Fructose 1,6-diphosphate alleviates myocardial ischemia reperfusion injury in rats through JAK2/STAT3 pathway
2018-03-08JuFeiWangChengJiang
Ju-Fei Wang, Cheng Jiang
Department of Cardiovascular Medicine, People's Hospital of Fenghua District Ningbo Zhejiang Province, Fenghua 315500, Zhejiang Province, China
1. Introduction
Ischemia/reperfusion (I/R) injury refers to the pathophysiological phenomenon that the metabolism dysfunction occurs and the structural damage is further aggravated after the recovery of blood perfusion in ischemic tissue. The I/R injury in patients with coronary heart disease after reperfusion treatment can affect the therapeutic effect, aggravate myocardial injury, and increase the occurrence risk of serious complications such as malignant arrhythmia and cardiac sudden death[1,2]. Anaerobic glycolysis enhancement and energy metabolism disorder are the basis of I/R injury in myocardial tissue, so improving cell energy metabolism is an effective means to prevent I/R injury after myocardial reperfusion therapy. Fructose 1,6-diphosphate (FDP) is the intermediate product of glucose metabolism that can activate intracellular phosphofructokinase and pyruvate kinase to improve the energy metabolism of cells [3,4]. In recent years, the value of FDP for reducing myocardial ischemia reperfusion injury has received more and more attention, but the specific molecular mechanism remains unclear. Janus kinase 2-Signal transducer and activator of transcription 3 (JAK2- STAT3)pathway is an important signaling pathway that regulates cell survival and cell function and is closely related to the myocardial cell damage. In the following study, we specifically explored whether FDP could alleviate the myocardial ischemia reperfusion injury in rats through the JAK2/STAT3 pathway.
2. Materials and methods
2.1. Experimental animals
Male SPF SD rats weighing 250-350 g were selected as the experimental animals, and were bought from Laboratory Animal Center of Ningbo University with permit SYXK2013-0191. The rats had free to eat and drink. The animal experiment was approved by the Hospital Ethical Review, and the animal experiments and treatment after death were conducted following standard rules.
2.2. Experimental materials
FDP and JAK2 inhibitor AG490 were bought from the Sigma Company, enzyme-linked immunosorbent assay kits were purchased from Shanghai Westang Biotechnology Company, and the first antibodies and HRP-labeled second antibodies of p-JAK2, JAK2,p-STAT3, STAT3, Bcl-2, Bax, Caspase-3 and β-actin were bought from Santa Cruz Company.
2.3. Experimental methods
2.3.1. Animal experiment methods
The experimental animals were randomly divided into Sham group,I/R group, FDP group and FDP+AG490 group, with 8 in each group.I/R group, FDP group and FDP+AG490 group were established as myocardial ischemia-reperfusion injury models according to the following method: after intraperitoneal injection of 5 mL/kg 10% chloral hydrate for anesthesia, endotracheal intubation was performed and small animal ventilator was connected, then No.3-5 left ribs were sheared, the heart was exposed, the left anterior descending coronary artery was separated, 6-0 suture was used to cross through the blood vessels, the rubber band was padded at the bottom, the blood vessel was ligatured for 30 min of myocardial ischemia, and then the suture was loosened for 120 min of myocardial blood reperfusion. FDP group were given intraperitoneal injection of 150 mg/kg FDP before operation; FDP+AG490 group were given intraperitoneal injection of 150 mg/kg FDP before operation and then intraperitoneal injection of 1.5 mg/kg AG490.Sham group were given Sham operation; the left anterior descending coronary artery was separated only, but not ligatured.
2.3.2. Serum index detection
Peripheral blood specimens were collected from the rats after decapitation, let stand for coagulation and then centrifuged in the centrifuge for 10 min at 3 000 r/min to separate serum specimens,and the enzyme-linked immunosorbent assay kit instructions were followed to determine CK, CK-MB, cTnI and LDH levels.
2.3.3. Gene expression detection
Myocardial tissue was collected from the ischemia-reperfusion area, cut into pieces, then added in RIPA lysate and fully split. The obtained tissue suspension was centrifuged under 4 ℃ centrifuge for 20 min at 12 000 r/min. After that, the upper clear protein suspension was separated and mixed with the loading buffer for Western-blot electrophoresis and then protein sample was transferred to the NC membrane. After the NC membrane was closed in 5% skim milk for 2 h, the first antibodies of p-JAK2, JAK2, p-STAT3, STAT3, Bcl-2,Bax, Caspase-3 and β-actin were incubated overnight. The HRP-labeled second antibodies were incubated the second day; after that,development was done to get the protein bands and then scan the grey value. JAK2 and STAT3 were used as reference respectively to calculate the protein expression of p-JAK2 and p-STAT3, and β-actin was used as reference to calculate the protein expression of Bcl-2, Bax and Caspase-3.
2.4. Statistical methods
SPSS23.0 software was used to process the experimental data.Variance analysis was used for the measurement data comparison among three groups, whilettest was applied for data comparison between two groups.P<0.05 indicated statistical significance in the differences.
3. Results
3.1. Regulating effect of FDP on serum myocardial injury markers in I/R model rats
Analysis of serum myocardial injury markers CK, CK-MB, cTnI and LDH contents among three groups of rats was as follows:CK, CK-MB, cTnI and LDH contents in serum of I/R group were significantly higher than those of Sham group; CK, CK-MB, cTnI and LDH contents in serum of FDP group were significantly lower than those of I/R group (Table 1).
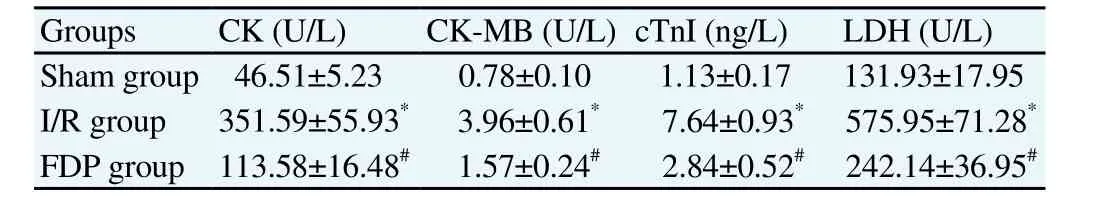
Table 1Effect of FDP on serum myocardial injury markers in I/R model rats (n=8,mean±SD).
3.2. Regulating effect of FDP on apoptosis gene expression in myocardial tissue of I/R model rats
Analysis of apoptosis genes Bcl-2, Bax and Caspase-3 expression in myocardial tissue among three groups of rats was as follows:Bcl-2 protein expression in myocardial tissue of I/R group was significantly lower than that of Sham group whereas Bax and Caspase-3 protein expression were significantly higher than those of Sham group; Bcl-2 protein expression in myocardial tissue of FDP group was significantly higher than that of I/R group whereas Bax and Caspase-3 protein expression were significantly lower than those of I/R group (Table 2).

Table 2Effect of FDP on apoptosis gene expression and JAK2/STAT3 pathway molecules in myocardial tissue of I/R model rats (n=8, mean±SD).
3.3. Regulating effect of FDP on JAK2/STAT3 pathway molecules in myocardial tissue of I/R model rats
Analysis of p-JAK2 and p-STAT3 expression in myocardial tissue among three groups of rats was as follows: p-JAK2 and p-STAT3 protein expression in myocardial tissue of I/R group were significantly lower than those of Sham group; p-JAK2 and p-STAT3 protein expression in myocardial tissue of FDP group were significantly higher than those of I/R group (Table 2).
3.4. Effect of JAK2 inhibitor AG490 on myocardial injury markers in serum and apoptosis genes in myocardial tissue of rats
CK, CK-MB, cTnI and LDH contents in serum as well as Bax and Caspase-3 protein expression in myocardial tissue of FDP+AG490 group were significantly higher than those of FDP group whereas Bcl-2 protein expression in myocardial tissue was significantly lower than that of FDP group (Table 3).
4. Discussion
Ischemia reperfusion injury is an important pathophysiological process that affects the efficacy of reperfusion therapy in patients with coronary heart disease, and its occurrence is related to the energy metabolism disorder of myocardial cells[5,6]. FDP is widely used in clinical treatment of myocardial ischemia;the drug’s active ingredients are the intermediate products in the process of glucose metabolism, and exogenous FDP can not only directly enter into the glycolysis process and provide energy for cellular metabolism[7], but also reactively activate the phosphofructokinase and pyruvate kinase,enhance glycolysis and increase the generation of ATP[8]. In recent years,in vitrostudy has confirmed that the FDP can alleviate the ischemia reperfusion injury of the isolated myocardium[9], but the effect of FDP on the myocardial ischemia in vivo reperfusion injury remains unclear. In the study, in order to determine whether the FDP had protective effect on the myocardial ischemia reperfusion injury, the ischemia-reperfusion models were made at first, and the analysis of serum myocardial injury marker contents in I/R group showed that CK, CK-MB, cTnI and LDH contents in serum of I/R group were significantly higher than those of Sham group. This shows that myocardial injury can occur in the myocardial ischemia reperfusion models produced in the study. On this basis, we analyzed the effect of FDP on the release of myocardial injury markers during myocardial ischemia reperfusion to reflect the cardioprotective effects of FDP, and the results showed that CK, CK-MB, cTnI and LDH contents in serum of FDP group were significantly lower than those of I/R group. This indicates that the FDP can significantly reduce the myocardial ischemia-reperfusion injury in rats.
Mitochondria are the intracellular organelles of myocardial cells,and the pathologic conditions of ischemia and ischemia reperfusion can cause mitochondrial damage and activate mitochondrial pathway apoptosis[10-12]. That the cytochrome C in the mitochondria enters into the cytoplasm is the initiation link of mitochondrial pathway apoptosis, which is regulated by the mitochondrial membrane proteins Bcl-2 and Bax[13,14]. Bax can polymerize into homodimer on mitochondrial membrane and become the pore for the cytochrome C to enter into the cytoplasm, thus it can promote cytochrome C to enter into the cytoplasm and then start the cascade,activate Caspase-3 and cause apoptosis[15,16]; Bcl-2 can form heterodimer with Bax and block the formation of cytochrome C pore to antagonize apoptosis[17-19]. Analysis of the changes in above mitochondrial pathway apoptosis molecule expression in myocardial tissue of ischemia-reperfusion rats in the study showed that Bcl-2 protein expression in myocardial tissue of I/R group was significantly lower than that of Sham group whereas Bax and Caspase-3 protein expression were significantly higher than those of Sham group. This indicates that the ischemia reperfusion injury can affect the balance of Bax/Bcl-2 to activate Caspase-3 and lead to the apoptosis of myocardial cells. FDP has the effect of regulating intracellular energy metabolism. Further analysis of the FDP’s effects on mitochondrial pathway apoptosis molecule expression in the process of myocardial tissue ischemia-reperfusion showed that Bcl-2 protein expression in myocardial tissue of FDP group was significantly higher than that of I/R group whereas Bax and Caspase-3 protein expression were significantly lower than those of I/R group. This indicated that the FDP could regulate the balance of Bax/bcl-2 and inhibit the expression of Caspase-3 so as to reduce the mitochondrial pathway apoptosis in myocardial ischemia reperfusion process.
JAK2/STAT3 is the signaling pathway in the cells that regulates mitochondrial pathway apoptosis, the stimulating signal of upstream growth factors can cause the phosphorylated activation of JAK2,and the phosphorylated JAK2 can cause STAT3 phosphorylation and translocation into the nucleus to activate the Bcl-2 expression and promote cell proliferation[20-22]. When the cells are in the pathological conditions of ischemia and ischemia reperfusion,the activity of the JAK2/STAT3 pathway in the cells is affected and it could cause changes in the expression of downstream Bcl-2 and Bax[23-25]. Analysis of the changes in above signaling pathway molecule expression in myocardial tissue with ischemiareperfusion in the study showed that p-JAK2 and p-STAT3 protein expression in myocardial tissue of I/R group were significantly lower than those of Sham group; p-JAK2 and p-STAT3 protein expression in myocardial tissue of FDP group were significantly higher than those of I/R group. This indicates that ischemiareperfusion can inhibit the activation of JAK2/STAT3 pathway in myocardial tissue, and the FDP intervention can activate the JAK2/STAT3 pathway in myocardial tissue with ischemia reperfusion.In order to further clarify whether the FDP directly alleviated the myocardial injury during ischemia reperfusion through the JAK2/STAT3 pathway, JAK2 inhibitor AG490 combined with FDP was used for the intervention on myocardial ischemia reperfusion rats,AG490 is the inhibitor of JAK2 phosphorylation activation, which can inhibit the phosphorylation process of JAK2 to block the JAK2/STAT3 signaling pathway activation. After AG490 intervention, and comparison with the FDP intervention alone showed that CK, CKMB, cTnI and LDH contents in serum as well as Bax and Caspase-3 protein expression in myocardial tissue of FDP+AG490 group were significantly higher than those of FDP group whereas Bcl-2 protein expression in myocardial tissue was significantly lower than that of FDP group. It means that JAK2 inhibitors can weaken the effects of FDP on reducing myocardial injury marker release and inhibiting mitochondrial apoptosis, which also shows that the FDP can alleviate the myocardial ischemia-reperfusion injury through the JAK2/STAT3 pathway.

Table 3Effect of JAK2 inhibitor AG490 on myocardial injury markers in serum and apoptosis gene expression in myocardial tissue of rats (n=8, mean±SD).
Based on above experimental studies, it can be concluded that JAK2/STAT3 pathway inhibition and mitochondrial apoptosis pathway activation are closely related to myocardial ischemia reperfusion injury; the FDP can activate the JAK2/STAT3 pathway and inhibit the mitochondrial pathway apoptosis to reduce the myocardial ischemia reperfusion injury.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Lakota J. Molecular mechanism of ischemia - Reperfusion injury after myocardial infarction and its possible targeted treatment.Int J Cardiol2016; 29(220): 571-572.
[2] Hashmi S, Al-Salam S. Acute myocardial infarction and myocardial ischemia-reperfusion injury: a comparison.Int J Clin Exp Pathol2015;8(8): 8786-8796.
[3] Veras FP, Peres RS, Saraiva AL, Pinto LG, Louzada-Junior P, Cunha TM,et al. Fructose 1,6-bisphosphate, a high-energy intermediate of glycolysis,attenuates experimental arthritis by activating anti-inflammatory adenosinergic pathway.Sci Rep2015; 19(5): 15171.
[4] Seok SM, Park TY, Park HS, Baik EJ, Lee SH. Fructose-1,6-bisphosphate suppresses lipopolysaccharide-induced expression of ICAM-1 through modulation of toll-like receptor-4 signaling in brain endothelial cells. Int Immunopharmacol2015; 26(1): 203-211.
[5] Spath NB, Mills NL, Cruden NL. Novel cardioprotective and regenerative therapies in acute myocardial infarction: a review of recent and ongoing clinical trials.Future Cardiol2016; 12(6): 655-672.
[6] Hollander MR, de Waard GA, Konijnenberg LS, Meijer-van Putten RM,van den Brom CE, Paauw N, et al. Dissecting the effects of ischemia and reperfusion on the coronary microcirculation in a rat model of acute myocardial infarction.PLoS One2016; 11(7): e0157233. DOI:10.1371/journal.pone.0157233
[7] Wang W, Liu M, You C, Li Z, Zhang YP. ATP-free biosynthesis of a high-energy phosphate metabolite fructose 1,6-diphosphate byin vitrometabolic engineering.Metab Eng2017; 42: 168-174.
[8] Alva N, Alva R, Carbonell T. fructose 1,6-bisphosphate: a summary of its cytoprotective mechanism.Curr Med Chem2016; 23(39): 4396-4417.
[9] Yuan H, Qian R, Chen Q, Xue Y, Chen Y. Fructose 1, 6-diphosphate with preconditioning on the effects of ischemia/reperfusion injury in isolated rat hearts.Chin J Clin (Electronic Edition)2015; 9(23): 4368-4372.
[10] Dong Y, Undyala VV, Przyklenk K. Inhibition of mitochondrial fission as a molecular target for cardioprotection: critical importance of the timing of treatment.Basic Res Cardiol2016; 111(5): 59.
[11] Torrealba N, Aranguiz P, Alonso C, Rothermel BA, Lavandero S.Mitochondria in structural and functional cardiac remodeling.Adv Exp Med Biol2017; 982: 277-306.
[12] Consolini AE, Ragone MI, Bonazzola P, Colareda GA. Mitochondrial bioenergetics during ischemia and reperfusion.Adv Exp Med Biol2017;982: 141-167.
[13] Zhang WZ, Li R, Liu S, Zhang JD, Ning XF, Cai SL. Effects of renal ischemic postconditioning on myocardial ultrastructural organization and myocardial expression of Bcl-2/Bax in rabbits.Biomed Res Int2016;2016: 9349437.
[14] Liang J, Yin K, Cao X, Han Z, Huang Q, Zhang L, et al. Attenuation of low ambient temperature-induced myocardial hypertrophy by atorvastatin via promoting Bcl-2 expression.Cell Physiol Biochem2017; 41(1): 286-295.
[15] Zhou T, Guo S, Wang S, Li Q, Zhang M. Protective effect of sevoflurane on myocardial ischemia-reperfusion injury in rat hearts and its impact on HIF-1α and caspase-3 expression.Exp Ther Med2017; 14(5): 4307-4311.
[16] Wang Y, Zhang H, Chai F, Liu X, Berk M. The effects of escitalopram on myocardial apoptosis and the expression of Bax and Bcl-2 during myocardial ischemia/reperfusion in a model of rats with depression.BMC Psychiatry2014; 14: 349.
[17] Zhang N, Ye F, Zhu W, Hu D, Xiao C, Nan J, et al. Cardiac ankyrin repeat protein attenuates cardiomyocyte apoptosis by upregulation of Bcl-2 expression.Biochim Biophys Acta2016; 1863(12): 3040-3049.
[18] Su F, Myers VD, Knezevic T, Wang J, Gao E, Madesh M, et al. Bcl-2-associated athanogene 3 protects the heart from ischemia/reperfusion injury.JCI Insight2016; 1(19): e90931.
[19] Thukkani AK, Shoghi KI, Zhou D, Xu J, Chu W, Novak E, et al. PET imaging of in vivo caspase-3/7 activity following myocardial ischemiareperfusion injury with the radiolabeled isatin sulfonamide analogue[(18)F]WC-4-116.Am J Nucl MedMol Imaging 2016; 6(2): 110-119.
[20] Tang Y, Tong X, Li Y, Jiang G, Yu M, Chen Y, et al. JAK2/STAT3 pathway is involved in the protective effects of epidermal growth factor receptor activation against cerebral ischemia/reperfusion injury in rats.Neurosci Lett2018; 662: 219-226.
[21] Xia Y, Xia H, Chen D, Liao Z, Yan Y. Mechanisms of autophagy and apoptosis mediated by JAK2 signaling pathway after spinal cord injury of rats.Exp Ther Med2017; 14(2): 1589-1593.
[22] Zhang ZM, Shen C, Li H, Fan Q, Ding J, Jin FC, et al. Leptin induces the apoptosis of chondrocytes in anin vitromodel of osteoarthritis via the JAK2 STAT3 signaling pathway.Mol Med Rep2016; 13(4): 3684-3690.
[23] Liao Y, Hu X, Guo X, Zhang B, Xu W, Jiang H. Promoting effects of IL 23 on myocardial ischemia and reperfusion are associated with increased expression of IL 17A and upregulation of the JAK2 STAT3 signaling pathway.Mol Med Rep2017; 16(6): 9309-9316.
[24] Li J, Xiang X, Gong X, Shi Y, Yang J, Xu Z. Cilostazol protects mice against myocardium ischemic/reperfusion injury by activating a PPARγ/JAK2/STAT3 pathway.Biomed Pharmacother2017; 94: 995-1001.
[25] Xue R, Lei S, Xia ZY, Wu Y, Meng Q, Zhan L, et al. Selective inhibition of PTEN preserves ischaemic post-conditioning cardioprotection in STZ-induced Type 1 diabetic rats: role of the PI3K/Akt and JAK2/STAT3 pathways.Clin Sci (Lond)2016; 130(5): 377-392.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- A hiddenly high hepatitis C virus related liver disease burden among Chinese patients with non-liver disease complaints: A hospital based study from 2013 to 2017
- Visceral leishmaniasis: An immunological viewpoint on asymptomatic infections and post kala azar dermal leishmaniasis
- In vitro fungistatic activity of 36 traditional oriental medicines and their synergistic effect against Trichophyton rubrume
- Antidiabetic effects of galactomannans from Adenanthera pavonina L. in streptozotocin-induced diabetic mice
- Syzygium aromaticum ethanol extract reduces AlCl3-induced neurotoxicity in mice brain through regulation of amyloid precursor protein and oxidative stress gene expression
- Phlebotomus (Adlerius) kabulensis (Diptera: Psychodidae) a new record sand fly species from Iran: Morphological and molecular aspects
