Analysis of iodinated quorum sensing peptides by LC–UV/ESI ion trap mass spectrometry
2018-03-06YorickJnssensFrederickVerekeNthnDeunneEvelienWynendeleKthelijnePeremnsBrtDeSpiegeleer
Yorick Jnssens,Frederick Vereke,Nthn Deunne,Evelien Wynendele,Kthelijne Peremns,Brt De Spiegeleer,✽
aDrug Quality and Registration(DruQuaR)Group,Faculty of Pharmaceutical Sciences,Ghent University,Ottergemsesteenweg 460,B-9000 Ghent,Belgium
bDepartment of Veterinary Medical Imaging and Small Animal Orthopaedics,Faculty of Veterinary Medicine,Ghent University,Salisburylaan 133,B-9820 Merelbeke,Belgium
1.Introduction
Quorum sensing peptides(QSP)are auto-inducing peptides produced by gram-positive bacteria and are used in bacterial communication[1].These peptides are secreted as large pro-peptides by ATP-binding cassette transporters,extracellularly hydrolyzed and uptaken through permeases directly interacting with cytoplasmic receptors such as the RNPP proteins and/or sensed by membrane located receptors.Binding of the QSP to its membrane receptor causes auto-phosphorylation on a histidine residue and subsequent transfer of a phosphoryl group to a response regulator in the cytoplasm.Following phosphorylation,this response regulator will activate or deactivate transcription of target genes[2,3].Recently,it has been demonstrated that some of these QSP are also able to influence human host cells,promoting invasion and angiogenesis of breast and colon cancer cells,thereby potentially stimulating tumor metastasis[4,5].Radiolabeling of QSP is a convenient way to study in vitro ligand interactions as well as in vivo pharmacokinetics.Iodination of QSP with125I has already been performed to investigate the bloodbrain barrier influx properties of some selected QSP;however,analytical quality information is lacking[6].Moreover,Verbeke et al.[7]give an overview of the currently used detection methods for unmodified QSP,while their chromatographic analysis is detailed by Debunne et al.[8].
Radio-iodination(i.e.incorporation of radioactive iodine such as123I,125I or131I)is a technique commonly used for radioligand investigations,medical imaging and therapy[9,10].Several direct and indirect iodination methods for peptides currently exist.Direct labelling is based on the iodination of tyrosine and histidine residues using Chloramine-T(CAT)[11],lactoperoxidase[12]and the Iodo-Gen®method[13].Indirect labelling is performed by conjugation of a small radio-iodinated molecule such as the Bolton-Hunter reagent(N-hydroxysuccinimide ester of 3-(4-hydroxyphenyl)propionic acid)[14].This method can be used in case of absence of tyrosine and histidine residues[15].Iodination with125I is the method of choice if peptides are to be radiolabeled due to the high specific radioactivity and ease of-use in counting gamma-radiation.Moreover,non-radioactive iodination is also used in the elucidation of structures of peptides and related products like foldamers[16].While iodination is generally considered as a minimal structural modification of the peptide,it may well induce functional differences[17,18]as a consequence of the various noncovalent intermolecular interactions involving iodine atoms[19].However,the iodination reaction can result in a mixture of peptides:unmodified as well as mono-and multiple-iodinated species,as tyrosine and histidine amino acids each can give rise to 3-and 5-diiodotyrosine,and 2-and 4-diiodohistidines,respectively[20].
Analytical characterization of iodinated peptides has been scarcely reported.Vergote et al.compared the iodination of obestatin using the lactoperoxidase,Iodo-Gen®and chloramine-Tmethods[21].A comparison of different iodination procedures using a variety of peptides has also been made by this group[20].Loot et al.reported the HPLC analysis of iodinated angiotensin-(1-7)using the Chloramine-T method[18].The analysis of iodinated salmon calcitonin using reversed phase HPLC has been reported by Lee et al.[22].De Blois et al.[23]give a clear overview of iodinated somatostatin analogues.However,the analytical characterization of iodinated QSP has not yet been reported.In this study, five QSP were iodinated using different techniques and their analytical characterization was investigated.These five peptides are situated in three different clusters(clusters 2A,2B and 3A)of the quorum sensing peptide chemical space[24]and are involved in mediating cell death(Q19)[25],plasmid transfer(Q132,Q184)[26,27],expressing virulence factors(Q164)[28]and inhibition of Rap phosphatases(Q206)[29].Q19 and Q164 bind a membrane associated receptor while Q132,Q184 and Q206 are internalized and bind to a cytoplasmic receptor.
2.Materials and methods
2.1.Reagents and peptides
Bolton-Hunter reagent(N-Succinimidyl 3-(4-hydroxyphenyl)propionate)and disodium hydrogen phosphate dihydrate were purchased from VWR(Oud-Heverlee,Belgium).Pre-coated Iodo-Gen®tubes were purchased from Thermo scientific(Erembodegem,Belgium).ULC/MS grade acetonitrile and LC–MS grade formic acid were purchased from Biosolve(Valkenswaard,The Netherlands).Chloramine-T(N-Chloro-ptoluenesulfonamide sodium salt hydrate)and DMSO were purchased from Sigma-Aldrich(Diegem,Belgium).All other reagents were purchased from Merck(Darmstadt,Germany).Water(18.2 MΩ/cm)was purified in the lab by a Sartorius arium®pro purification system(Goettingen,Germany).All peptides were purchased from GL Biochem(Shanghai,China)(Table 1).The peptide numbering used in the Quorumpeps®database is retained in this study[30].
2.2.Peptide iodination using Bolton-Hunter
To 10 μL of sodium iodide(4.5 mg/mL),15 μL of Bolton-Hunter reagent(0.5 mg/mL),15 μL of sodium hydroxide(0.1 M)and 15 μL of chloramine-T(4 mg/mL)were added.After 60 s,15 μL of sodiummetabisulphite(8 mg/mL)was added to stop the reaction.Finally,50 μL of the peptide solution(1 mM in 25 mM sodium phosphate buffer pH 7.4)was added to the mixture and incubated overnight at room temperature.
2.3.Peptide iodination using Iodo-Gen®
50 μL of sodium iodide(1.1 mM)and 15 μL of 0.1 M sodium hydroxide were added to a rinsed pre-coated Iodo-Gen®tube.This mixture was incubated for 6 min at room temperature.The reaction mixture was added to 50 μL of peptide solution(1 mM in 25 mM sodium phosphate buffer pH 7.4)and further incubated for 6 min at room temperature.
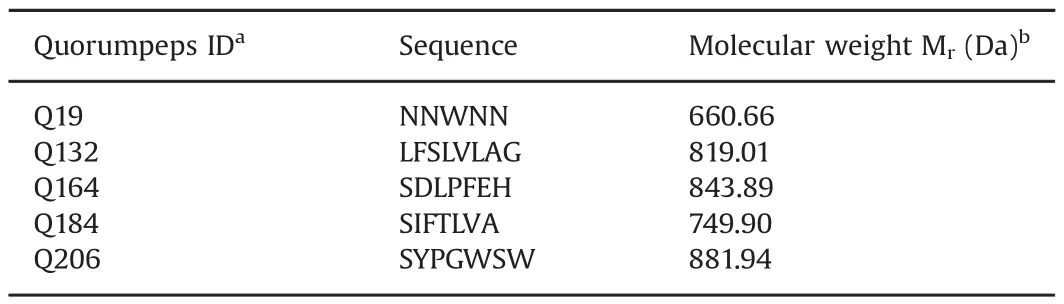
Table 1Quorum sensing peptides under investigation.
2.4.Peptide iodination using Chloramine-T(CAT)
This method was based on the method described by Wynendaele et al.[6].briefly,to 50 μL of 1 mM peptide solution,20 μL of 4.5 mg/mL NaI in 100 mM sodium phosphate buffer,30 μL of 4 mg/mL chloramine-T in 100 mM phosphate buffer and 17 μL of a 0.1 M sodium hydroxide solution were added.After 40 s incubation,the reaction was stopped by adding 30 μL of sodiummetabisulphite solution(8 mg/mL).
2.5.LC–MS instrumentation and conditions
The HPLC–UV/MS apparatus consisted of a Spectra System SN4000 interface,a Spectra System SCM1000 degasser,a Spectra System P1000XR pump,a Spectra System AS3000 auto sampler and a Finnigan LCQ Classic ion trap mass spectrometer in positive ion mode(Thermo,San José,CA,USA)equipped with a Waters 2487 dual wavelength UV detector(Waters,Milford,MA,USA)and XCalibur 2.0 software(Thermo,San José,CA,USA)for data acquisition.The dwell volume of this setup was 1.7 mL.
Different HPLC methods were optimized for the different peptides to obtain the shortest run time as possible.The first method used an XBridge™ BEH300 C18(250 mm × 4.6 mm,5 μm)stationary phase(Waters,Milford,MA,USA).Column temperature was maintained at 45°C.The mobile phase consisted of(A)95/5%(v/v)H2O/acetonitrile supplemented with 0.1%(m/v)formic acid and(B)5/95%(v/v)H2O/acetonitrile supplemented with 0.1%(m/v)formic acid.Elution was performed as described in Table 2 using a flow rate of 1 mL/min.
The second and third methods used a Vydac Everest C18column(250 mm × 4.6 mm i.d.,5 μm)(Grace,Columbia,MD,USA).Column temperature was maintained at 30°C.The mobile phases consisted of H2O and acetonitrile supplemented with 0.1%(m/v)formic acid.Elution was performed as described in Table 2 using a flow rate of 1 mL/min.Detection was performed in all these methods using UV at 210 nm and ESI-MS set in positive mode(m/z:100–2000).The ESI-MS used a spray voltage of 4.5 kV and a capillary temperature of 250°C.N2was used as sheath and auxiliary gas with flow rates of respectively 80 and 20 mL/min.Peptide solutions resulting from the iodination were directly injected on the LC–MS apparatus without prior purification.
3.Results and discussion
3.1.MS spectral data
The experimental MS spectra obtained with the peptides and the iodinated derivatives corresponded to their respective molecular structures(Table 3).The addition of a Bolton-Hunter molecule caused am/zshift in[M+H]+of+148 Da,while addition of a iodine-labeled Bolton-Hunter molecule caused am/zshift of+274 Da.Using the Iodo-Gen®method and CAT method,a shift of+126 Da was observed from non-iodinated peptide(NIP)to mono-iodinated peptide(MIP)and a further shift of+126 Da to di-iodinated peptide(DIP).The chemistry ofthese iodination reactions is displayed in Fig.1.A typical MS spectrum for the MIP of Q164 using the Iodo-Gen®method,together with its corresponding MS2spectrum is shown in Fig.2.Peaks were observed atm/zvalues 970.10 Da and 485.73 Da,attributed to the[M+H]+and[M+2 H]2+ions,respectively.MS spectra of the other peptides are given in the Supplementary material.
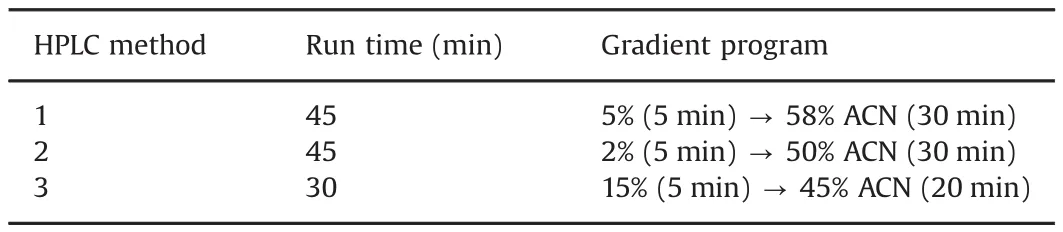
Table 2Gradient program of HPLC methods.
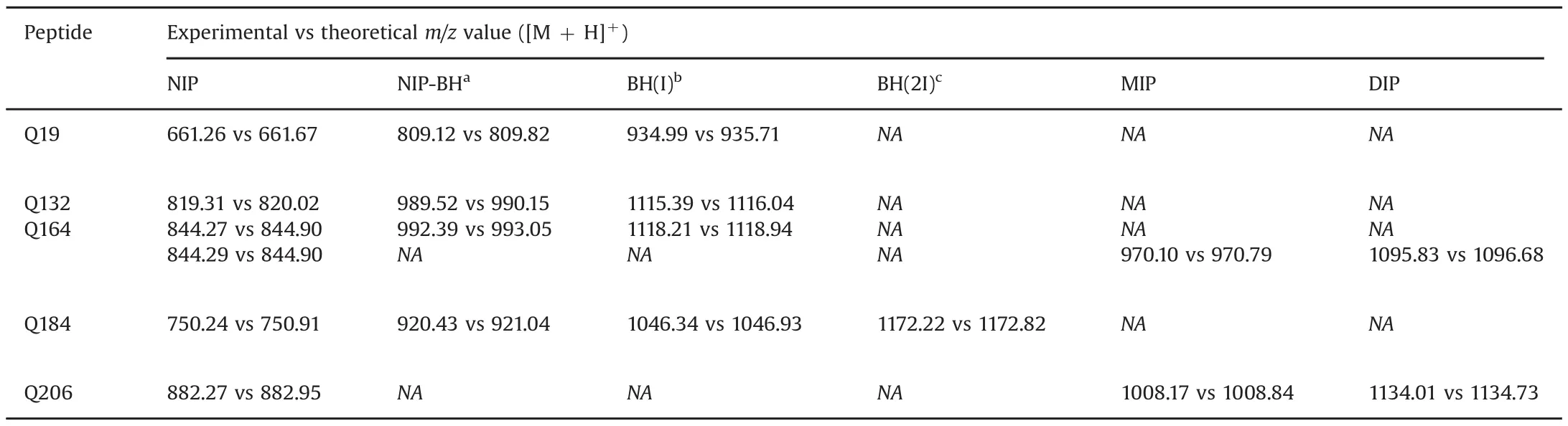
Table 3Experimental MS spectral data obtained on iodinated QSP vs expected theoretical data.
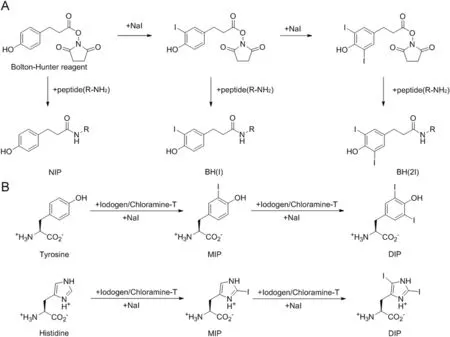
Fig.1.(A)Iodination using the Bolton-Hunter method.The Bolton-Hunter reagent was iodinated using NaI,the iodinated Bolton-Hunter molecule reacted with the aminoterminus of the peptides.(B)Iodination using the Iodo-Gen®-or Chloramine-T method.Peptides were iodinated on the Tyrosine(Q206)and or Histidine(Q164)residues.
3.2.Chromatography
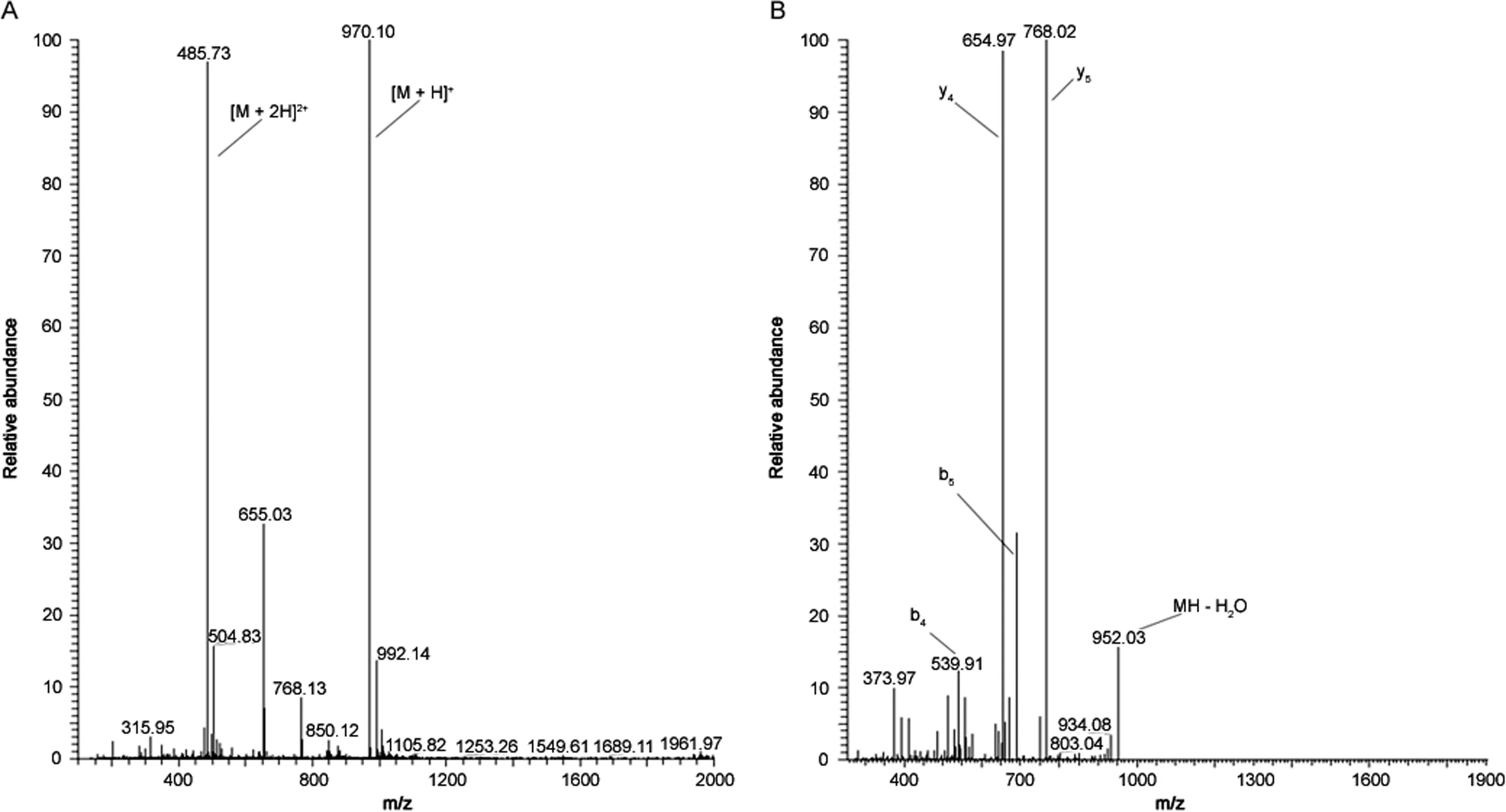
Fig.2.(A)LC-MS spectrum of mono-iodinated Q164 using the Iodo-Gen®method and(B)MS2spectrum of mono-iodinated Q164 with[M+H]+as parent-ion.
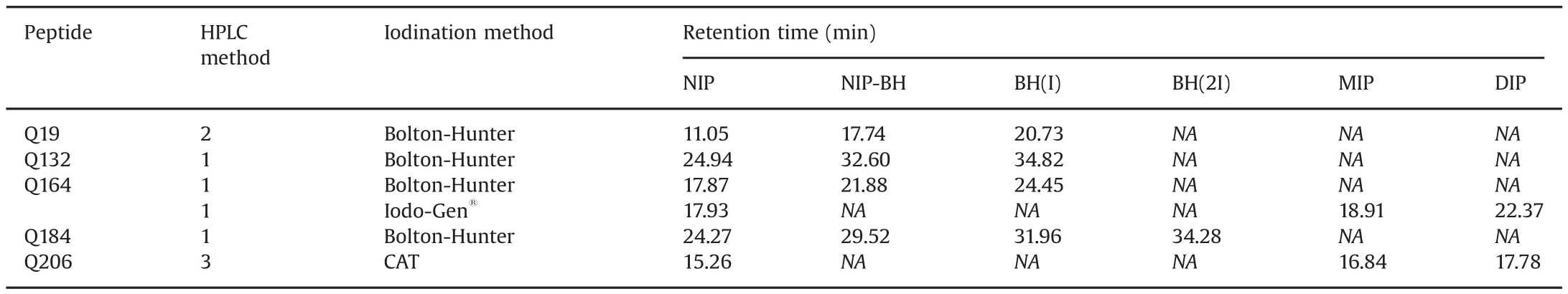
Table 4LC data on iodinated QSP.
Due to the addition of Bolton-Hunter reagent and iodine molecules,lipophilicity of the peptides increased and an increase in retention time of these iodinated peptides was expected.An overview of the retention time of iodinated peptides is given in Table 4.All peptides could be analyzed using standard HPLC methods with acetonitrile,water and 0.1%(m/v)formic acid as mobile phase constituent.A relationship between the ratio of the percentage acetonitrile at the retention time of MIP vs NIP and DIP vs MIP and the molecular mass of the iodinated peptide divided by the number of charges in acidic medium has been observed by Vergote et al.[20].An example of the UV chromatogram together with a total ion current(TIC)chromatogram of iodinated Q164 using the Iodo-Gen®method is given in Fig.3.Chromatograms of the other peptides are given in the Supplementary material.
3.3.Iodination yields
Due to the lack of tyrosine and histidine residues,the peptides Q19,Q132 and Q184 were iodinated using the Bolton-Hunter method[14].Q164 and Q206 contain a histidine or tyrosine residue and were iodinated using a direct method.The iodination yields are given in Table 5.Yields are calculated by comparing the peak area of the iodinated peptide to the total peak area of the peptides in the TIC spectrum.Iodination of Q19 using Bolton-Hunter resulted in an iodination yield of around 2.4%.For Q132,a low iodination yield of around 6%was obtained.For Q164,two iodination methods could be used.Using the Iodo-Gen®method,an iodination yield of±20%could be obtained compared to only±2%using the Bolton-Hunter method.For Q184 a total iodination yield of around 5%was obtained.The Chloramine-T method for Q206 resulted in a high iodination yield of±57%.These iodination yields using Bolton-Hunter are rather low as literature shows that yields up to 40%can be achieved using Bolton-Hunter[32].
In most pharmacokinetic studies using iodine labelling,no isolation of mono-iodinated peptides is performed.A mixture of noniodinated,mono-iodinated and multiple-iodinated peptides is used[6,33,34].When saturable transport mechanisms are used,the presence of non-labeled peptide will underestimate the transport properties of the peptide as less radio-labeled peptide is transported due to competition of the NIP transport[35].The observed effect can also be the result of multiple-iodinated peptides.These peptides underwent the biggest changes as iodination with multiple125I atoms increases the peptide size and hydrophobicity and can also affect the secondary structure of the peptide,thereby also changing the physico-chemical as well as the pharmacokinetic and pharmacodynamic properties[36,37].Furthermore,radiolabeling of peptides can influence the biological properties such as receptor affinity,biodistribution,internalization and cell dissociation[15].For most biomedical and pharmaceutical applications,single iodinated peptides are desirable as they are expected to functionally behave closest to the unmodified peptide compared to the multiple-iodinated species.Therefore,it is advantageous to isolate the mono-iodinated peptide for biomedical studies of these QSP.
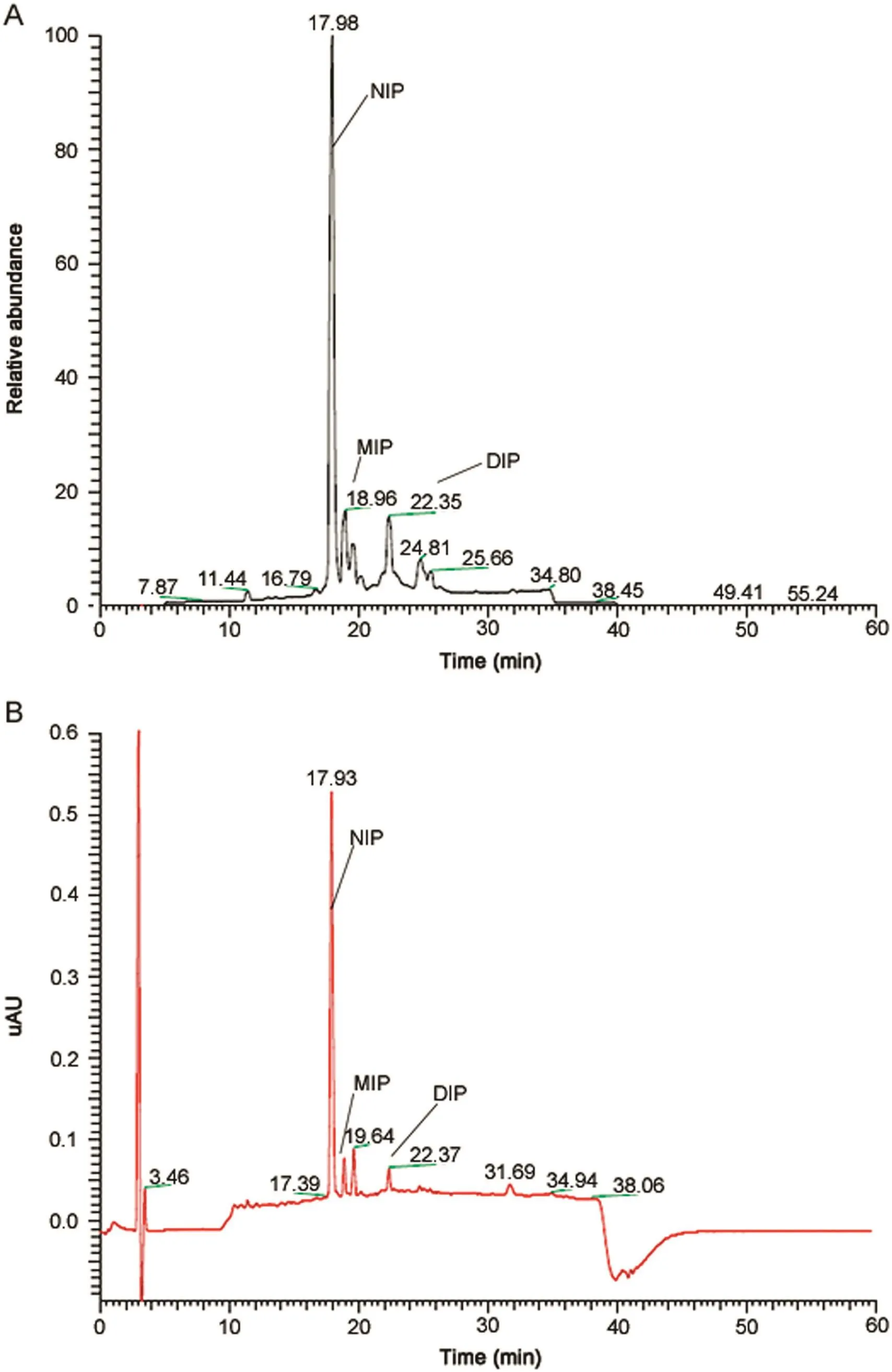
Fig.3.(A)TIC chromatogram of Q164 using the Iodo-Gen®method and(B)UV chromatogram of Q164.Peaks at 17.93,18.91 and 22.37 min were identified as NIP,MIP and DIP respectively.
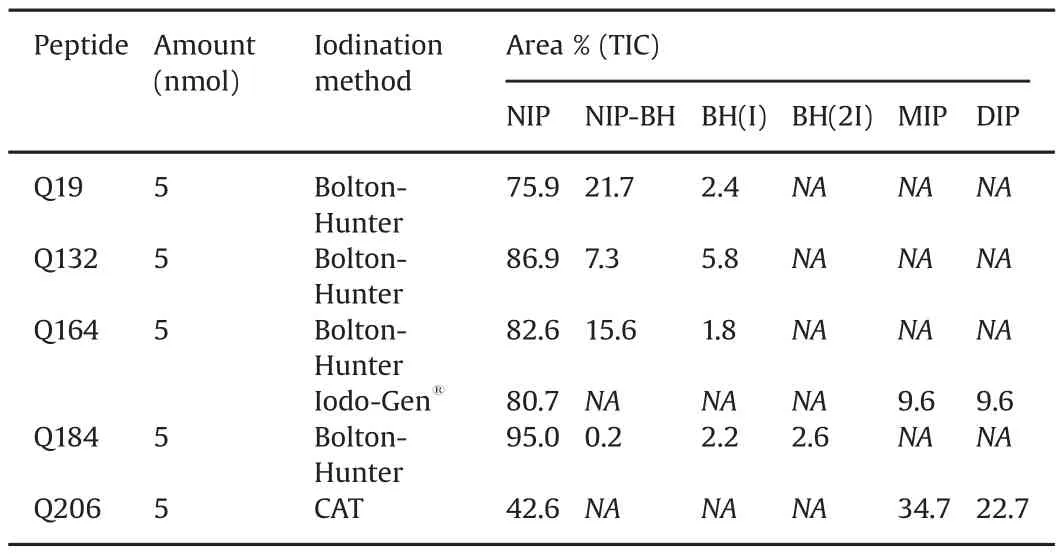
Table 5Iodination yields of quorum sensing peptides.
4.Conclusions
Iodinated quorum sensing peptides were separated by HPLC using a C18column with water-acetonitrile mobile phase gradients and 0.1%(m/v)formic acid.Iodinated peptides were identified using mass spectrometry and quantitatively estimated using normalization of the peak areas from the totalion current chromatograms.Iodination yields were variable,ranging from 2%to 57%depending on the used iodination method.
Conflicts of interest
The authors declare that there are no Conflicts of interest.
The authors thank the ‘Research Foundation–Flanders(FWO)’(Grant no.1S21017N to Nathan Debunne)and the‘Institute for the Promotion of Innovation through Science and Technology in Flanders(IWT-Vlaanderen)’(Grant no.131356 to Frederick Verbeke).
Appendix A.Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2017.09.001.
[1]M.H.Sturme,M.Kleerebezem,J.Nakayama,et al.,Cell to cell communication by autoinducing peptides in gram-positive bacteria,Antonie Van Leeuwenhoek 81(1–4)(2002)233–243.
[2]M.B.Miller,B.L.Bassler,Quorum sensing in bacteria,Annu.Rev.Microbiol.55(2001)165–199.
[3]J.Rocha-Estrada,A.E.Aceves-Diez,G.Guarneros,et al.,The RNPP family of quorum-sensing proteins in gram-positive bacteria,Appl.Microbiol.Biotechnol.87(3)(2010)913–923.
[4]B.De Spiegeleer,F.Verbeke,M.D'Hondt,et al.,The quorum sensing peptides PhrG,CSP and EDF promote angiogenesis and invasion of breast cancer cells in vitro,PLoS One 10(3)(2015)e0119471.
[5]E.Wynendaele,F.Verbeke,M.D'Hondt,et al.,Crosstalk between the microbiome and cancer cells by quorum sensing peptides,Peptides 64(2015)40–48.
[6]E.Wynendaele,F.Verbeke,S.Stalmans,et al.,Quorum sensing peptides selectively penetrate the blood-brain barrier,PLoS One 10(11)(2015)e0142071.
[7]F.Verbeke,S.De Craemer,N.Debunne,et al.,Peptides as quorum sensing molecules:measurement techniques and obtained levels in vitro and in vivo,Front.Neurosci.11(2017)183.
[8]N.Debunne,F.Verbeke,Y.Janssens,et al.,Chromatography of quorum sensing peptides,an important functional class of the bacterial peptidome,Chromatographia(2017)(Manuscript submitted for publication).
[9]L.Chen,X.Zhong,X.Yi,et al.,Radionuclide(131)I labeled reduced graphene oxide for nuclear imaging guided combined radio-and photo thermal therapy of cancer,Biomaterials 66(2015)21–28.
[10]L.Li,C.L.Zhang,L.Kang,et al.,Enhanced EJ cell killing of(125)I radiation by combining with cytosine deaminase gene therapy regulated by synthetic radio-responsive promoter,Cancer Biother.Radiopharm.30(8)(2015)342–348.
[11]R.Hunter,Standardization of the chloramine-T method of protein iodination,Proc.Soc.Exp.Biol.Med.133(3)(1970)989–992.
[12]J.J.Marchalonis,An enzymic method for the trace iodination of immunoglobulins and other proteins,Biochem.J.113(2)(1969)299–305.
[13]J.M.Conlon,The use of IODO-GEN for preparing 125I-labeled peptides and their purification by reversed-phase high performance liquid chromatography,Methods Mol.Biol.73(1997)231–237.
[14]A.E.Bolton,W.M.Hunter,The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent,Biochem.J.133(3)(1973)529–539.
[15]E.Wynendaele,N.Bracke,S.Stalmans,et al.,Development of peptide and protein based radiopharmaceuticals,Curr.Pharm.Des.20(14)(2014)2250–2267.
[16]G.W.Collie,K.Pulka-Ziach,G.Guichard,In situ iodination and X-ray crystal structure of a foldamer helix bundle,Chem.Commun.52(6)(2016)1202–1205.
[17]A.Bertolani,L.Pirrie,L.Stefan,et al.,Supramolecular amplification of amyloid self-assembly by iodination,Nat.Commun.6(2015)7574.
[18]A.E.Loot,A.van Buiten,A.J.Roks,et al.,The suitability of iodinated Angiotensin-(1-7)peptides as pharmacological tools,J.Pharmacol.Toxicol.Methods 51(1)(2005)51–55.
[19]R.Glaser,R.Murphy,What's in a name?Noncovalent Ar-Cl(H-Ar')n interactions and terminology based on structure and nature of the bonding,CrystEngComm 8(2006)948–951.
[20]V.Vergote,S.Bode,K.Peremans,et al.,Analysis of iodinated peptides by LC-DAD/ESI ion trap mass spectrometry,J.Chromatogr.B Anal.Technol.Biomed.Life Sci.850(1–2)(2007)213–220.
[21]V.Vergote,B.Baert,E.Vandermeulen,et al.,LC–UV/MS characterization and DOE optimization of the iodinated peptide obestatin,J.Pharm.Biomed.Anal.46(1)(2008)127–136.
[22]K.C.Lee,T.S.Kang,B.H.Woo,et al.,Reversed-phase high-performance liquid chromatography of radioiodinated salmon calcitonins,J.Chromatogr.B Biomed.Sci.Appl.694(1)(1997)31–37.
[23]E.de Blois,H.S.Chan,W.A.Breeman,Iodination and stability of somatostatin analogues:comparison of iodination techniques.A practical overview,Curr.Top.Med.Chem.12(23)(2012)2668–2676.
[24]E.Wynendaele,B.Gevaert,S.Stalmans,et al.,Exploring the chemical space of quorum sensing peptides,Biopolymers 104(5)(2015)544–551.
[25]I.Kolodkin-Gal,R.Hazan,A.Gaathon,et al.,A linear pentapeptide is a quorum-sensing factor required for mazEF-mediated cell death in Escherichia coli,Science 318(5850)(2007)652–655.
[26]J.Nakayama,Y.Ono,A.Suzuki,Isolation and structure of the sex pheromone inhibitor,iAM373,of Enterococcus faecalis,Biosci.Biotechnol.Biochem.59(7)(1995)1358–1359.
[27]R.Wirth,The sex pheromone system of Enterococcus faecalis.More than just a plasmid-collection mechanism?Eur.J.Biochem.222(2)(1994)235–246.
[28]L.Bouillaut,S.Perchat,S.Arold,et al.,Molecular basis for group-specific activation of the virulence regulator PlcR by PapR heptapeptides,Nucleic Acids Res.36(11)(2008)3791–3801.
[29]M.Perego,J.A.Brannigan,Pentapeptide regulation of aspartyl-phosphate phosphatases,Peptides 22(10)(2001)1541–1547.
[30]E.Wynendaele,A.Bronselaer,J.Nielandt,et al.,Quorumpeps database:chemical space,microbial origin and functionality of quorum sensing peptides,Nucleic Acids Res.41(2013)D655–D659.
[31]B.De Spiegeleer,M.D'Hondt,Molecular weights under discussion?Pharmeuropa 24(2012)1–3.
[32]J.Russell,J.A.O'Donoghue,R.Finn,et al.,Iodination of annexin V for imaging apoptosis,J.Nucl.Med.43(5)(2002)671–677.
[33]B.Gevaert,E.Wynendaele,S.Stalmans,et al.,Blood-brain barrier transport kinetics of the neuromedin peptides NMU,NMN,NMB and NT,Neuropharmacology 107(2016)460–470.
[34]S.Stalmans,N.Bracke,E.Wynendaele,et al.,Cell-penetrating peptides selectively cross the blood-brain barrier in vivo,PLoS One 10(10)(2015)e0139652.
[35]A.J.Kastin,V.Akerstrom,W.Pan,Validity of multiple-time regression analysis in measurement of tritiated and iodinated leptin crossing the blood-brain barrier:meaningful controls,Peptides 22(12)(2001)2127–2136.
[36]Y.E fimova,B.Wierczinski,S.Haemers,et al.,Changes in the secondary structure of proteins with125I:CD spectroscopy and enzymatic activity studies,J.Radioanal.Nucl.Chem.264(2005)91–96.
[37]R.J.Bauer,S.D.Leigh,C.A.Birr,et al.,Alteration of the pharmacokinetics of small proteins by iodination,Biopharm.Drug Dispos.17(9)(1996)761–774.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- S-Nitroso-N-acetyl-L-cysteine ethyl ester(SNACET)and N-acetyl-L-cysteine ethyl ester(NACET)–Cysteine-based drug candidates with unique pharmacological profiles for oral use as NO,H2S and GSH suppliers and as antioxidants:Results and overview
- Tissue-based metabolite profiling and qualitative comparison of two species of Achyranthes roots by use of UHPLC-QTOF MS and laser micro-dissection
- A liquid chromatography with tandem mass spectrometry method for quantitating total and unbound ceritinib in patient plasma and brain tumor
- Denaturation studies on bovine serum albumin–bile salt system:Bile salt stabilizes bovine serum albumin through hydrophobicity
- Insight into the interaction of inhaled corticosteroids with human serum albumin:A spectroscopic-based study
- Effect of nonionic surfactants in release media on accelerated in-vitro release profile of sirolimus eluting stents with biodegradable polymeric coating
