Effect of RNA interference on WD101 gene of Schistosoma japonicum
2018-03-08PengZhangGangFengWeiNaZhangYingYingZhangChunShengLiu
Peng Zhang, Gang Feng, Wei-Na Zhang, Ying-Ying Zhang, Chun-Sheng Liu
Department of Clinical Laboratory, Yijishan Hospital of Wanan Medical College, Wuhu, Anhui, China
1. Introduction
Schistosomiasis is a public health problem throughout the world[1,2]. The disease afflicts approximately 240 million individuals globally, causing the loss of approximately 70 million disabilityadjusted life years[3]. In China, schistosomiasis is caused by the continental strain ofSchistosoma japonicum(S. japonicum). Despite the tremendous decrease of schistosomiasis incidence after almost 60 years of control, the schistosomiasis has been still increasing recently along the middle and downward of Yangtz River due to the fast changes of the biology, nature, society and economics. The continuous surveillance with a focus on schistosomiasis should be enhanced in potential risk areas in China[4,5]. The life cycle of schistosomes is complex. In intermediate host or mammalian definitive host, they become adult worms that are reproductive active for years. The eggs are the agent of schistosomiasis transmission and lead to granulomatous reaction. In order to prevent schistosomiasis, controlling sexual maturation, sexual dimorphism and labour division may be effective means.
The protein encoded by WD101 gene inS. japonicumhas 6 WD-repeats function domain and 6 WD40 structure domain. The protein that has specific spatial structure HMM model can lead the interactions among the proteins[6,7]. Previous studies in laboratories have shown the significance role of WD protein family in adjusting cell information transduction, transcription, processing precursor mRNA[8], but the function of WD101 gene in reproductive system has not been verified inS. japonicum.
In this study, we aimed to identify the effect of RNA interference(RNAi) on WD101 gene and its effect on the expression of WD101 mRNA and protein inS. japonicum.
2. Material and methods
2.1. Preparation of double-stranded RNA (dsRNA)
The WD101 gene template was generated by PCR fromS.japonicumusing gene-targeted primers (Table 1). The negative control group, pcDNA™1.2/V5-GW/lacZ plasmid was provided by the BLOCK-iTTMRNAi TOPO®Transcription Kit. The control group gene was also amplified by PCR using gene-targeted primers (Table 1). Then both of them were linked with T7 promoter. Then the genes that were linked with T7 promoter were amplified separately by PCR using each primer (WD101 or LacZ) and T7 promoter primer(Table 1). Each single-stranded RNA (ssRNA) was received through transcription of PCR production, and then ssRNA were purified and mingled correspondently. Finally ssRNA transformed into dsRNA.In the process of preparing dsRNA, the BLOCK-iTTMRNAi TOPO®Transcription Kit (Invitrogen, Carlsbad, CA) was used according to the manufacturer’s instructions.

Table 1The primers for WD101 and control gene, T7 promoter.
2.2. Parasite preparation, eletroporation and culture in vivo/vitro
The continental strain ofS. japonicummaintained in the Institution of Jiangxi Parasitic Disease Prevention was used for this study. After exposing two hundreds infectOncomelaniato light for 3 h (22-26 ℃), cercariae were collected in a centrifuge tube and placed on ice for 10 min. Next, the cercariae were collected by centrifugation at 1 500 rpm for 10 min and washed twice with RPMI 1640 (Invitrogen)containing 200 U/mL penicillin G sulfate, 200 μg/mL streptomycin sulfate and 500 ng/mL amphotericin B (Sangon). The cercarial tails were sheared off by passing the suspension through a 20-gauge needle 30 times. Schistosomula were isolated from the free tails by swirling and pipetting. All worms were incubated in 10 mL of the medium-841 containing 10.4 g/L RPMI 1640, 10% (v/v) rabbit serum (Invitrogen), 0.1% (w/v) lactalbumin hydrolysate (Hyclone),10-6M hydrocortisone (Sigma), 10-6M 5-hydroxytryptamine(Sigma), 0.5×10-6M hypoxanthine (Sigma), 0.2U/mL bovine insulin (Sigma), additionally with 100 U/mL Penicillin G sulfate,100 μg/mL streptomycin sulfate, 250 ng/mL amphotericin B and 2.0 g sodium bicarbonate in an atmosphere of 37 ℃, 5% CO2for 3 h to prepare for electroporation. For electroporation, parasites were washed twice and resuspended at 20 000/mL in wash medium containing dsRNA of WD101 and LacZ (40 μg). The suspension of this type were transferred into 4 mm gap cuvettes (BTX) and incubated on ice for 10 min, and electroporated at 120 V for 20 ms square-wave pulse using an Electro Square PoratorTMECM830(BTX), respectively. After electroporation, all parasites were divided into two groups. One group were transferred to pre-warmed 841 medium in 12 well plates, cultured as described above, and supplied with fresh medium every 2 d. Aliquots of parasites were harvested on day 1, 3, 5 after electroporation, respectively (1 500 parasites/well). Another group were injected into the rear thigh muscles of Balb/c mice (two injection sites and 500 parasites/site).
2.3. Isolation of nucleic acid and protein for analysis
Before collected, schistosomula were washed twice with PBS.Total RNA, DNA and protein were isolated using TRIzol reagent(Invitrogen, USA) according to the manufacturer’s guidelines.
2.4. Quantitative real-time PCR analysis
The cDNAs were synthesized using a reverse transcription kit(Promega, USA) according to the manufacturer’s protocols. Then the cDNAs were experimented for qPCR on a ABI Prism 7500 Sequence Detector (Applied Biosystem, USA). The probes and primers designed by our group using Primer Premier 5.0 software(Table 2) and synthesized by Shanghai Sangon Company (China).A final volume of 50 μL containing Premix Ex TaqTM2× (Takara),10 μM forward primer, 10 μM reverse primer, ROC×Reference DyeⅡ 50 × (Takara), 2 μL probe, 15 μL sterile water and 5 μL template DNA (cDNA diluted 1:5). Each sample was performed in triplicate.As the following was the cycling conditions: 95 ℃, 45 s; 58 ℃, 45 s;72 ℃ , 45 s for 40 cycles. According to the manufacture’s protocol,GAPDH which served as standard curves indicating the template quantity and Ct value was obtained.R2>0.99 was determined using linear equations. The qPCR results were analyzed with ABI Prism 7500 SDS software based on a relative standard curve. The experiment was repeated three times.

Table 2The primers and TaqMan probes for real-time qPCR.
2.5. Western blotting analysis
Total protein from WD101 dsRNA-containing schistosomula were quantfied with Lowry method. The samples heated at 100 ℃ for 10 min after mixing the equal volume of 2×SDS loading buffer.Then the protein were separated with 12% SDS-PAGE (15 μg/well), transferred to a nitrocellulose membrane. Then the membrane containing WD101 protein was incubated with the anti-SjWD101 antibody diluted 1:2 000 for 1 h at room temperature with gentle shaking, while the membrane containing an endogenous control protein β-actin was incubated with rabbit anti-actin antibody(Bioss, China) diluted 1:2 000 for 1 h at room temperature with gentle shaking. After washed with PBST, the membranes were incubated in HRP-conjugated goat anti-mouse and anti-rabbit IgG(Zhongshan, China) in PBS buffer at a 1:100 000 dilution for 1 h at room temperature with gentle shaking separately. After washed with PBST, the protein-antibody complexes were visualized using chemiluminescence (SuperSignal®West Femto Maximum Sensitivity Substrate, Pierce). The results were analyzed using densitometry(Jieda image analysis system). The experiment was repeated three times.
2.6. Observation of parasite morphology change
Adult parasites of schistosome were obtained from the portal vasculature of the mice. Ten male and nine female parasites were obtained from test group, while eighteen male and sixteen female parasites from control group. All these parasites were fixed in FAA(10% formaldehyde, 2% acetic acid, 48% alcohol), stained with acetic acid carmine at 37 ℃ for 16 h, dehydrated in a series of alcohols (70%, 90%, 100%), clarified with methyl salicylate mixed with an equal amount of alcohol for 0.5 h and clarified again with methyl salicylate for 0.5 h, and whole-mounted on glass slides with neutral gum. Then observed the morphology of the parasites by confocal laser scanning microscopy (Leica).
2.7. Statistic analysis
All data were analyzed using statistical software SPSS 21.0.Independent samplet-tests were used to determine differences of WD101 mRNA and protein between different groups in bothin vivoandin vitroexperiments at various times of post-dsRNA treatment.Differences were considered significant at a level ofP< 0.05.
3. Results
3.1. Reduction of WD101 mRNA levels after dsRNA interference
After 1, 3 and 5 d of RNAi, WD101 mRNA level was decreased by 15%, 39% and 58% respectively, compared to that in control group(Table 3). The mRNA levels of endogenous GAPDH gene in two groups showed no significant differences (P>0.05).

Table 3The percentage of WD101 mRNA level compared with control (LacZ) at day 1, 3, and 5 after RNAi (%).
3.2. WD101 protein expression levels after dsRNA interference
After 1, 3 and 5 d of RNAi, WD101 protein level was decreased by 11%, 28% and 43% respectively compared to that in control group(Table 4). The protein levels of endogenous β-actin gene in two groups showed no differences(P>0.05).

Table 4The percentage of WD101 protein level compared with control (LacZ) at day 1, 3, and 5 after RNAi (%).
3.3. Morphological change after dsRNA interference
The characteristics variation statistics of worms in two groups are summarized in Table 5. There were more sperms in testicular lobes in experiment group than that in control group, while there were no differences in ovary and vitelline glands between two groups (Figure 1).
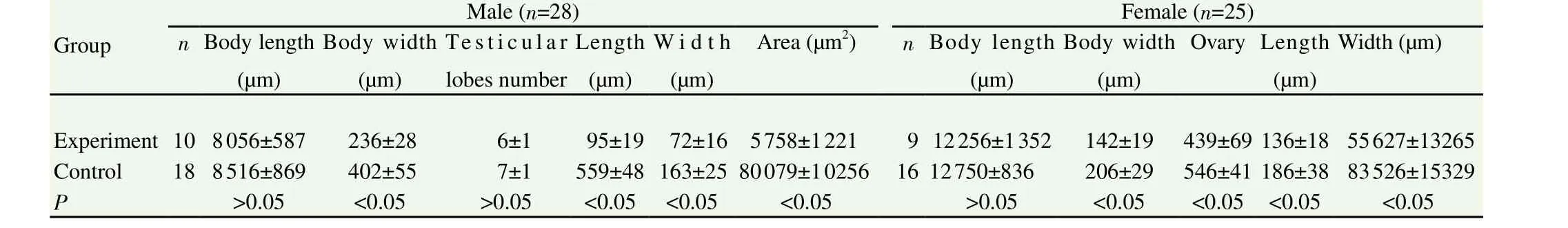
Table 5The characteristic variation statistics of worms in two groups.
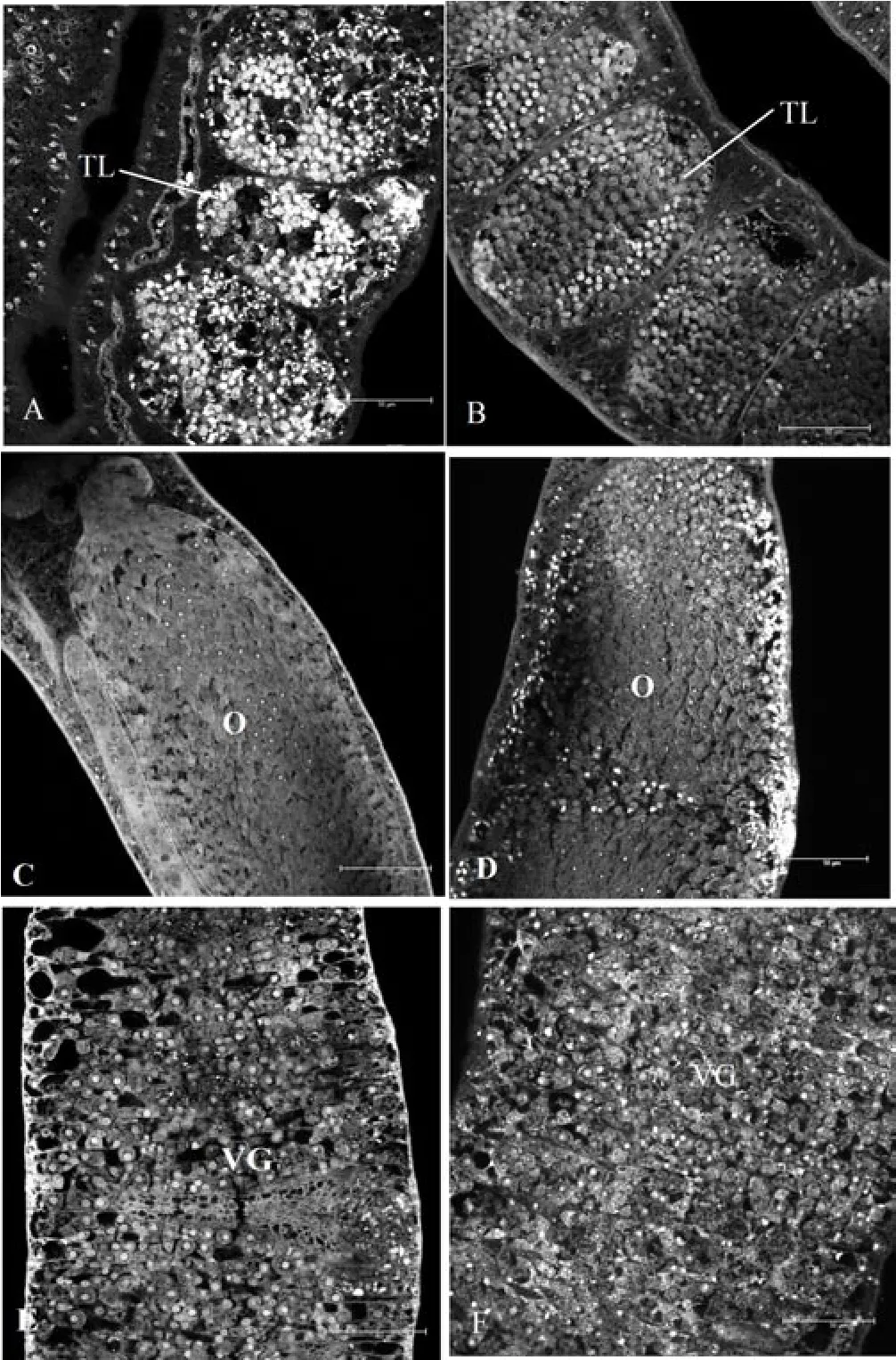
Figure 1. Confocal scanning laser microscope images of S. Japonicum isolated from experiment group and control group (bar: 50 μm).
4. Discussion
The eggs produced by the female worms are the main cause of the granulomatous, as it can lead to pathology damage and spread diseases[9]. Thus the molecular level research on the development of the female worms could provide a new means in prevention and treatment of schistosomiasis. Previous study shown that there are high homologous among WD101 gene and GTP binding-protein gene, mRNA precursor processing factor gene of other species[10].The WD101 protein has an important role in cell development,movement and information interaction. But in process of reproductive system development, the role of WD101 gene has not been verified.
dsRNA-WD101 which was synthesized by chemical method was electroporated into the body ofS. japonicumto inhibit gene specifically[11-14]. In this experiment, the results showed there were significant changes in gene, protein and morphology in WD101 dsRNA interference group. In order to improve the purity, recombined plasmids of WD101 and GAPDH as standard curves were used. If the coefficient correlation is above 0.99, it demonstrates the quantitation of standard curves is accuracy and the result is highreliablility. The results showed that WD101 mRNA levels were decreased in the dsRNA-WD101 transfected group. It indicated that dsRNA of WD101 could silence the expression of WD101 gene transcription specifically. Correspondingly, accompanied by the decreases in its mRNA levels, WD101 protein expression levels were also decreased in the dsRNA-WD101 transfected group. It indicated that dsRNA-WD101 could reduce the protein expression of WD101 gene specifically. After 6 weeks, the difference in morphology of theS. japonicumbetween test and control groups were observed, the length, width of worms and the length, width, area of testicular lobes and the length, width, area of ovary were tested. The results showed there were more specimen in the dsRNA-WD101 group than that in control group, while there were no significant differences in ovary and vitelline gland between two groups. The width of worms and the length, width, area of testicular lobes and ovary have significant differences between two groups. From the research, it is concluded the WD101 gene had tight correlation to reproductive development.
RNAi could been used widely in many fields because of the degradation of homologous mRNA triggered by RNAi[15,16]. In schistosoma, Zhao used short hairpin RNA (shRNA) to inhibit the Mago nashi gene expression[17], Ren and Zhang used dsRNA to inhibit the Tsunagi gene expression[18]. Tran and colleagues soaked 3-hour-old larval parasites in 1 mg/mL of dsRNA against tetraspanin 1 or 2 (Sm-tsp-1 or Sm-tsp-2) and culturedin vitroat 37 ℃ for 7,14, and 21 d, with fresh changes of media, blood, and dsRNAs every second day. As a result, transcript levels decreased to less than 33%compared to control and schistosomula displayed a significantly thinner and more vacuolated tegument[19]. Compared these methods,shRNA need vector to transducted into cell and take advantage of Enzyme digestion mechanism to obtain its function, the process was complicated and has many influencing factors, while dsRNA which was synthesizedin vitroand could silence target gene through many ways directly.
The study ofSchistosomaspecies and schistosomiasis has undergone a dramatic change in recent years[20]. RNAi has significantly helped the advancement in the study of non-model species. Since RNAi is a process which apparently depends on total complementarity between siRNA and target RNA, more specific drugs may arise from this technology.
In this research, dsRNA was applied to interfere the target gene and the results of this experiment was satisfactory. Our study has several possible limitations. We only tested RNAi for a short time because of the culture condition limitation and the few samples of worms,a part of schistosomula died after electroporation, thus the results could not reflect the total real status, the transfection method need improvement in the future. Considering the large amount of genomic data that might be available in the future, it is clear that there are a number of important biological questions that could be addressed using this type of gene silencing technology.
In conclusion, the dsWD101-RNAi can induce effective suppression of WD101 gene expression at both mRNA and protein levels. WD101 gene might be a reproduction-related gene inSchistosoma japonicum.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
This research was supported by Natural Science Foundation of Anhui Education Department (KJ2013B318).
[1] Mintsa-Nguéma R, Moné H, Ibikounlé M, Mengué-Ngou-Milama K,Kombila M, Mouahid G. Cercarial emergence pattern ofSchistosoma haematobiumfrom Libreville, Gabon.Parasite2014; 21: 3.
[2] Alemayehu B, Tomass Z.Schistosoma mansoniinfection prevalence and associated risk factors among schoolchildren in Demba Girara, Damot Woide District of Wolaita Zone, Southern Ethiopia.Asian Pac J Trop Med2015; 8(6): 457-463.
[3] Olveda DU, Olveda RM, McManus DP, Cai P, Chau TN, Lam AK, et al. The chonic enteropathogenic disease schistosomiasis.International Journal of Infectious Diseases2014; 28: 193-203.
[4] Qian YJ, Li SZ, Xu J, Yang K, Huang YX, Cao ZG, et al. Potential schistosomiasis foci in China: A prospective study for schistosomiasis surveillance and response.Acta Tropica2015; 141(PtB): 342-348.
[5] Wang W, Li Y, Li H, Xing Y, Qu G, Dai J, et al. Immunodiagnostic efficacy of detection ofSchistosoma japonicumhuman infections in China:a meta analysis.Asian Pac J Trop Med2012; 5(1): 15-23.
[6] Cho PY, Kim TI, Yoo WG, Li S, Hong SJ, Kim TY, et al. Molecular cloning and characterization of WD40-repeat protein fromClonorchis sinensis.Parasitology Research2007; 102(1): 53-56.
[7] Cho PY, Kim TI, Li S, Hong SJ, Choi MH, Hong ST, et al. Metacercarial proteins interacting with WD40-repeat protein ofClonorchis sinensis.Korean journal of parasitology2007; 45(3): 229-232.
[8] Singh VP, Katta S, Kumar S. WD-repeat protein WDR13 is a novel transcriptional regulator of c-Jun and modulates intestinal homeostasis in mice.BMC Cancer2017; 17(1): 148.
[9] Yao Y, Zhou CQ, Chu DY. The effect of recombinant sTGFβ1RII and sIL13R 2 receptor proteins on schistosomiasis japonica, hepatic fibrosis and signal transduction in a mouse model of schistosome disease.Experimental Parasitology2014; 142: 17-26.
[10] Li D and Roberts R. WD-repeat proteins: structure characteristics,biological function, and their involvement in human diseases.Cellular and Molecular life Sciences2001; 58(14): 2085-2097.
[11] Forrester SJ and Hall N. The revolution of whole genome sequencing to study parasites.Molecular and Biochemical Parasitology2014; 195(2):77-81.
[12] Ayuk MA, Suttiprapa S, Rinaldi G, Mann VH, Lee CM, Brindley PJ.Schistosoma mansoniU6 gene promoter-driven short hairpin RNA induces RNA interference in human fibrosarcoma cells and schistosomules.International journal for parasitology2011; 41(7): 783-789.
[13] Beckmann S, Long T, Scheld C, Geyer R, Caffrey CR, Grevelding CG.Serum albumin and -1 acid glycoprotein impede the killing ofSchistosoma mansoniby the tyrosine kinase inhibitor Imatinib.International Journal for Parasitology-drugs and drug Resistance2014; 4(3): 287-295.
[14] Patocka N and Ribeiro P. The functional role of a serotonin transporter inSchistosoma mansonielucidated through immunolocalization and RNA interference.Molecular and Biochemical Parasitology2013; 187(1): 32-42.
[15] Gobert GN, You H, McManus DP. Gaining biological perspectives from schistosome genomes.Molecular and Biochemical Parasitology2014;196(1): 21-28.
[16] He Y, Cai G, Ni Y, Li Y, Zong H, He L. siRNA-mediated knockdown of two tyrosinase genes fromSchistosoma japonicumculturedin vitro.Experimental Parasitology2012; 132(4): 394-402.
[17] Zhao ZR, Lei L, Liu M, Zhu SC, Ren CP, Wang XN, et al.Schistosoma japonicum: Inhibition of Mago nashi gene expression by shRNA-mediated RNA interference.Experimental Parasitology2008; 119(3):379-384.
[18] Ren CP, Zhang P, Zhang WN, Huang DK, Jia XM, Gui L, et al.Schistosoma japonicum: Tsunagi/Y14 protein plays a critical role in the development of the reproductive organs and eggs.Experimental Parasitology2013; 135(2): 430-436.
[19] Tran MH, Freitas TC, Cooper L, Gaze S, Gatton ML, Jones MK, et al.Suppression of mRNAs encoding tegument tetraspanins fromSchistosoma mansoniresults in impaired tegument turnover.PloS Pathogens2010;6(4): e1000840.
[20] Merrick JM, Osman A, Tsai J, Quackenbush J, LoVerde PT, Lee NH.TheSchistosoma mansonigene index: gene discovery and biology by reconstruction and analysis of expressed gene sequences.J Parasitol2003; 89(2): 261-269.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- A hiddenly high hepatitis C virus related liver disease burden among Chinese patients with non-liver disease complaints: A hospital based study from 2013 to 2017
- Visceral leishmaniasis: An immunological viewpoint on asymptomatic infections and post kala azar dermal leishmaniasis
- In vitro fungistatic activity of 36 traditional oriental medicines and their synergistic effect against Trichophyton rubrume
- Antidiabetic effects of galactomannans from Adenanthera pavonina L. in streptozotocin-induced diabetic mice
- Syzygium aromaticum ethanol extract reduces AlCl3-induced neurotoxicity in mice brain through regulation of amyloid precursor protein and oxidative stress gene expression
- Phlebotomus (Adlerius) kabulensis (Diptera: Psychodidae) a new record sand fly species from Iran: Morphological and molecular aspects
