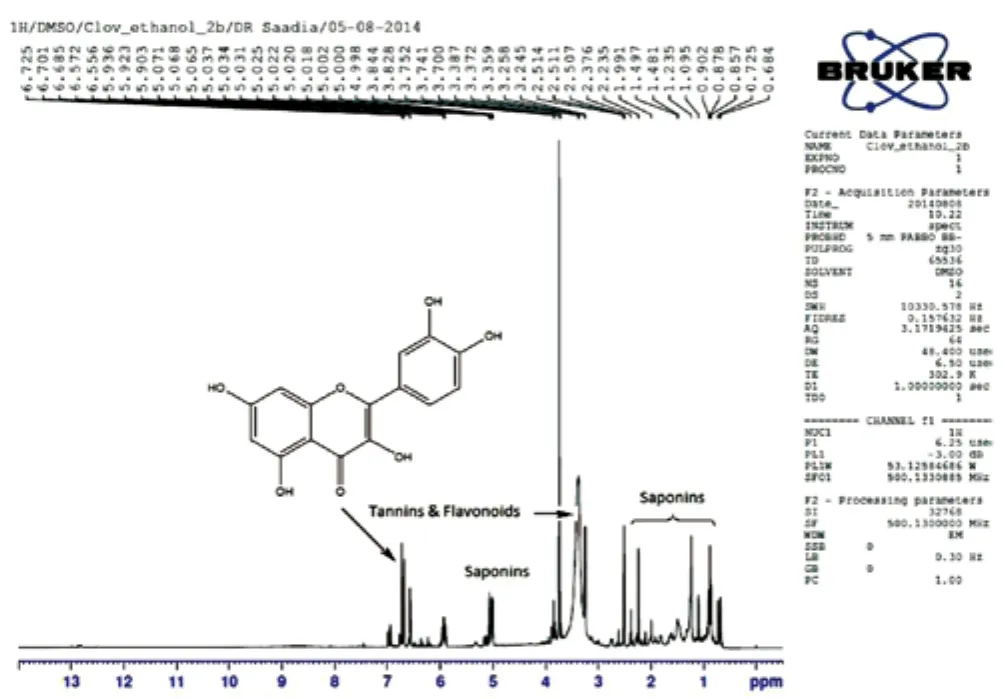
Figure 2. 1H-NMR spectrum of S. aromaticum extract.
3.3. Histopathological assessment of cresyl violet stained cortical sections
A marked reduction in Nissl substances was observed in the AlCl3·6H2O-treated and self-recovery group as compared to the negative control (P<0.05).S. aromaticumtreatment post AlCl3exposure showed a significant increase in number of Nissl bodies(P<0.05). On comparison of the self-recovery group with the positive control, no significant difference was found. Shrinkage of cell bodies was evident in the AlCl3·6H2O-treated groups (Figure 3).
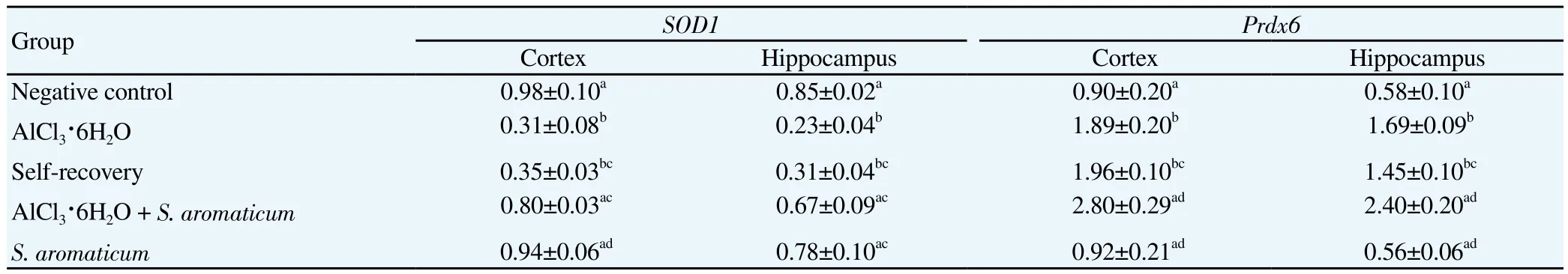
Table 2Results of gene expression analysis of oxidative stress markers, SOD1 and Prdx6 (mean±SEM).

Table 3Results of gene expression analysis of amyloid precursor protein isoforms, APP695 and APP770 (mean±SEM).
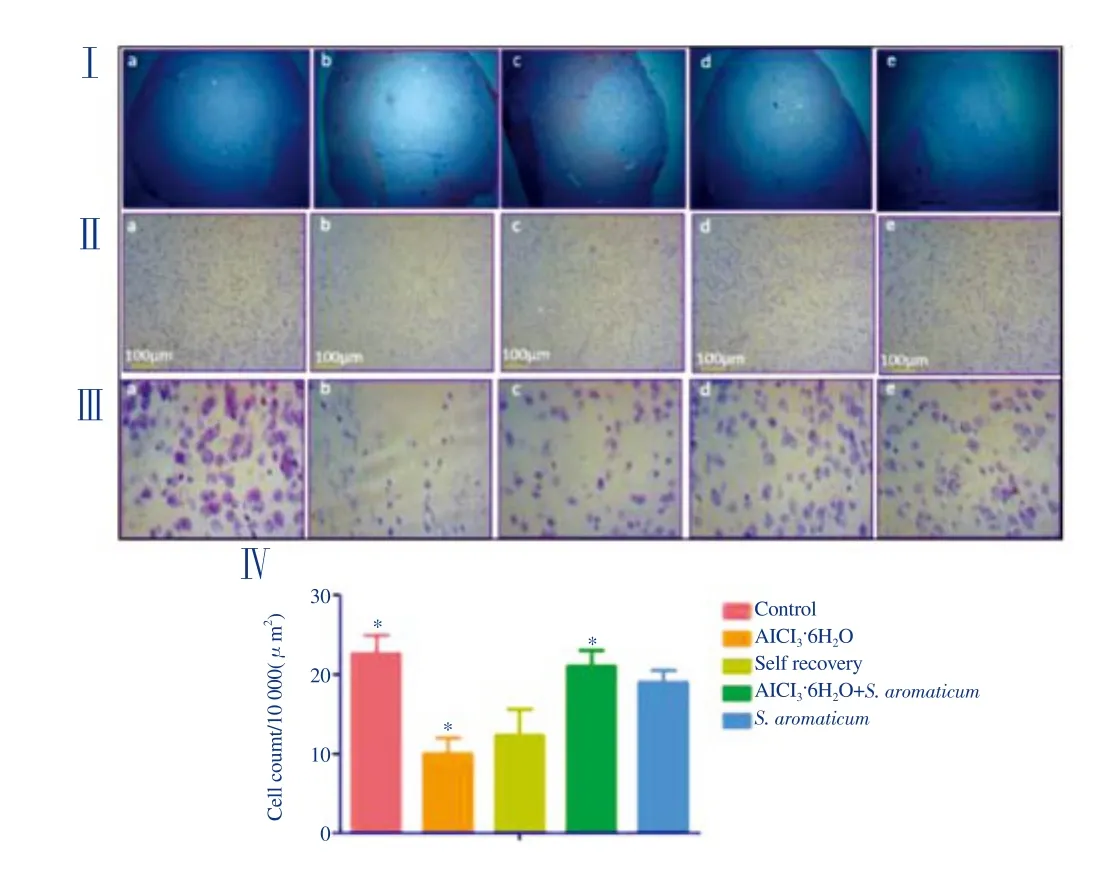
Figure 3. Nissl stained sections of mice cortex.
3.4. Effect of AlCl3·6H2O and S. aromaticum extract on SOD1 and Prdx6 expression
Significant differences in the expression level of oxidative stress markers, i.e.,SOD1andPrdx6were observed in both the regions(Table 2). The expression level ofSOD1significantly decreased following AlCl3·6H2O as compared to the negative control (P<0.05 cortex;P<0.05 hippocampus). The group treated with AlCl3·6H2O followed byS. aromaticumextract treatment showed significantly normalized expression ofSOD1gene (P<0.05 cortex;P<0.05 hippocampus). There was no significant difference in the expression ofSOD1between the negative control and theS. aromaticum-treated group as well between the self-recovery and the AlCl3·6H2O-treated group. On the other hand, exposure with AlCl3·6H2O significantly raised the expression ofPrdx6in both the regions of the treated groups (P<0.05 cortex;P<0.05 hippocampus). Interestingly,treatment withS. aromaticumfollowing AlCl3·6H2O exposure further increased its expression (P<0.05 cortex;P<0.05 hippocampus) as compared to the negative control. However, no significant difference in thePrdx6expression was observed between the negative control and theS. aromaticum-treated group. Similarly, the self-recovery and the AlCl3·6H2O-treated groups revealed no significant differences in the expression levels ofPrdx6.
3.5. Effect of AlCl3·6H2O and S. aromaticum extract on APP isoforms
Gene expression analysis ofAPPisoforms (APPCommon,APP770,APP695) was performed by semi quantitative PCR to determine the effect ofS. aromaticumextract on AlCl3·6H2O-induced neurotoxicity. The expression level of cortical and hippocampalAPPcommon gene did not show variation in expression for any of the tested groups. A significant decrease (P<0.05) in expression was observed forAPP695in mice cortex treated with AlCl3·6H2O when compared with the negative control. Whereas, in the group treated withS. aromaticumpost AlCl3·6H2O exposure, there was a significant increase in theAPP695expression (P<0.05) which was approximately similar to the negative control (Table 3). The group treated withS. aromaticumshowed no variation in its level of expression as compared to the negative control. The effects of AlCl3·6H2O onAPP695expression were similar in the self-recovery group and the AlCl3·6H2O-treated group. A similar expression pattern ofAPP695was observed in the hippocampus in all the experimental groups, indicating a potential positive effect ofS.aromaticumtreatment.
Expression analysis ofAPP770showed a remarkable increase in the AlCl3·6H2O-treated and the self-recovery group as compared to the negative control (P<0.05 cortex;P<0.05 hippocampus). TheS. aromaticumtreatment post AlCl3·6H2O exposure significantly normalized the altered expression ofAPP770(P<0.05 cortex;P<0.05 hippocampus) (Table 3). No significant difference was observed between the negative control andS. aromaticum-treated group.
4. Discussion
A number of studies reported the neurotoxic effects of Al and its association with cognitive impairment, Aβ accumulation and oxidative stress in various neurological disorders[25-28]. Al impairs the integrity and permeability of blood brain barrier and facilitates iron-mediated oxidative reactions[29,30]. Our findings showed that Al exacerbates the expression levels of antioxidant enzymes and amyloid precursor protein isoforms. On the other hand, the administration ofS. aromaticumextract (500 mg/kg) to Al fed mice showed a reversal of the deleterious effects of Al. This shows potential protective action ofS. aromaticumagainst neurotoxicity.The free radical quenching activity ofS. aromaticumextract was confirmed by applying a DPPH assay and its1H-NMR analysis showed some polar and potent chemical entities including flavonoids such as quercetin, tannins and saponins.
Flavonoids exert a protective effect by directly scavenging ROS,by activating antioxidant enzymes[31] or through metal chelating activity[32]. In our study, a significant increase (P<0.05 cortex;P<0.05 hippocampus) in the expression level ofSOD1followingS. aromaticumextract treatment can be attributed to the potential antioxidant properties of the flavonoids identified inS. aromaticumextract.
Quercetin, a potential flavonoid, known for its iron-chelating and stabilizing properties[33], is suggested to play an important role in protection against oxidative damage. It significantly increases the activities of SOD1 and GSH-Px, and markedly reduces the level of malondialdehyde and may ameliorate brain damage. Quercetin inhibits free radical generation by enhancing the activity of endogenous antioxidant enzymes; thus providing neuroprotection[34].Quercetin also provides resistance against neurodegeneration by reducing microglial killing of damaged neurons[35]. Based on the findings of our study, we therefore proposed that the improved expression of the antioxidant enzymes may possibly be due to the metal chelating abilities of quercetin present in theS. aromaticumextract.
Tannin is another major chemical constituent identified during this study. It has the potential to alleviate a sodium fluoride-induced oxidative state in the brain, and is suggested to be more effective at retrieving SOD activity than vitamin C[36]. Our results showed that there is substantial decrease inSOD1expression following AlCl3·6H2O exposure while theS. aromaticumextract significantly increasedSOD1expression in both the cortex and hippocampus(P<0.05 cortex;P<0.05 hippocampus). Self-recovery of mice against AlCl3-induced toxicity was also assessed but it was not promising,and made us conclude that although the body is no longer exposed to oxidative insult, it is still unable to cope with the consequences of the insult and an exogenous source of antioxidants is required.Saponins (steroid or triterpenoid glycosides) identified in theS.aromaticumethanolic extracts are common to a large number of plants and their products, and are an important component of human and animal nutrition[37]. A group of saponins containing 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP), (an antioxidant moiety attached at C23) allows saponins to scavenge super oxides thus preventing cellular damage by ROS[38].
The cerebral cortex and hippocampus are severely affected by Al as compared to other areas of the central nervous system with marked histopathological alterations, including neuronal degeneration[39-41]. The histopathological assessment of cortex of the AlCl3·6H2O-treated group showed aberrations in neuronal structure. The marked difference in the density of Nissl granules among different groups is suggestive of the fact that Al toxicity may led to degeneration of neurons resulting in a decreased content of Nissl bodies (P<0.05). These results are in accordance with previously published studies wherein AlCl3caused significant decrease in the number of nissl granules[24,42]. The non-significant difference found in the density of Nissl bodies between AlCl3·6H2O-treated group and self-recovery group indicates that the damage caused is irreversible and the body/brain itself cannot overcome the deleterious effects of Al toxicity. However, promising observations were noted for the tissue sections treated withS. aromaticum, which showed a marked increase in the density of Nissl substances as compared to the AlCl3-treated group (P<0.05). This revealed its potential neuroprotective role. Our results vividly demonstrate that Al exposure is involved in neurodegeneration and oxidative stress while administration ofS. aromaticumcan counteract the damage inflicted by Al in the brain of mice.
The expression level of potent antioxidant enzyme Prdx6 was substantially higher in the AlCl3·6H2O-treated group as compared to the negative control group (P<0.05 cortex;P<0.05 hippocampus).This over expression could be due to the resistance produced against membrane damage associated with phospholipid peroxidation during the early stages of oxidative stress[43]. Interestingly, treatment withS. aromaticumfurther increases the concentration of enzyme which may help it to cope with oxidative stress (P<0.05 cortex;P<0.05 hippocampus). While the group of mice treated withS. aromaticum(without prior treatment to Al) did not show any significant difference in the expression ofPrdx6as compared to the negative control group.
We have also examined the expression ofAPPcommon following Al andS. aromaticumextract administration; results indicated that theAPPcommon expression level did not vary significantly among the groups in both the cortex and hippocampus. This shows that although the concentration of the different isoforms varies among the groups, overall expression ofAPPremains the same in accordance with an earlier study[44]. APP isoforms plays a critical role in memory formation, synaptic plasticity and brain development.Among the various APP isoforms,APP695is abundantly present in cerebral cortex and some other brain regions[45]. Increased expression ofAPP695was observed in negative control which is suggestive of its neuroprotective effects. However, the expression ofAPP695was significantly decreased in the AlCl3·6H2O-treated group(P<0.05 cortex;P<0.05 hippocampus). This decreasedAPP695expression in the AlCl3-treated group is concomitant with neuronal loss in very same group as APP695 resides inside the neurons[46].TheS. aromaticumextract significantly increased the expression ofAPP695, which indicates its neuroprotective features (P<0.05 cortex;P<0.05 hippocampus).
Moreover, there was a high level ofAPP770expression in the cortex and hippocampus of AlCl3·6H2O-induced oxidative stress mice as compared to the negative control (P<0.05 cortex;P<0.05 hippocampus). Previously, Iqbalet al. has reported similar effects of AlCl3·6H2O administration onAPP770expression levels in mice brain cortex, hippocampus and amygdala[46]. These results demonstrate that Al exerts neurotoxic effects in brain via up regulation of amyloidogenicAPP770gene expression. Decreased expression ofAPP770gene expression is observed inS. aromaticumtreated mice. It has been reported that quercetin exhibits protective effects against Aβ-induced toxicity on both neurons and endothelial cells by reducing the production of prostaglandin E2 in response to amyloid-β1-42[47]. Tannins have potent antioxidant motifs that could bind specifically to Aβ fibrils and inhibit fibril formation and/or destabilize preformed Aβ fibrils through mechanisms yet unknown[48]. Based on the findings from our study it is proposed that the decreased expression ofAPP770inS. aromaticum-treated mice bodes well for the capacity of active phytochemicals like flavonoids,tannins and saponins to combat Aβ related neurotoxicity. This proves its therapeutic potential to intervene against neurodegenerative disorders.
This study reveals that Al has strong potential to exert oxidative stress in the brain as well as having deleterious effects on the neurons leading to neurodegeneration. The neuroprotective role ofS. aromaticum, by preventing AlCl3-induced neurotoxicity in the studied brain regions, is an important finding of the present work. In addition to stabilizing the histopathological alterations,S.aromaticumalso showed promising results in normalizing the altered levels of oxidative stress markers and amyloid precursor protein isoforms. However, further characterization of the compounds ofS. aromaticumextract is required to determine the active principle and to determine the exact mechanism by which the phytochemicals exert pharmacological and neuroprotective effects.
Author contribution
S.Z., substantial contribution to conception and design of the study and finalization of the manuscript; S.A.A.S., substantial contribution of acquisition and interpretation of1H NMR data; T.A., analysis of gene expression data, histological assessment; S.A., all experimental work, analysis and interpretation of data, drafting the article. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
The work was supported by research grant by National University of Sciences and Technology (NUST), Islamabad, Pakistan. We thank taxonomist Dr. Muhammad Qasim Hayat, expert in medicinal plant chemistry, Plant Biotechnology Department, ASAB, NUST, for verifying plant specimen and providing laboratory facilities for plant extract preparation. We also thank Prof. Dr. Thomas Nugent, Jacobs University, Bremen Germany, for proof reading the manuscript.
[1] Jansson ET. Aluminum exposure and Alzheimer's disease.J Alzheimer's Dis2001; 3(6): 541-549.
[2] Bondy SC. The neurotoxicity of environmental aluminum is still an issue.Neurotoxicology2010; 31(5): 575-581.
[3] Pratico D, Uryu K, Sung S, Tang S, Trojanowski JQ, Lee VMY.Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice.FASEB J2002; 16(9): 1138-1140.
[4] Praticò D, Uryu K, Leight S, Trojanoswki JQ, Lee VMY. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis.J Neurosci2001; 21(12): 4183-4187.
[5] Julka D, Gill KD. Altered calcium homeostasis: a possible mechanism of aluminium-induced neurotoxicity.Biochim Biophys Acta1996; 1315(1):47-54.
[6] Yousef MI. Aluminium-induced changes in hemato-biochemical parameters, lipid peroxidation and enzyme activities of male rabbits:protective role of ascorbic acid. Toxicology2004; 199(1): 47-57.
[7] Lucca G, Comim CM, Valvassori SS, Réus GZ, Vuolo F, Petronilho F,et al. Effects of chronic mild stress on the oxidative parameters in the rat brain.Neurochem Int2009; 54(5): 358-362.
[8] Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing.Nature2000; 408(6809): 239-247.
[9] Luque-Contreras D, Carvajal K, Toral-Rios D, Franco-Bocanegra D,Campos-Pena V. Oxidative stress and metabolic syndrome: Cause or consequence of Alzheimer's disease?Oxid Med Cell Longev2014; 2014:497802.
[10] Adler MJ, Coronel C, Shelton E, Seegmiller J, Dewji NN. Increased gene expression of Alzheimer disease beta-amyloid precursor protein in senescent cultured fibroblasts.Proc Natl Acad Sci1991; 88(1): 16-20.
[11] Yoon EJ, Park HJ, Kim GY, Cho H, Choi JH, Park HY, et al. Intracellular amyloid beta interacts withSOD1and impairs the enzymatic activity ofSOD1: implications for the pathogenesis of amyotrophic lateral sclerosis.Exp Mol Med2009; 41(9): 611-617.
[12] Menendez-Gonzalez M, Perez-Pinera P, Martinez-Rivera M, Calatayud M, Blazquez MB. APP processing and the APP-KPI domain involvement in the amyloid cascade.Neurodegener Dis2005; 2(6): 277-283.
[13] Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M,et al. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation.J Neurochem1995;65(5): 2146-2156.
[14] Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): A precious spice.Asian Pac J Trop Biomed2014; 4(2): 90-96.
[15] Beltran-Villalobos KL, Déciga-Campos M, Aguilar-Mariscal H,González-Trujano M, Martínez-Salazar MF, de los Ángeles Ramírez-Cisneros M, et al. Synergistic antinociceptive interaction ofSyzygium aromaticumorRosmarinus officinaliscoadministered with ketorolac in rats.Biomed Pharmacother2017; 94: 858-864.
[16] Sahin S, Eulenburg V, Heinlein A, Villmann C, Pischetsrieder M.Identification of eugenol as the major determinant of GABA A-receptor activation by aqueousSyzygium aromaticumL.(clove buds) extract.JFunct Foods2017; 37: 641-649.
[17] El-Maati MF, Mahgoub SA, Labib SM, Al-Gaby AM, Ramadan MF.Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities.Eur J Integr Med2016; 8(4): 494-504.
[18] Santamarina MP, Roselló J, Giménez S, Blázquez MA. CommercialLaurus nobilisL. andSyzygium aromaticumL. Merr. & Perry essential oils against post-harvest phytopathogenic fungi on rice.LWT-Food Sci Technol2016; 65: 325-332.
[19] Taher YA, Samud AM, El-Taher FE, ben-Hussin G, Elmezogi JS, Al-Mehdawi BF, et al. Experimental evaluation of anti-inflammatory,antinociceptive and antipyretic activities of clove oil in mice.Libyan J Med2015; 10(1): 28685.
[20] Ryu B, Kim HM, Lee JS, Lee CK, Sezirahiga J, Woo JH, et al. New flavonol glucuronides from the flower buds ofSyzygium aromaticum(Clove).J Agric Food Chem2016; 64(15): 3048-3053.
[21] Halder S, Mehta AK, Kar R, Mustafa M, Mediratta PK, Sharma KK.Clove oil reverses learning and memory deficits in scopolamine-treated mice.Planta Med2011; 77 (8): 830-834.
[22] Singh R, Singh N, Saini B, Rao HS.In vitroantioxidant activity of pet ether extract of black pepper.Indian J Pharmacol2008; 40(4): 147-151.
[23] Gage GJ, Kipke DR, Shain W. Whole animal perfusion fixation for rodents.J Vis Exp2012; (65): e3564.
[24] Farhat SM, Mahboob A, Ahmed T. Cortex and amygdala-dependent learning and nicotinic acetylcholine receptor gene expression is severely impaired in mice orally treated with AlCl3.Biol Trace Elem Res2017; 18:1-11.
[25] Borsani E, Ricci F, Favero G, Bianchi R, Foglio E, Rodella L, et al.Aluminium exposure induces Alzheimer s disease-like histopathological alterations in mouse brain.Histol Histopathol2008; 23(4): 433-439.
[26] Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases.Front Cell Neurosci2015; 9: 124-146.
[27] Thenmozhi AJ, Raja TRW, Janakiraman U, Manivasagam T.Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats.Neurochem Res2015; 40(4): 767-776.
[28] Tripathi S, Mahdi AA, Nawab A, Chander R, Hasan M, Siddiqui MS,et al. Influence of age on aluminum induced lipid peroxidation and neurolipofuscin in frontal cortex of rat brain: A behavioral, biochemical and ultrastructural study.Brain Res2009; 1253: 107-116.
[29] Song Y, Xue Y, Liu X, Wang P, Liu L. Effects of acute exposure to aluminum on blood-brain barrier and the protection of zinc.Neurosci Lett2008; 445(1): 42-46.
[30] Zatta P, Ibn-Lkhayat-Idrissi M, Zambenedetti P, Kilyen M, Kiss T.In vivoandin vitroeffects of aluminum on the activity of mouse brain acetylcholinesterase.Brain Res Bull2002; 59(1): 41-45.
[31] Nijveldt RJ, Van Nood E, Van Hoorn DE, Boelens PG, Van Norren K,Van Leeuwen PA. Flavonoids: A review of probable mechanisms of action and potential applications.Am J Clin Nutr2001; 74(4): 418-425.
[32] Ferrali M, Signorini C, Caciotti B, Sugherini L, Ciccoli L, Giachetti D, et al. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity.FEBS Lett1997; 416(2): 123-129.
[33] Szelag A, Magdalan J, Kopacz M, Kuzniar A, Kowalski PA, Piesniewska M. Assessment of efficacy of quercetin-5'-sulfonic acid sodium salt in the treatment of acute chromium poisoning: Experimental studies.Pol J Pharmacol2003; 55(6): 1097-1104.
[34] Dong Y, Wang J, Feng D, Qin H, Wen H, Yin Z, et al. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage.Int J Med Sci2014; 11(3): 282-290.
[35] Bate C, Salmona M, Williams A. Ginkgolide B inhibits the neurotoxicity of prions or amyloid-β 1-42.J Neuroinflammation2004; 1(1): 4-12.
[36] Nabavi SF, Habtemariam S, Jafari M, Sureda A, Nabavi SM. Protective role of gallic acid on sodium fluoride induced oxidative stress in rat brain.Bull Environ Contam Toxicol2012; 89(1): 73-77.
[37] Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: A review.Br J Nutr2002; 88(6): 587-605.
[38] Yoshiki Y, Kudou S, Okubo K. Relationship between chemical structures and biological activities of triterpenoid saponins from soybean.Biosci Biotechnol Biochem1998; 62(12): 2291-2299.
[39] Matyja E. Aluminum enhances glutamate-mediated neurotoxicity in organotypic cultures of rat hippocampus.Folia neuropathol2000; 38(2):47-53.
[40] Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: A review of progress.J Neurol Neurosurg Psychiatry1999; 66(2): 137-147.
[41] Silva AF, Aguiar MSS, Carvalho OS, Luana de Nazaré SS, Franco EC, Lima RR, et al. Hippocampal neuronal loss, decreased GFAP immunoreactivity and cognitive impairment following experimental intoxication of rats with aluminum citrate.Brain Res2013; 1491: 23-33.
[42] Ahmed S, Mahmood Z, Javed A, Hashmi SN, Zerr I, Zafar S, et al. Effect of metformin on adult hippocampal neurogenesis: Comparison with donepezil and links to cognition.J Mol Neurosci2017; 62(1): 88-98.
[43] Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin,functions in antioxidant defense and lung phospholipid metabolism.Free Radical Biol Med2005; 38(11): 1422-1432.
[44] Bordji K, Becerril-Ortega J, Nicole O, Buisson A. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-β production.J Neurosci2010; 30(47): 15927-15942.
[45] Nalivaeva NN, Turner AJ. The amyloid precursor protein: A biochemical enigma in brain development, function and disease.FEBS letters2013;587(13): 2046-2054.
[46] Iqbal G, Iqbal A, Mahboob A, Farhat SM, Ahmed T. Memory enhancing effect of black pepper in the AlCl3induced neurotoxicity mouse model is mediated through its active component chavicine.Curr pharm biotechnol2016; 17(11): 962-73.
[47] Liu R, Zhang TT, Zhou D, Bai XY, Zhou WI, Huang C, et al. Quercetin protects against the Aβ 25-35-induced amnesic injury: involvement of inactivation of rage-mediated pathway and conservation of the NVU.Neuropharmacology2013; 67: 419-431.
[48] Ono K, Hasegawa K, Naiki H, Yamada M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer's β-amyloid fibrilsin vitro.Biochimica Biophysica Acta2004; 1690(3): 193-202.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- A hiddenly high hepatitis C virus related liver disease burden among Chinese patients with non-liver disease complaints: A hospital based study from 2013 to 2017
- Visceral leishmaniasis: An immunological viewpoint on asymptomatic infections and post kala azar dermal leishmaniasis
- In vitro fungistatic activity of 36 traditional oriental medicines and their synergistic effect against Trichophyton rubrume
- Antidiabetic effects of galactomannans from Adenanthera pavonina L. in streptozotocin-induced diabetic mice
- Phlebotomus (Adlerius) kabulensis (Diptera: Psychodidae) a new record sand fly species from Iran: Morphological and molecular aspects
- Effect of RNA interference on WD101 gene of Schistosoma japonicum
