Antidiabetic effects of galactomannans from Adenanthera pavonina L. in streptozotocin-induced diabetic mice
2018-03-08caroGusmPintoVieiraFranciscaNoliaPereiraMendesSabrinasardaSilvaRaquelTeixeiraTerceiroPaimBrunoBezerradaSilvaStephenRathinarajBenjaminEridanOrlandoPereiraTramontinaFloreanMariaIzabelFlorindoGuedes
Ícaro Gusmão Pinto Vieira, Francisca Noélia Pereira Mendes, Sabrina César da Silva, Raquel Teixeira Terceiro Paim, Bruno Bezerra da Silva, Stephen Rathinaraj Benjamin✉, Eridan Orlando Pereira Tramontina Florean, Maria Izabel Florindo Guedes
1Technological Development Park (PADETEC), Federal University of Ceará, Pici Campus, 60455-970, Fortaleza, Ceará, Brazil
2Department of Health and Nutrition, State University of Ceará, Av. Dr. Silas Munguba 1700, Itaperi campus, 60714-903, Fortaleza, Ceará, Brazil
1. Introduction
Diabetes mellitus (DM) is a chronic disease associated with metabolic dysfunction, generally followed by severe complications,which results in loss of life quality and premature mortality[1].Hyperglycemia is the main DM symptom, and is responsible for tissue damage and vascular complications, which limits professional performance in diverse degrees and generats a social cost beyond the just of the outpatient. In 2015, according to the International Diabetes Federation, the diabetic population was 415 million people worldwide, with a prospect of increasing to 642 million by the year 2040.
Therapeutic treatments include commercial insulin and oral hypoglycemic that help to control the patients glycaemia, however physical activity and healthy nourishment are important to their life quality. Among the nutrients highly relevant for the treatment of DM,dietary fibers, especially soluble fibers, stand out and represent an important part of a healthy diet because of their action in increasing peripheral insulin sensitivity and reducing blood lipid levels[2].
Galactomannans are soluble fibres, commonly found in the seeds of Fabaceae, although they may be obtained from other plants and fungi[3]. Their dietary consumption has hypoglycemic and hypocholesterolemic potential.
Adenanthera pavonina(A. pavonina) (Fabaceae family, subfamily Mimosoideae, known as dragon’s eye) seeds are a promising source for galactomannans in Brazil, because, in addition to its richness in this polysaccharide[4], this plant is well adapted to Brazilian cultivation conditions[5]. Beacause of their chemical structures silmilar to galactomannans used in glycemic and metabolic DM control - guar gum and fenugreek[6], galactomannans fromA.pavoninahave potential to serve as a soluble fibre supplement for diabetic patients.
The objective of this study was to characterize and evaluate the effect of galactomannans extracted fromA. pavonina’s L. seeds(GAP) on diabetic mice’s glycaemia.
2. Materials and methods
2.1. Drugs and chemicals
Streptozotocin (STZ) was supplied by Sigma, Co (St Louis, MO,USA). Metformin hydrochloride was purchased from Medley Pharmaceutical, Co (Campinas, SP, Brazil). Total cholesterol(TC) and triacylglycerol (TAG) were determined by an enzymatic and colorimetric method using a commercial kit (Bioclin, Belo Horizonte, MG, Brazil). All other reagents of analytical grade were purchased locally.
2.2. Isolation and purification of galactomannans
A. pavoninaseeds were collected in Fortaleza/Ceará/Brazil between September to October in 2014. The identification of the botanical species was performed by Herbarium Prisco Bezerra of the Federal University of Ceará, and deposited under number 56964. The isolation procedure of galactomannans from the seeds ofA. pavoninawas performed according to Vieiraet al[7]. Initially,150 g (seeds) were boiled in 500 mL distilled water for 2 h. After 12 h resting at 24 ℃, the endosperm was removed and lyophilized.For galactomannan’s extraction, 2.5 g of the lyophilized endosperm were submitted to four consecutive extractions with water. At each extraction, 100 mL of distilled water at 80 ℃ for 4 h was used.After this procedure, the aqueous extract was vacuum-filtered with polyester net. The material retained in the net was submitted again to the extraction process repeatedly until the galactomannans were fully extracted. At the end of the aqueous extractions, the material obtained was filtered through a celite layer, frozen and lyophilized.
2.3. Moisture, ash, protein and carbohydrate content
Moisture and ash content were determined according to methods described by Instituto Adolfo Lutz (2008)[8]. Total carbohydrate content was quantified using the method reported by DuBois, Gilles,Hamilton, Rebers, & Smith (1956)[9] and protein content was determined according to Bradford (1976)[10].
2.4. Molar weight estimation through gel permeation chromatography
Gel permeation chromatography was performed in a SHIMADZU LC-10AD chromatograph with refraction index detector RID-10A at 40 ℃. A linear type ultrahydrogel 250 column (waters) with 7.8 mm × 300 mm was used, with a mobile phase of sodium nitrate solution (0.1 mol/L), and a flow rate 0.5 mL/min, a sample volume of 20 μL ran through the column. GAP sample was solubilized in 1.0 mL deionized water, resulting in a 1 mg/mL solution that was then filtered in a 0.45 μm Millipore® membrane. Pullulan (Shodex Denko®) samples of molecular weight (Mw) 5.90 × 103g/mol,4.73× 104g/mol , 1.12 × 105g/mol, 4.04 × 105g/mol and 7.88 × 105g/ mol were used as molecular standard[11].
2.5. GAP monosaccharide content
GAP monosaccharide content was determined through its hydrolysis[12], followed by thin layer chromatography (TLC)analysis. In a silica gel 60 TLC chromatoplate, 4 μL of mannose and galactose standard solutions and hydrolyzed galactomannans at 10 mg/mL were applied. After elution with an acetic acid glacial,chloroform: Water as mobile phase (74:65:11 v/v), the plate was dried at 100 ℃ for 2 min and pulverized with orcinol-sulfuric acid spray reagent for detection.
2.6. Nuclear magnetic resonance (NMR) (1H-NMR) and mannose: Galactose ratio determination
GAP sample (20 mg/mL) was dissolved in deuterium oxide (D2O)and the solution was put in a 5 mm NMR tube at 85 ℃ under constant agitation. Proton resonance spectra were obtained in an Avance DRX-500 Bruker Spectrometers® operating in the hydrogen frequency, at 500 MHz. The spectra were registered at 85 ℃, and for its acquisition, 5 s relaxation periods and 30 pulse sequences were used[13].
2.7. Fourier transformation infrared absorption
The infrared spectra were obtained in a Perkin-Elmer 1320 Spectrometers®, with wavelengths of 400 cm-1to 4 000 cm-1. The GAP sample was analysed in KBr pellets[13].
2.8. Experimental animals
Fifty female Swiss mice (25 g - 30 g with ages ranging from 8 wk - 12 wk) were kept at ± 22 ℃, with 12/12 light/dark cycle[14].All animals were kept in Polyacrylic cages and received water and foodad libitum. The experimental procedure performed on animals was in accordance with the policy of the Institutional Animal Ethical Committee of the State University of Ceará (UECE) (No.0874146/2015.).
2.9. Diabetes induction
Diabetes was induced with an intraperitoneal injection of 120 mg/ kg of STZ (Sigma®) in aqueous solution, at 24 mg/mL. After 7d, diabetes was confirmed by measuring the serum glucose level.Only mice whose post-induction glycaemia was above 200 mg/dL were considered diabetic[15].
2.10. Experimental design
Animals were distributed into five groups (n=10): NC group- normal control (healthy mice); DC Group - Diabetic control(diabetic mice); GAP 1% group - diabetic mice and treated with 1%GAP enriched food; GAP 2% group - diabetic mice and treated with 2% GAP enriched food; MET group - diabetic mice treated with metformin® (200 mg/kg).
2.11. Food preparation
The standard animal food used was from Presence Animal Nutrition®, purchased at a local market. Standard food presents 13%moisture, 23% total protein, 4% ethereal extract, 6% fibre and 10%mineral matter content. GAP 1% and GAP 2% were produced from crushed standard food with 1% (w/w) and 2% (w/w) GAP addition,respectively. After homogenization, food with GAP added was compressed into pellets and dried at 50 ℃ for 48 h.
2.12.Body weight water and food ingestion
The animals had their weight measured on the day before treatment, considered as day 0, 21,and 28 of treatment. Water and food ingestion were measured weekly during the 30 d of treatment.
2.13. Biochemical parameters analysis
Blood samples were collected on day 0, 21 and 30 of the treatment for evaluation of fasting glycaemia. TC and TAG were evaluated at the end of treatment. These biochemical parameters were analysed through enzymatic and colorimetric techniques using commercial Bioclin® specific kits.
2.14. Toxicity evaluation
Toxicity study was performed by relative liver weight (RLW)comparisons between groups and a long time and histological cuts from the animal’s pancreas and liver. After being euthanised, the animal’s organs were weighed, fixed with 10% formaldehyde for 24 h and treated with microscope slides preparation according to Michanaly[16].
2.15. Statistical analysis
Data were demonstrated as a mean ± standard deviation. Analysis of variance followed by a performance of Newman-Keuls test, as aP<0.05 being significant. GraphPad Prism® 6.0 software was used in the application of tests and graphic presentation.
3. Results
3.1. Isolation and characterization of galactomannan from the A. pavonina
The product of the extraction was a galactomannan free from insoluble fibres and with clear aspect in solution. A total of 11.00% yield of galactomannans was obtained in relation to seed total weight followed by endosperm/seed (13.83%) and GAP/endosperm (80.10%). The galactomannans were determined and the results revealed that the moisture percentage and ash content were 13.87% and 3.20%, respectively. For macronutrients, the values were 72.290% and 0.034% for carbohydrate and proteins respectively. High carbohydrate and low protein content confirmed the high purity of the extracted material. TLC showed that GAP was constituted of mannose and galactose unities. Gel permeation chromatography analysis presented a molar weight (Mw) for GAP of 1.45 × 107g/mol. The1H NMR spectrum was 500 MHz,A. pavoninaL. galactomannan and its anomeric region in the spectrum were shown in Figure 1. The resonances of the anomeric protons were well separated, and their identification was self-evident from the known monomeric compositions of the samples. Through1H NMR spectra interpretation, the M/G (D-mannose/D-galactose) ratio was calculated, resulting in a 1.46:1.00 value. By analyzing the1H NMR spectrum of galactomannan and expansion, it could be confirmed that the galacto pyranosyl obtainedA. pavoninaL. signals around were related to alpha-D-galacto pyranosyl: H-1, 5.02 ppm (G1);H-2, 3.80 ppm; H-3, 3.92 ppm; H-4, 4.00 ppm; H-5,3.89 ppm, and H-6, 3.76 ppm, and the beta -D-manopyranosyl, H-1, 4.74 ppm; H-2,4.12 ppm; H-3, 3.79 ppm; H-4, 3.89 ppm; H-5,3.54 ppm (M5), and H-6, 3.90 ppm. The IR result showed that GAPs (Figure 2) presented relative absorption bands to hydroxyl groups (3 461 cm-1), alkane groups (2 933 cm-1), C-O and C-O-H groups (1 145 and 1 043 cm-1)and D-galactopyranose unities (810 cm-1).

Figure 1. Spectra result.
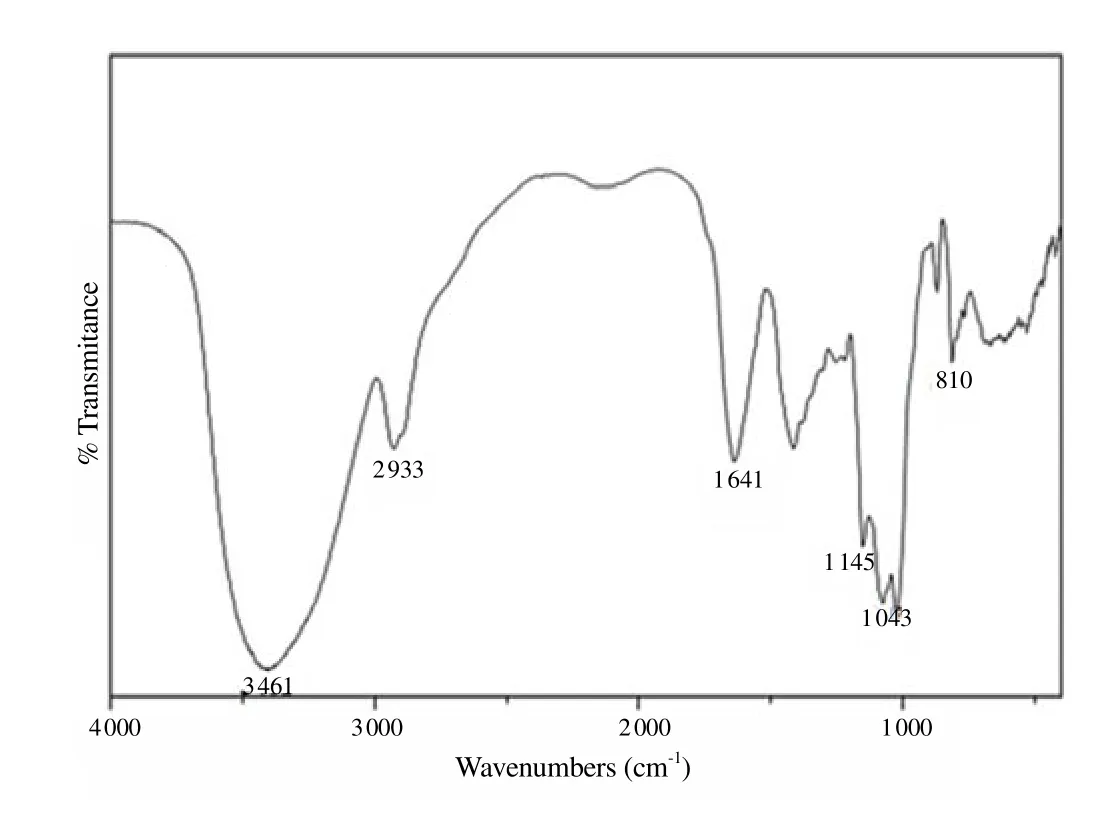
Figure 2. FT-IR spectra of galactomannan obtained from A. pavonina L. (cm-1 KBr).
3.2. Biochemical results
Glycaemia values (Table 1) showed that after 21 treatment days,both GAP 1% and GAP 2% exhibited significant glycaemia reduction in relation to diabetic control, which was kept until the end of the experiment, on the 30th day of treatment. GAP enriched food consumption during the 30 days resulted in lower TAG plasma levels(Table 2) in mice (P<0.001), although the only GAP 2% presented lower TC levels (Table 2) compared to diabetic control (P<0.05).

Table 1Effect of A. pavonina galactomannan-enriched food on blood total cholesterol(mg/dL), triacylglycerols (mg/dL), water (mL/animal/day) and food ingestion (g/animal/day).

Table 2Effect of A. pavonina galactomannan-enriched food on fasting glycaemia of diabetic mice (mg/dL).
3.3. Water and feed intake
Galactomannans food enrichment didn’t change the animals feeding behavior (Table 1), yet both GAP 1% and GAP 2% group had higher water intake (23.40 ± 2.56) mL/animal/d and (21.20± 3.69) mL/animal/d, respectively, (Table 2) significantly superior(P<0.001;P<0.01, respectively) to DC group [(14.2 ± 2.45) mL/animal/d].
3.4. Body weight
Between diabetic groups, mice body weight (Table 3) wasn’t significantly affected by GAP supplementation, however, it was seen that GAP 2% body weight was significantly similar to the NC group (P>0.05), revealing a tendency of these animals to recover the body lost due to the diabetic state. GAP 1% and GAP 2% groups presented a RLW (Table 4) higher than NC group, but similar to diabetic group (P>0.05) and inferior, to metformin, thus indicating hepatotoxicity lower than the standard drug to diabetes.
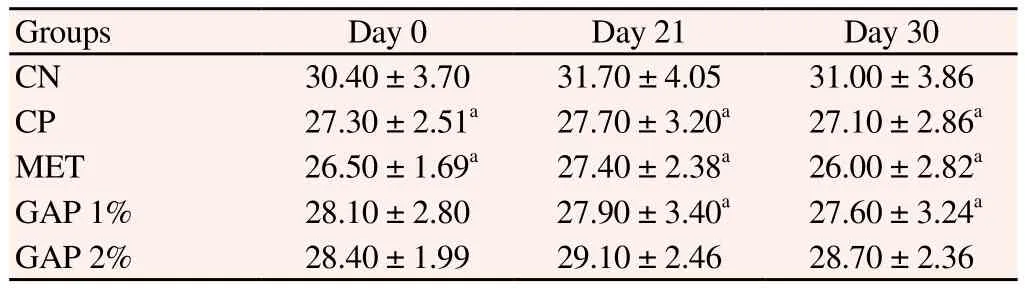
Table 3Effect of A. pavonina galactomannan-enriched food on body weight (g).
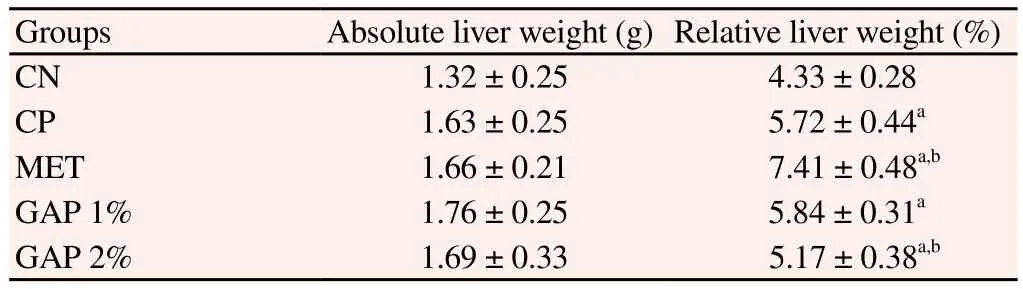
Table 4Effect of A. pavonina galactomannan-enriched food on liver weight.
3.5. Histopathological results
The pancreatic tissue showed no architectural alteration in the NC animals. In the DC group, there was only a slight decrease in the size of the islets with cell degeneration. In the MET group, there was a drastic reduction in the number and size of islets with evident cellular degeneration. In the GAP-1 group, discrete cell degeneration was visualised in all animals, however, only 2 animals showed an apparent decrease in the number of pancreatic islets. In the GAP-2 group, there was discrete degeneration in all animals, however,4 animals showed an apparent decrease in the number of islets(Figure 3). Regarding the hepatic tissue, there were no architectural changes in the animals of the NC group, however, some animals in the group exhibited hydropic degeneration that varied from mild to moderate. There were also discrete inflammatory foci consisting of lymphocytes and plasma cells (Figure 4).
In 13 animals from this experiment, 2 animals in the DC group, 3 in the MET group, all from the GAP-1 group and 3 in the GAP-2 group, there was neoplasia that reproduced malignant hepatocytes of severe anisocytosis, with a massive cytoplasm and nucleus with irregular contours, grossly distributed chromatin with evident nucleoli (irregular and sometimes macronuclei). There were also binucleation, the presence of atypical mitoses and multiple foci of apoptosis. Bile duct hyperplasia was also seen in 1 of GAP-1 and 1 of GAP-2 animals. The remaining liver tissue was thirsty for hydropic degeneration (Figure 4).
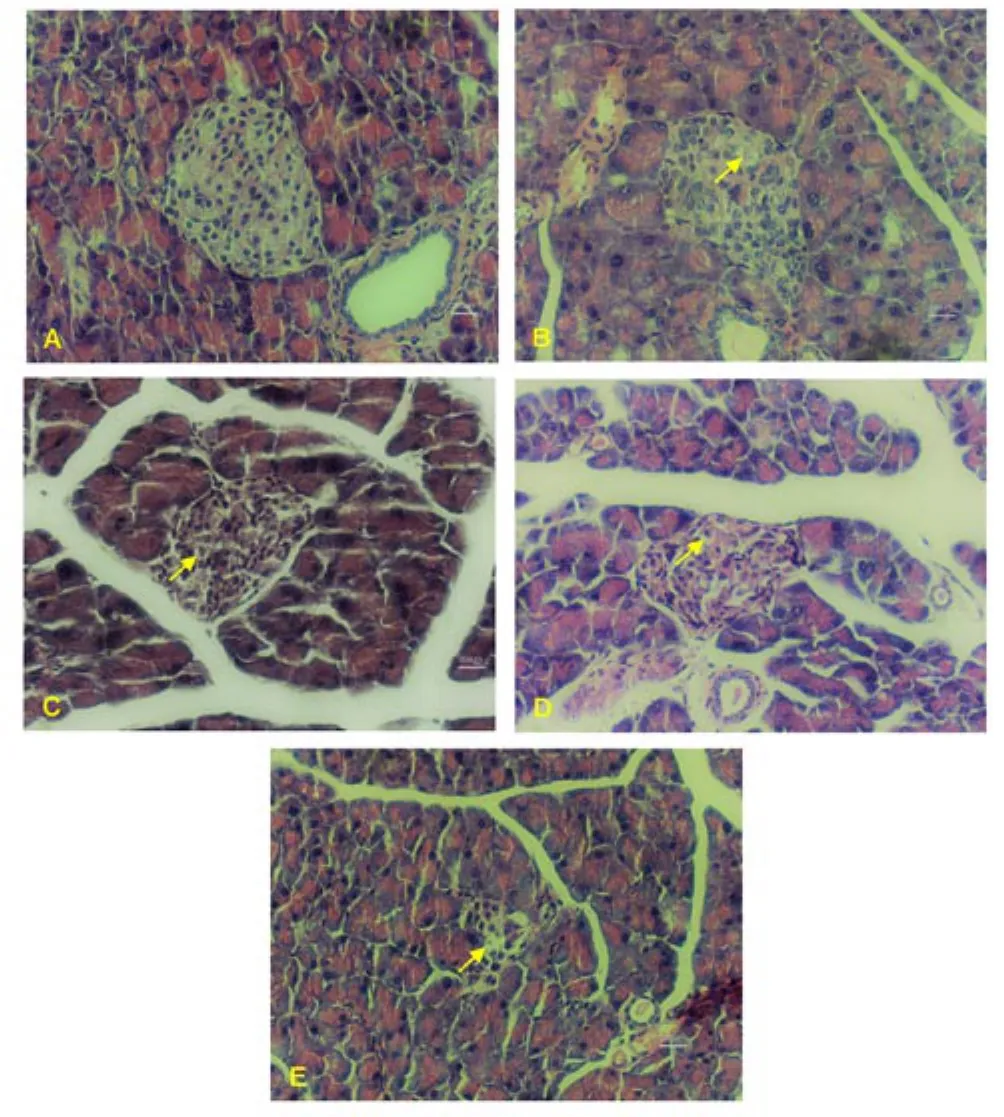
Figure 3. Pancreas photomicrograph of diabetic animals treated with galactomannan enriched food.
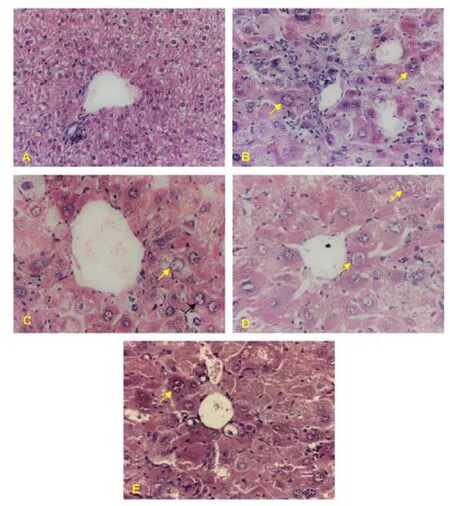
Figure 4. Liver photomicrograph of diabetic animals treated with galactomannan enriched food.
4. Discussion
DM is chronic, and becomes the most common disease, especially in developing nations. The disease is featured for the severity of its complications, and has been considered as a public health issue regarding populace development and maturing, more prominent urbanization, the expanding pervasiveness of obesity and sedentarism, and the expanded survival rate from individuals with DM[17]. Traditional plant remedies have been used for centuries in the treatment of diabetes[18], but only a few of these plants have been scientifically evaluated by researchers[19-21]. Therefore, we have investigated the effect of galactomannans fromA. pavoninaL. on STZ-induced diabetic mice.
In the present study, isolated endosperm ofA. pavoninaL. seeds contains water-soluble galactomannan. The product extracted fromA. pavoninaL. seed comprises a galactomannan free of insoluble fibers in solution, and with clear appearance. The yield was 11.00%galactomannan based on the total seed weight. This value is different from the yield reported in the literature forA. pavonina- 17.1%[4].The differences in yield may be related to factors such as the source species of galactomannan, the seed source or maturation stage of the endosperm[13].
TLC shows that chemical structure of GAP is compatible with galactomannan, which has a main chain composed of mannose with ramifications formed by one unity of galactose[22]. The higher molar weight presented by GAP implies a higher interaction between polysaccharide chains, which results in increased molecular aggregation, and a decrease on intrinsic galactomannans viscosity. However GAP presents a Mw similar to guar gum - 1.3× 107g/mol[23], to carob gum - 1.2 × 106g/mol and to fenugreek galactomannans - 1.4 × 106g/mol[24]. The M/G ratio of GAP is similar to the ones found for galactomannans with known hypoglycemic activity, like fenugreek galactomannans[25], which has a 1:1 M/G ratio[26,27], and guar gum[26] whose M/G ratio is 1.43:1 or 1.2:1[28]. Lateral galactose groups interfere in galactomannan’s water solubility, as they make polysaccharide crystallisation and interchain interactions harder, to ease its water solubilization. The detection of absorption bands, 810 cm-1, in the GAPs Fourier transformation infrared absorption suggest the presence of D-galactopyranose unities[29]. And a study withA. pavonina(L.) films detected two absorption bands, 812 cm-1and 871 cm-1, suggesting the presence of α-D-galactopyranose units and β-linked D-mannopyranose units, respectively. Glycaemia values show that both GAP 1%and GAP 2% exhibit significant glycaemia reduction in relation to diabetic control. Studies imply that concentration and viscosity contribute directly to hypoglycemic activity because it slows digestive enzymatic activity and glucose absorption in the gut[25,30].Galactomannans viscosity is influenced by its M/G ratio. The higher the galactose content in the molecule, the easier the water access to the molecule. Galactomannan prevents interactions with other polysaccharides and their crystallisation[3]. The lower TAG plasma levels and lower TC levels may be a reflex of animals glycaemia diminution, due to a lower intestinal glucose absorption tax. Once minor blood glucose reduces insulin excretion and consequently makes hepatic TAG and cholesterol production lower. Other studies indicate that the decrease of digestive lipases activity and high 3-hydroxy-3-methylglutaryl-CoA reductase activity, hepatic enzyme are responsible for bile acid production and neutral steroids[31], this explains the hypocholesterolemic action of galactomannans.
The weight of the organs provides information for preliminary evaluations of toxicological tests. It may indicate lesions to the systems, as we can observe, for example, elevation of liver weight due to hepatocellular hypertrophy[32]. The results of RLW indicated GAP hepatotoxicity was lower than the standard drug of diabetes,and these results were confirmed by the histological analysis of hepatic and pancreatic tissue. These changes may be justified by administration of STZ, a drug with diabetogenic properties because of its pancreatic β-cell selectivity. However, STZ induces sublethal DNA alkylation in important organs such as the liver and can promote carcinogenesis by inducing sub-lethal DNA alkylation[33].In conclusion, our results in this study suggest that galactomannans extracted fromA. pavoninaL. seeds have antidiabetic activity, by regulating glycaemia and diabetic mice lipedema, not interfering with the animals food behaviour and body weight, beyond that no toxicity signals are observed. This work evidences thatA. pavoninaL. galactomannans have potential as a therapeutic alternative for the control and treatment of DM. For being soluble fibres, the antidiabetic effect of galactomannans may be related to intestinal glucose and lipid absorption inhibition, and is potentially involved in the reabsorption inhibition of biliary salts, but further studies are necessary to perform this analysis.
Conflict of interest statement
The authors declared that there is no conflict of interest.
Acknowledgements
The authors thank the Brazilian governmental agency FUNCAP(Ceará State Research Funding) for their financial support.
[1] International Diabetes Federation.IDF diabetes atlas.7th ed. Brussels:International Diabetes Federation; 2015, p.1-140.
[2] Timm DA, Slavin JL. Dietary fiber and the relationship to chronic diseases.Am J Lifestyle Med2008; 2(3): 233-240.
[3] Prajapati VD, Jani GK, Moradiya NG, Randeria NP, Nagar BJ, Naikwadi NN, et al. Galactomannan: A versatile biodegradable seed polysaccharide.Int J Biol Macromolec2013; 60: 83-92.
[4] Cerqueira MA, Pinheiro AC, Souza BWS, Lima ÁMP, Ribeiro C, Miranda C, et al. Extraction, purification and characterization of galactomannans from non-traditional sources.Carbohydr Polym2009; 75(3): 408-414.
[5] Contreiras Rodrigues APD, Oliveira AKM de, Laura VA, Yamamoto CR, Chermouth K da S, Freitas MH. Tratamentos para superação da dormência de sementes deAdenanthera pavoninaL.Rev Árvore2009;33(4): 617-623.
[6] Fuller S, Stephens JM. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: Mechanisms of actions and potential effects on metabolic syndrome.Adv Nutr An Int Rev J2015; 6(2): 189-197.
[7] Vieira ÍGP, Mendes FNP, Gallão MI, de Brito ES. NMR study of galactomannans from the seeds of mesquite tree [Prosopis juliflora(Sw)DC].Food Chem2006; 101(1): 70-73.
[8] Lutz IA. Métodos físicos-quimicos para análise de Alimentos.Métodos físicos-quimicos para análise Aliment. 4th ed. São paulo: Instituto Adolfo Lutz; 2008, p. 589-625.
[9] Dubois M, Gilles K, Hamilton J, Rebers P, Smith F. Colorimetric method for determination of sugars and related substances.Anal Chem1956;28(3): 350-356.
[10] Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding.Anal Biochem1976; 72(1-2): 248-254.
[11] Almeida RR, Magalhães HS, de Souza JRR, Trevisan MTS, Vieira ÍG.,Feitosa JPA, et al. Exploring the potential ofDimorphandra gardnerianagalactomannans as drug delivery systems.Ind Crops Prod2015; 69: 284-289.
[12] Kapoor VP, Chanzy H, Taravel FR. X-ray diffraction studies on some seed galactomannans from India.Carbohydr Polym1995; 27(3): 229-233.
[13] Mulimani VH, Prashanth SJ. Investigating plant galactomannans.Biochem Mol Biol2002; 30(2): 101-103.
[14] Cavalli VL de LO, Sordi C, Tonini K, Grando A, Muneron T, Guigi A, et al.In vivostudy of the hypoglycemiant efect of extracts from the root and leaf of the bardanaArctium minus(Hill.) Bernh.Brazilian J Pharmacogn2007; 17(1): 64-70.
[15] Barbosa AP de O, Silveira G de O, de Menezes IAC, Rezende Neto JM, Bitencurt JLC, Estavam C dos S, et al. Antidiabetic effect of theChrysobalanus icacoL. aqueous rxtract in rats.J Med Food2013 ; 16(6):538-543.
[16] Michalany J.Técnica histológica em anatomia patológica. São Paulo:Editora Michalany; 1998, p. 295.
[17] Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030.Diabetes Res Clin Pract2011; 94(3): 311-321.
[18] Rai PK, Srivastava AK, Sharma B, Dhar P, Mishra AK, Watal G. Use of laser-induced breakdown spectroscopy for the detection of glycemic elements in Indian medicinal plants.Evid Based Complement Alternat Med2013; 2013: 1-9.
[19] Kamble H, Kandhare AD, Bodhankar S, Mohan V, Thakurdesai P.Effect of low molecular weight galactomannans from fenugreek seeds on animal models of diabetes mellitus.Biomed Aging Pathol2013; 3(3):145-151.
[20] Jiang W, Gao L, Li P, Kan H, Qu J, Men L, et al. Metabonomics study of the therapeutic mechanism of fenugreek galactomannan on diabetic hyperglycemia in rats, by ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry.J Chromatogr B2017; 15(1044-1045): 8-16.
[21] Pandhare RB, Sangameswaran B, Mohite PB, Khanage SG. Antihyperglycaemic and lipid lowering potential ofAdenanthera pavoninaLinn. in streptozotocin induced diabetic rats.Orient Pharm Exp Med2012; 12(3): 197-203.
[22] López-Franco YL, Cervantes-Montaño CI, Martínez-Robinson KG,Lizardi-Mendoza J, Robles-Ozuna LE. Physicochemical characterization and functional properties of galactomannans from mesquite seeds(Prosopisspp.).Food Hydrocoll2013; 30(2): 656-660.
[23] Cunha PLR, Castro RR, Rocha FAC, De Paula RCM, Feitosa JPA.Low viscosity hydrogel of guar gum: Preparation and physicochemical characterization.Int J Biol Macromol2005; 37(1-2): 99-104.
[24] Srivastava M, Kapoor VP. Seed galactomannans: An overview.Chem Biodivers [Internet]2005; 2(3): 295-317.
[25] Srichamroen A, Thomson ABR, Field CJ, Basu TK.In vitrointestinal glucose uptake is inhibited by galactomannan from Canadian fenugreek seed (Trigonella foenum graecumL) in genetically lean and obese rats.Nutr Res2009; 29(1): 49-54.
[26] Dos Santos VRF, Souza BWS, Teixeira JA, Vicente AA, Cerqueira MA.Relationship between galactomannan structure and physicochemical properties of films produced thereof.J Food Sci Technol2015; 52(12):8292-8299.
[27] Mathur V, Mathur NK. Fenugreek and other lesser known legume galactomannan-polysaccharides: Scope for developments.J Sci Ind Res2005; 64: 475-481.
[28] Hussain M, Bakalis S, Gouseti O, Zahoor T, Anjum FM, Shahid M.Dynamic and shear stress rheological properties of guar galactomannans and its hydrolyzed derivatives.Int J Biol Macromol2015; 72: 687-691.
[29] Figueiró SD, Góes JC, Moreira RA, Sombra ASB. On the physicochemical and dielectric properties of glutaraldehyde crosslinked galactomannan-collagen films.Carbohydr Polym2004; 56(3): 313-320.
[30] Jenkins DJ, Wolever TM, Nineham R, Taylor R, Metz GL, Bacon S, et al.Guar crispbread in the diabetic diet.Br Med J1978; 2(6154): 1744-1746.
[31] Ramulu P, Giridharan NV, Udayasekhararao P. Hypolipidemic effect of soluble dietary fiber (galactomannan) isolated from fenugreek seeds in wnin (GR-Ob) obese rats.J Med Plant Res2011; 5(19): 4804-4813.
[32] Sellers RS, Mortan D, Michael B, Roome N, Johnson JK, Yano BL,et al. Society of toxicologic pathology position paper: Organ weight recommendations for toxicology studies.Toxicol Pathol2007; 35(5):751-755.
[33] Vinerean HV, Gazda LS, Hall RD, Smith BH. Streptozotocin is responsible for the induction and progression of renal tumorigenesis in diabetic wistar-furth rats treated with insulin or transplanted with agarose encapsulated porcine islets.Islets2011; 3(4): 196-203.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- A hiddenly high hepatitis C virus related liver disease burden among Chinese patients with non-liver disease complaints: A hospital based study from 2013 to 2017
- Visceral leishmaniasis: An immunological viewpoint on asymptomatic infections and post kala azar dermal leishmaniasis
- In vitro fungistatic activity of 36 traditional oriental medicines and their synergistic effect against Trichophyton rubrume
- Syzygium aromaticum ethanol extract reduces AlCl3-induced neurotoxicity in mice brain through regulation of amyloid precursor protein and oxidative stress gene expression
- Phlebotomus (Adlerius) kabulensis (Diptera: Psychodidae) a new record sand fly species from Iran: Morphological and molecular aspects
- Effect of RNA interference on WD101 gene of Schistosoma japonicum
