Tissue-based metabolite profiling and qualitative comparison of two species of Achyranthes roots by use of UHPLC-QTOF MS and laser micro-dissection
2018-03-06YoginiJiswlZhitoLingAlnHoHuioChenLeonrdWillimsZhongzhenZho
Yogini Jiswl,Zhito Ling,Aln Ho,Huio Chen,Leonrd Willims,Zhongzhen Zho,✽
aCenter for Excellence in Post-Harvest Technologies,The North Carolina Research Campus,500 Laureate Way,Kannapolis,NC 2808,USA
bSchool of Chinese Medicine,Hong Kong Baptist University,Kowloon,Hong Kong Special Administrative Region,China
1.Introduction
Plants belonging to genus Achyranthes L.are saponin and steroid rich medicinal plants used for various therapeutic benefits in traditional systems of medicine worldwide[1–4].Achyranthes bidentataBlume andAchyranthes asperaLinn.are annual herbs belonging to the family Amaranthaceae[5,6].A.bidentateBlume,commonly known as“Niuxi”in Traditional Chinese Medicine(TCM),is used for its pharmacological activities as an anti-inflammatory,anti-cancer,immunomodulatory and anti-osteoporosis drug[6–12].A.asperaLinn.,commonly known as “Apamarga”in Ayurveda,is used for its antifungal,anti-bacterial,anti-inflammatory,anti-fertility,anti-cancer and abortification properties[13–19].Based on the literature survey of the reports cited above,it is found that these two species have been used in TCM and Ayurveda for two common therapeutic effectsviz.,anti inflammatory and anti-cancer activities.These plants have been reported for similar activities and thus do pose a possibility of having constituents with similar therapeutic activity or similar chemical class,although they may vary quantitatively in their presence.Thus,in this study we aim to provide scientific substantiation on the possibilities of their qualitative substitution by identifying and comparing their phytoconstituents,using microscopic and hyphenated chromatography techniques.There have been no studies carried out till date,for qualitative tissue specific metabolite analysis ofA.bidentataandA.asperato identify the possibilities of their substitution with each other.
Literature survey reveals that there are no synthetic drug formulations available for active constituents extracted from roots ofA.bidentataandA.aspera.The drug preparation methods in both TCM and Ayurveda differ considerably.In TCM the roots ofA.bidentataare collected in winter,separated from rootlets,sundried until wrinkled externally,and cut evenly for use as medicine.To prepare processed drug,the dried roots are stir baked in wine[20].In Ayurveda,the roots ofA.asperaare prepared into a formulation calledkshara.In this formulation the water soluble ashes of the roots are used to obtain alkaline substances[21].Thus,this study envisages to provide insights into the common actives that may be responsible for common therapeutic effect,although different preparation methods are used traditionally.This study provides inferences that may be employed in designing common preparation techniques for formulations from these plants.
A.asperais used in Ayurveda for treatment of kidney disorders by taking the dried powder of the roots,with a dose of about 5 g two times a day.For treatment of pain/swelling,the powdered roots are dipped in hot water and the resulting extract is applied on the inflamed regions[22].Its roots are used in the form of ointments to treat warts and as a tooth brush for its effective action against dental ache and plaque[23–25].The roots of this plant are also reported to be used in treatment of epilepsy,and its effect is known due to its saponins being able to facilitate the GABAergic neurotransmission[26].In several studies,the anti-inflammatory and anticancer properties ofA.asperaare attributed to the presence of steroidal saponins,phenolic acids,alkaloids,tannins,terpenoids and glycosides[27,28].The roots ofA.bidentataprocessed in wine are taken internally for the treatment of dysuresia,nourishment of kidneys and liver,pruritic urticaria,leprosy,aptha and malaria.The juice of the roots is used for treatment of pharyngitis,wounds and eye ailments.The aqueous decoction of the roots is used in the treatment of stranguria,mazischesis and wounds[28].In reports published till date for both these species and the whole genera of Achyranthes,it is suggested that the fructans and saponins have immunomodulatory effect due to their ability to selectively boost the immunological responses,increase the Th1 cytokine secretions and decrease the Th2 cytokine secretion[29,30].The anti-cancer activity of these plants has been demonstrated in various in-vitro and in-vivo models and it is suggested that this property can be attributed to the triterpenoid saponins and polysaccharides.These compounds inhibit tumour growth by activation of the immune system and induction of apoptosis in cancerous cells[9,31].
A recent publication by Tyler et al.[32]states thatA.bidentatais one of those plants in TCM which have a great trade value in the herbal drugmarket in the United States of America,and is vulnerable of being endangered.The National Medicinal Plants Board,New Delhi,India,and Foundation for Revitalisation of Local Health Traditions,Bangalore,India,published a report which states thatA.asperais one of the 46 important Indian medicinal plant species that are sourced from the wastelands[33].These species may not warrant immediate attention for the threat of being endangered,but they do demand implementation of actions to enhance their cultivation outputs and strict quality control standards to avoid adulteration.
There is a rising need to identify qualitative substitutes of plants with such varied and beneficial therapeutic effects and whose natural habitats are being exploited for meeting the needs of herbal drug market.Tissue specific metabolite analysis can help identify constituent rich parts of the plant that can be effectively used for enrichment of herbal drugs or health supplements.There have been no reports published to identify secondary metabolites in specific tissues of roots of these two selected Achyranthes species and provide their qualitative comparison.The UV laser dissection feature of laser macro-dissection(LMD)instrument was used to observe and dissect tissues for specific tissue metabolite analysis.Achyranthes species are well known for their pharmacological activities attributed to the presence of oleanolic acid glycosides(saponins),dammarane saponins,steroids,anthraquinones, flavonoids and phenol carboxylic acids.Based on the literature survey of various mass banks,databases,and scientific publications,we prepared a database of 95 constituents reported in Achyranthes species[34–38].The extraction solvent used for the present analysis was methanol,as its polarity was close to the solvent used for extraction of the roots in traditional systems of medicine,i.e.water.This solvent selection was suitable for liquid chromatography–mass spectrometry(LC–MS)analysis and could extract most of the constituents that could be extracted with water as a solvent.The present study is the first report that provides such a comparative approach and identification of tissue specific metabolites in these two species.
2.Materials and methods
2.1.Chemicals
Acetonitrile(HPLC grade)used for LC–MS analysis was obtained from E.Merck(Darmstadt,Germany).Other mobile phase modifiers and reagents including for micacid (HPLC grade,purity of 96.0%)were purchased from Tedia Company Inc(U.S.A.).Ultra-pure water used for plant sample treatment and for different experimental steps was obtained from in house installed Milli-Q water purification system(Millipore,Bedford,MA,U.S.A.).
2.2.Plant materials
Three sample sets of each of the dried roots ofA.asperaLinn.andA.bidentataBlume were collected from commercial herbal drug markets in the state of Maharashtra,India,and herbal drug stores from various regions of China.The authentication of samples was carried out by Professor Zhongzhen Zhao and the voucher specimens were deposited for records in the Chinese Medicines Centre of Hong Kong Baptist University,Hong Kong,China.
2.3.Preparation of microscopy sections
The dissection of microscopic tissues from roots of both the species ofAchyranthessamples was carried out as detailed by Jaiswal et al.[39,40].Prior to laser micro dissection,the root samples were conditioned by wrapping in non-cellulose paper soaked with ultrapure water.The soaked samples were then kept under vacuum at 25 inHg pressure for a period of 24 h,at room temperature.After softening,the roots were cut into small pieces of about 1–1.5 cm heights and embedded in cryogel matrix(Leica Microsystems,Germany)for cryo-sectioning.Sections of roots with thickness of about 20–25 μm were cut with a cryotome equipment and carefully placed on metallic PET slides,precooled at-15°C.
2.4.LMD of root tissues
Cryo-sectioning of roots and LMD of specific root tissues were carried out at- 15°C,by using a Thermo Shandon As620 Cryotome,UK and Leica LMD 7000 system(Leica,Benshein,Germany),respectively.By using the Leica LMD-BGR fluorescence filter system and various magnifications(6.3× and 10×)for tissue inspection,the target tissues were dissected and collected in Eppendorf tube caps prefixed in LMD collecting device.The total area dissected for each specific tissue was about 1 × 106μm2.The conditions used for the LMD instrument during dissection were aperture size of 1,exposure time of 115.9 ms,speed value 3 and 2.3×gain.
2.5.Extraction of LMD dissected tissue and the whole root sections
The laser dissected root tissues were centrifuged at 15,000 rpm for 10 min by a Centrifuge 5415R(Eppendorf,Hamburg,Germany)to reduce sample loss and ensure effective collection of tissues.After centrifugation,100μL of HPLC grade methanol was added into each Eppendorf tube,and the extraction process was facilitated by sonication of the tubes for 30 min using CREST 1875HTAG ultrasonic processor,USA.After 30 min of sonication,centrifugation of the tubes was carried out at 15,000 rpm for 10 min.The resultant supernatant extracts were collected in HPLC glass vials containing glass inserts with bottom springs.The samples were kept refrigerated at 4°C until analysis.
The whole root sections were extracted by collecting 5–6 sections of the cryo-dissected samples in Eppendorf tubes.These sections were then extracted with 500μL of HPLC grade methanol with the procedure identical to the one described for specific laser dissected tissues described above for isolated tissues.
2.6.UHPLC-QTOF MS analysis
The ultra-performance liquid chromatography(UPLC)analysis of samples was carried out by use of an Agilent 6540 accurate–mass Q-TOF LC/MS(Agilent Technologies,USA)system.Separation was carried out by use of a UPLC C18analytical column(2.1 mm×100 mm,I.D.1.7 μm,ACQUITY UPLC®BEH,Waters,U.S.A.)equipped with a C18pre-column.The LC–MS analysis was performed at room temperature of 20°C.The optimised chromatographic conditions used for MS analysis were as follows:dry gas(N2) flow rate 6 L/min,Vcap 4500,dry gas temperature 300°C,nozzle voltage of 500 V,fragmentor voltage 150 V,and nebulizer pressure 40 psi.To obtain the identification of the largest number of phytoconstituents and the most appropriate mode of acquisition,the mass spectra acquisition was carried out in both negative and positive modes.Mass scanning range for mass to charge ratio(m/z)selected for analysis was 110–1700.The solvent system used for the chromatographic analysis comprised a mixture of water(A)and acetonitrile(B),both containing 0.1%formic acid.The linear gradient conditions were as follows:0–8 min,2%–15%B;8–18 min,15%–55%B;18–23 min,55%–100%B;23–26 min,100%B;26–26.1 min,100%–2%B,26.1–30 min,2%B, flow rate 0.4 mL/min.The injection volume used for each sample was 2.0μL.Accurate mass calibration was done by using ESI-low concentration tuning mix solution(Agilent technologies,USA)with accuracy error threshold of 10 ppm.
2.7.Data analysis
Analysis of data obtained from LC–MS was carried out with Agilent Mass Hunter Workstation Software-Qualitative Analysis(version B 4.00,Build 4.0.479.5,Service Pack 3,Agilent Technologies,Inc.2011).The following settings were applied during data analysis:extraction restricted retention time of 1.0–30.0 min,charge state of+1 and-1 used for positive and negative modes,respectively.The peaks with height≥2000 counts were used for analysis.The elements of C,H,O,and N from 3 to 60,0 to 120,0 to 30 and 0 to 30,respectively,were used to generate formulae.The compound absolute height used was≥5000 counts and relative height was≥2.5%.Peak spacing tolerance used for analysis was 0.0025m/zplus 7.0 ppm.The peak lists from the data analysis with Mass Hunter Workstation software were prepared in Microsoft Excel software(Microsoft,Redmond,WA).The results of analyses are depicted with base peak chromatograms(BPCs,withm/zrange 150–950).
3.Results and discussion
3.1.Macroscopic and microscopic studies of the roots of A.bidentata and A.aspera
The roots ofA.bidentatawere cylindrical in shape,with frequent twisted regions and alternate striations(Fig.1A).Striations on the outer area of the root depicted the remnants of the rootlets.The roots were about 1.0–1.2 cm in width with some roots also appearing as broad as 1.5 cm.They appeared light brown in dried conditions.A.asperaroots appeared less parenchymatous and had fewer striations and rootlet remnants compared toA.bidentataroots.A.asperaroots were about 0.8–1.0 cm in thickness and likeA.bidentatahad occasionally twisted regions and wrinkles along the outer region of the roots(Fig.1B).The inner core of the roots appeared parenchymatous and fleshy around the cortex region for both the species.
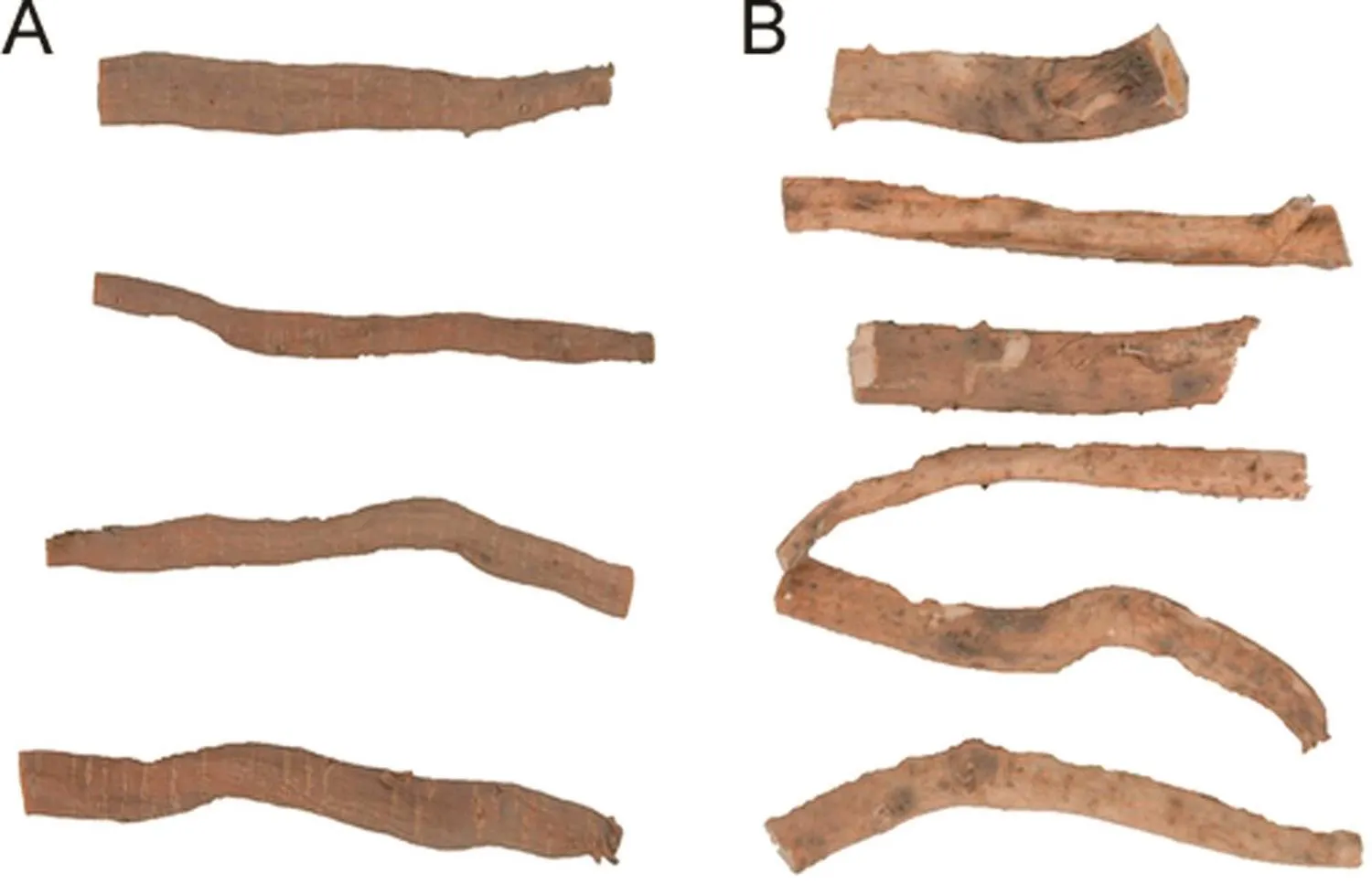
Fig.1.Pictorial representation of dried roots of(A)Achyranthes bidentate Blume and(B)Achyranthes aspera Linn.
In Figs.2 and 3,typical microscopic features ofA.bidentataandA.asperaroot sections are depicted under white light and blue fluorescence light,respectively.The roots ofA.bidentataexhibited a good development of tertiary structures formed by differentiation of cambium.Medullary rays and parenchyma cells separated the tertiary vascular bundles,and the tertiary vascular bundles were found more in number towards the centre.The outer peripheral region showed the presence of a thin indistinct epidermis,cortex and protoxylem.The secondary structures found were the medullary rays and the metaxylem interspersed with parenchyma cells.
In case ofA.aspera,the primary and secondary tissue structures were common to those ofA.bidentata.Difference was found in the placement of the tertiary vascular bundles.The tertiary vascular bundles were arranged radially along the centre of the roots.The parenchyma cells were not found to be as dense as observed inA.bidentatatowards the central region.Under blue fluorescence,the cortex cells exhibited bright fluorescence and the cork appeared dark reddish brown in colour.The tertiary vascular bundle cells exhibited a brighter fluorescence compared to the medullary rays and were prominent and easy to distinguish.
3.2.Characterization of metabolites
The constituents identified are listed in Table 1,with the calculated and observedm/zvalues,molecular formulae,retention time(Rt),and identified molecular species.Based on the reportedm/zvalues and the observedm/zvalues,tentative identification of the constituents was carried out.The constituents identified in both positive and negative modes are listed in Table 1.However,negative mode was found to identify the most constituents present in roots of both selected plant species,with good peak resolution.
3.3.Identification of metabolites by UHPLC-QTOF MS
Representative BPCs of whole root tissues of both the species,the cryogel used as control and methanol used as solvent blank are indicated in Fig.4.A database of 95 constituents was prepared and used for identification,of which 26 constituents were identified in the species studied.The database is provided for reference(Supplementary material).The cryogel used for cryo-sectioning contains glycols and polyvinyl alcohols which exhibited distinct peaks between 12 and 13 min retention time.Methanol used as solvent eluted few minor peaks after 18 min retention time,and did not show any interference with the identification of constituents.Negative mode resulted in identification of greater number of compounds with better resolution and chromatograms of negative mode are indicated in Fig.4.
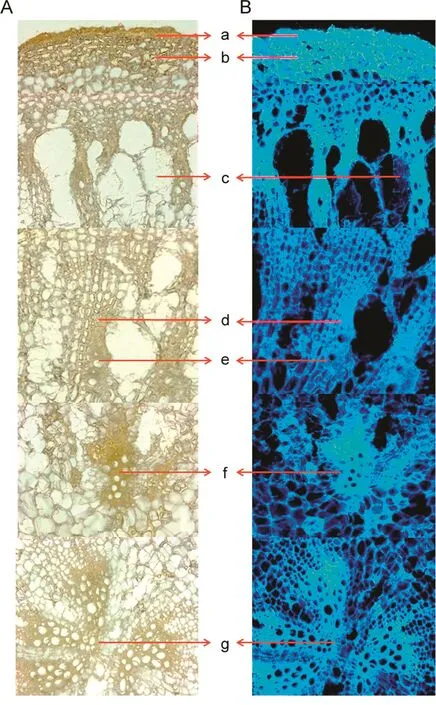
Fig.2.Microscopic characteristics of A.bidentata under(A)white light and(B)blue fluorescence.The various tissues denoted are:(a)cork,(b)cortex,(c)protoxylem,(d)medullary rays,(e)metaxylem,and(f and g)tertiary vascular bundles.
As observed in Fig.4,several constituents were found in common in bothA.bidentataandA.aspera.Out of the 26 identified constituents,23 constituents were found to be common between root samples of both the species.InA.aspera,1-Tritriacontanol,11-methyl-and chikusetsu saponin-IVa butyl ester were exclusively found.Palmitic acid was found to be present only in theA.bidentataspecies.The identified group of constituents can be classified into different classes,as listed in Table 2.
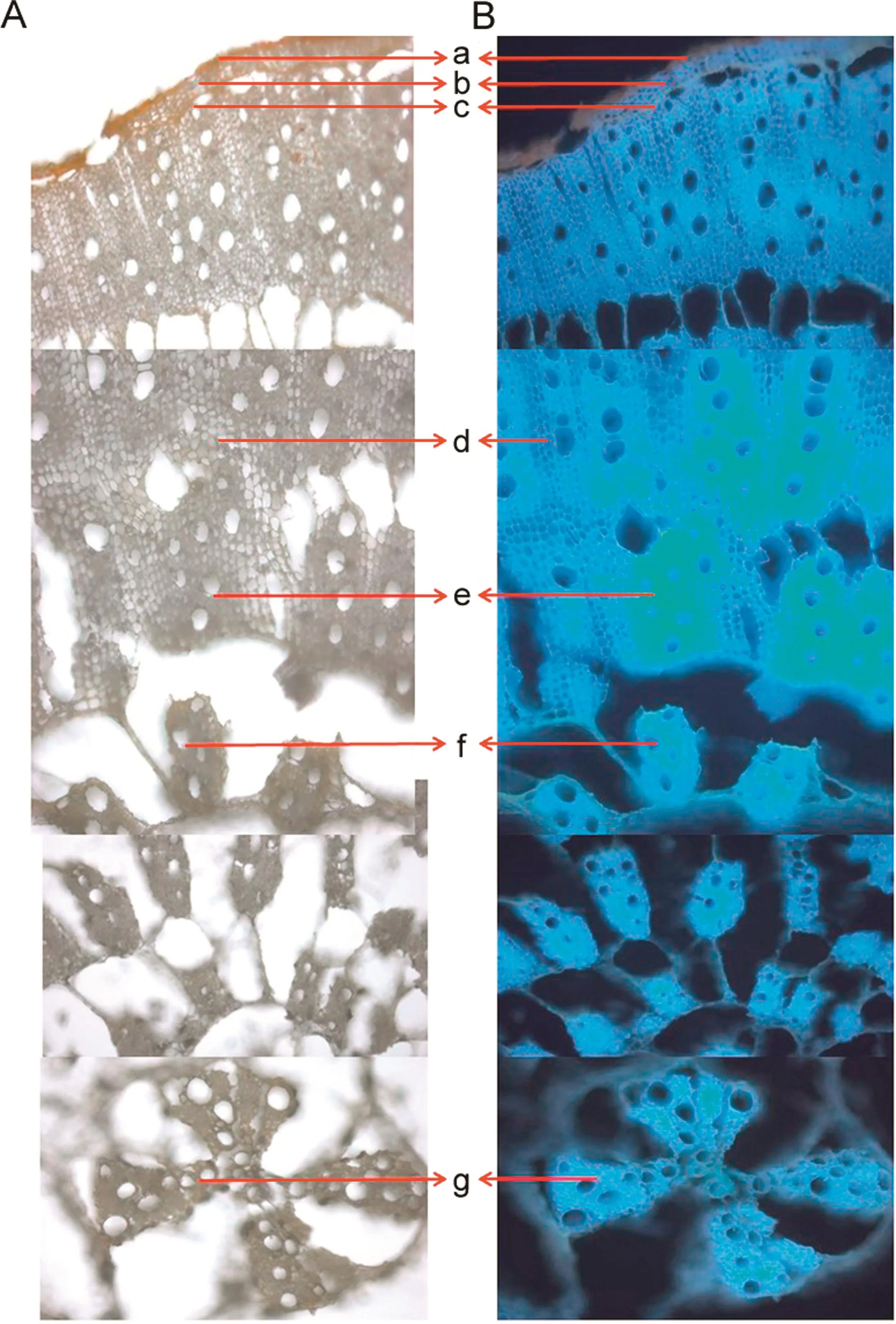
Fig.3.Microscopic characteristics of A.aspera under(A)white light and(B)blue fluorescence.The various tissues denoted are:(a)cork,(b)cortex,(c)protoxylem,(d)medullary rays,(e)metaxylem,and(f and g)tertiary vascular bundles.
The constituents,glutamic acid,niacin,achyranthine,5-hydroxymethyl-furfural,myristic acid,behenic acid and 1-Tritriacontanol,11-methyl-,were detectable only in the positive mode and hence are not represented in Figs.4–6.These components are important constituents of the plant as they include vitamins and fatty acids which contribute to other activities of the plants such as anti-oxidant effect.However,the most prominent and well-known constituents of the plant are its saponins and steroids detected in the negative mode.Overall,bothA.bidentataandA.asperaspecies have comparable number and most of their constituents are identical.Based on these findings we suggest that these species can be substituted for each other for specific therapeutic properties,which include their anti-inflammatory and anti-cancer properties that are attributed in literature due to the presence of saponins and steroids.In Fig.4,the constituents eluted in the whole extract of both the species are elucidated.In Figs.5 and 6,the tissue specific constituents that were identified are elucidated.These identifications provide information of the presence of constituents in specific tissues.This information can be used for further exploration by herbal drug formulators,herbal drug researchers and biotechnologists to enhance or modify the extraction of constituents from these tissues and prepared constituent-enriched products.
A tentative quantitation of the constituents can be carried out for the identified constituents based on the peak heights of the constituents,as the experimental conditions were the same for both the samples.It was observed that inA.bidentata,theginsenoside Ro,sulphachyranthoside D,and bidentatoside had peak heights about 4 times higher than inA.aspera.Ascorbic acid indicated peak height 3 times higher,and(25S)-inokosterone-20,22-acetonide exhibited peak height twice higher inA.bidentatathan inA.aspera.This is only the tentative estimation of the quantitative differences between these species,as the article focuses mainly upon qualitative differences between these two species,based on the identification of various constituents.
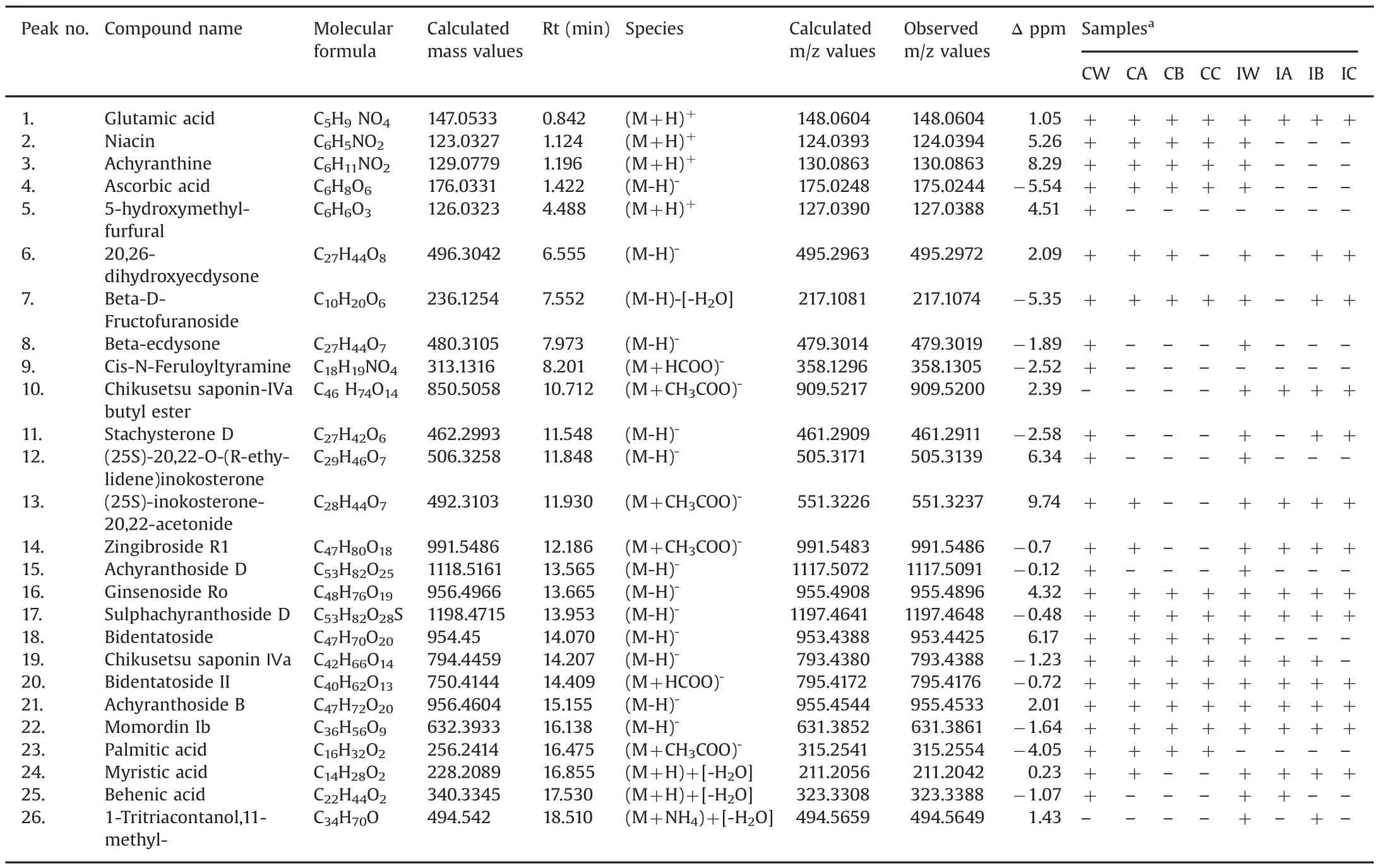
Table 1List of constituents identified in Achyranthes aspera and Achyranthes bidentata species.
Table 2Classification of constituents identified in A.bidentata and A.aspera in the developed LC–MS method.
3.4.Tissue-specific metabolite analysis by UHPLC-QTOF MS
For tissue-specific metabolite analysis,three tissues were selected for LMD from root samples of both the species.The cortex,the medullary rays and the tertiary vascular bundles were dissected from each of the three root samples of both the species.Representative BPCs for each of the tissues ofA.bidentataandA.asperaare shown in Figs.5 and 6,respectively.
The secondary metabolites belonging to the class dammarane saponins that include ginsenoside Ro,sulphachyranthoside D,achyranthoside B,and oleanolic acid glycoside saponins that include bidentatoside II and momordin Ib were found in all three isolated tissues of bothA.bidentataandA.aspera.InA.bidentata,in addition to the above-mentioned constituents,the common constituents between the cortex,medullary rays and the tertiary vascular bundles were ascorbic acid and palmitic acid.The cortex and medullary ray tissues exhibited the presence of the steroid 20,26-dihydrox-yecdysone,whereas the tertiary vascular bundles did not show the presence of it.The oleanolic acid glycoside saponin zingibroside R1 and the steroid(25S)-inokosterone-20,22-acetonide were found in the cortex,the medullary ray,and the tertiary vascular bundles ofA.asperaand the cortex tissue ofA.bidentata.The cortex ofA.asperadid not show the presence of the steroidal constituent,20,26-dihy-droxyecdysone.Based on the tissue specific metabolite analysis results,we infer that the cortex and medullary rays have higher number of dammarane and oleanolic acid glycoside saponins and steroids.Thus,the outer regions of the roots comprising the cortex and the medullary rays can be extruded and used exclusively for enrichment of herbal products for saponin and steroidal content.
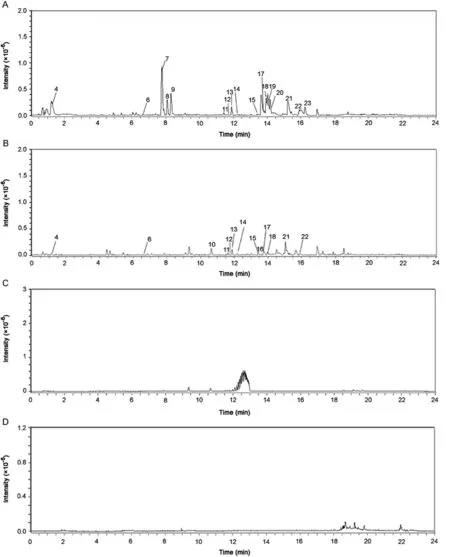
Fig.4.LC–MS base peak chromatograms of(A)whole tissue sections of A.bidentata,(B)whole tissue sections of A.aspera,(C)cryogel used as control,and(D)methanol used as solvent blank in negative mode.
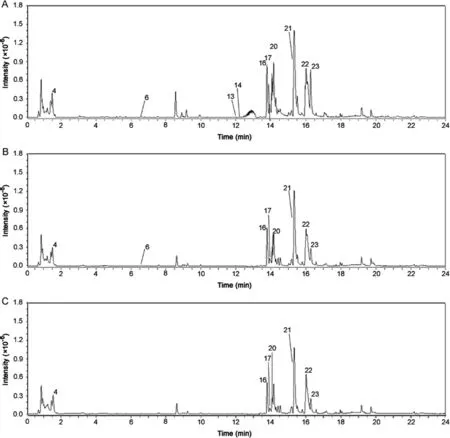
Fig.5.LC–MS base peak chromatograms of various laser dissected tissues of A.bidentata analysed in negative mode.(A)cortex,(B)medullary rays,and(C)tertiary vascular bundles.
A.bidentataandA.asperaare both widely used for the therapeutic benefits discussed above in TCM and Ayurveda,respectively.A.bidentatais reported to be one of the most extensively used medicinal plants in TCM that face the problem of being endangered due to exploitation of their natural habitats,to meet commercial demands for their medicinal products[32].In-vitro propagation of plants as an alternative to supplement for the demands of endangered species is a promising discipline.However,it faces the concern of being a time-consuming approach.The reduced adaptability of the plants grown through in-vitro propagation to the natural habitats is a major factor for commercial cultivation[41,42].Thus,identification of alternate species of the same genus and family,which have the same phytoconstituents,will be of significant help to provide qualitative substitute species for therapeutically beneficial but endangered medicinal plants[43,44].identification of substitute species will help herbal drug industrialists,researchers in the field of phyto-chemistry and pharmacology,traditional medicine practitioners and agriculturists to utilise and further investigate the applications of substitute species in traditional medicine and for commercial production of herbal products.
4.Conclusion
From this comparative study,we infer that inA.bidentataandA.asperathe cortex and the medullary rays are the tissues rich in saponin and steroidal contents and can be used for enrichment of herbal products.The roots of these two selected plant species can be used as qualitative substitutes for each other in traditional medicine practices and for commercial herbal drug production.
Conflicts of interest
The authors declare that there are no Conflicts of interest.
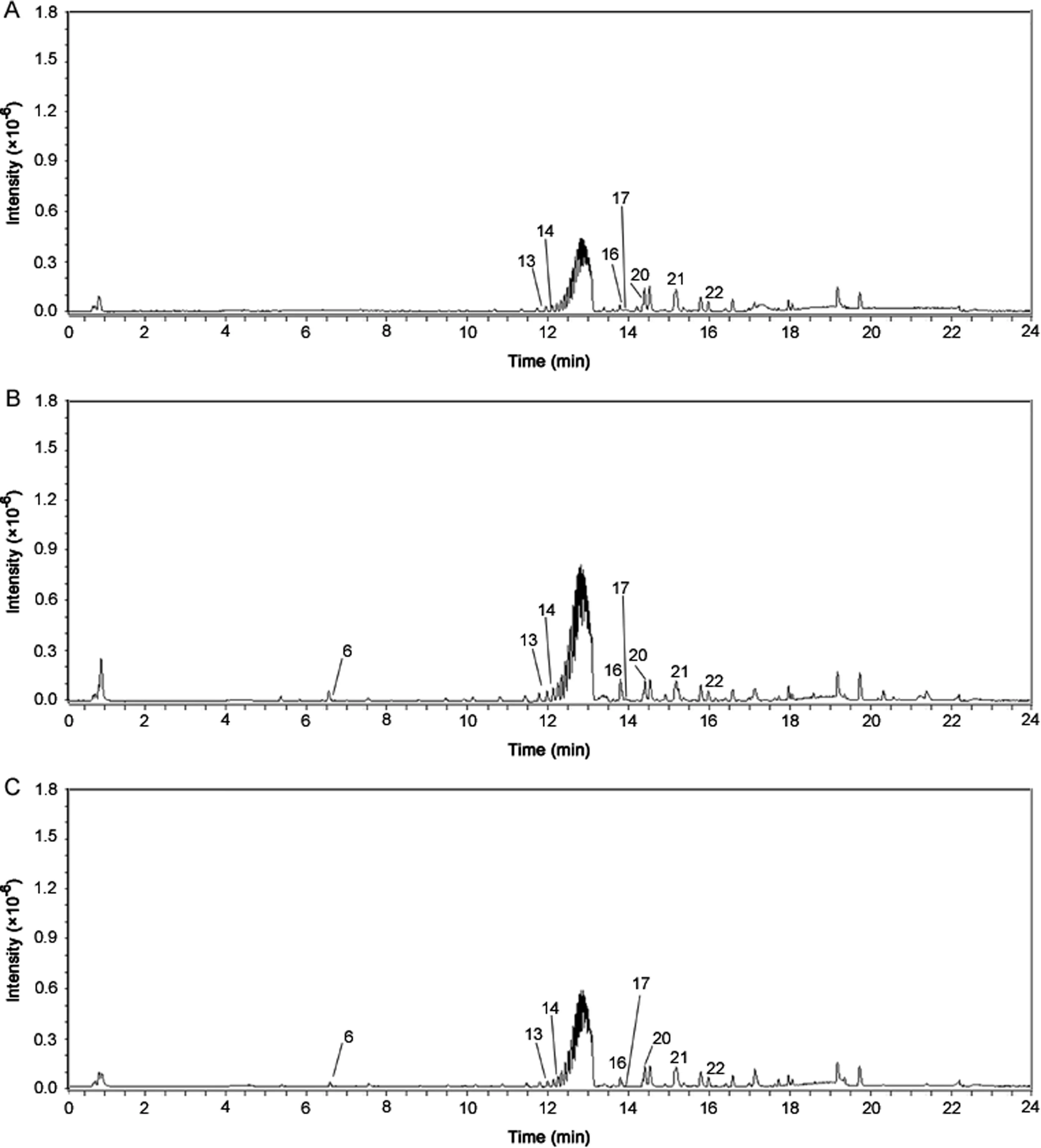
Fig.6.LC–MS base peak chromatograms of various laser dissected tissues of A.aspera analysed in negative mode.(A)cortex,(B)medullary rays,and(C)tertiary vascular bundles.
Appendix A.Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2017.06.006.
[1]V.Upadhya,S.R.Pai,G.Ankad,et al.,Phenolic contents and antioxidant properties from aerial parts of Achyranthes coynei Sant,Indian J.Pharm.Sci.75(2013)483–486.
[2]S.Bang,J.H.Kim,H.Y.Kim,et al.,Achyranthes japonica exhibits anti inflammatory effect via NF-??B suppression and HO-1 induction in macrophages,J.Ethnopharmacol.144(2012)109–117.
[3]B.Gokhale,S.Damre,K.R.Kulkami,et al.,Preliminary evaluation of anti-inflammatory and anti-arthritic activity of S.lappa,A.speciosa and A.aspera,Phytomedicine 9(2002)433–437.
[4]Y.Ida,Y.Satoh,M.Katsumata,et al.,Two novel oleanolic acid saponins having a sialyl Lewis X mimetic structure from Achyranthes fauriei root,Bioorg.Med.Chem.Lett.8(1998)2555–2558.
[5]S.Srivastav,P.Singh,G.Mishra,et al.,Achyranthes aspera-an important medicinal plant:a review,J.Nat.Prod.Plant Resour.1(2011)1–14.
[6]S.Zhou,X.Chen,X.Gu,et al.,Achyranthes bidentata Blume extract protects cultured hippocampal neurons against glutamate-induced neurotoxicity,J.Ethnopharmacol.122(2009)547–554.
[7]Q.H.Wang,L.Yang,H.Jiang,et al.,Three new phytoecdysteroids containing a furan ring from the roots of Achyranthes bidentata Bl,Molecules 16(2011)5989–5997.
[8]T.Vetrichelvan,M.Jegadeesan,Effect of alcoholic extract of Achyranthes bidentata Blume on acute and sub acute inflammation,Indian J.Pharmacol.34(2002)115–118.
[9]D.B.Xiang,X.Y.Li,Antitumor activity and immuno-potentiating actions of Achyranthes bidentata polysaccharides,Acta Pharmacol.Sin.14(1993)556–561.
[10]C.C.He,R.R.Hui,Y.Tezuka,et al.,Osteoprotective effect of extract from Achyranthes bidentata in ovariectomized rats,J.Ethnopharmacol.127(2010)229–234.
[11]S.Sun,Y.Bai,Z.Yu,et al.,Studies on Niuxi with the method of FT-IR spectroscopy and analyze technology auxiliary,Zhong Yao Cai.28(2005)181–184.
[12]S.Yu,Y.Zhang,Effect of Achyranthes bidentata polysaccharides(ABP)on antitumor activity and immune function of S180-bearing mice,Chin.J.Oncol.17(1995)275–278.
[13]A.Kumar,R.Londonkar,Potential antibacterial and antifungal activity of Achyranthes aspera L,Recent Res.Sci.Technol.3(2011)53–57.
[14]H.Mukherjee,D.Ojha,P.Bag,et al.,Anti-herpes virus activities of Achyranthes aspera:an Indian ethnomedicine,and its triterpene acid,Microbiol.Res.168(2013)238–244.
[15]S.Srivastav,P.Singh,K.K.Jha,et al.,Diuretic activity of whole plant extract of Achyranthes aspera Linn,Eur.J.Exp.Biol.1(2011)97–102.
[16]N.Vasudeva,S.K.Sharma,Post-coital antifertility activity of Achyranthes aspera Linn.root,J.Ethnopharmacol.107(2006)179–181.
[17]W.Shibeshi,E.Makonnen,L.Zerihun,et al.,Effect of Achyranthes aspera L.on fetal abortion,uterine and pituitary weights,serum lipids and hormones,Afr.Health Sci.6(2006)108–112.
[18]P.R.Subbarayan,M.Sarkar,S.Impellizzeri,et al.,Anti-proliferative and anticancer properties of Achyranthes aspera:specific inhibitory activity against pancreatic cancer cells,J.Ethnopharmacol.131(2010)78–82.
[19]S.Vijaya Kumar,P.Sankar,R.Varatharajan,Anti-inflammatory activity of roots of Achyranthes aspera,Pharm.Biol.47(2009)973–975.
[20]A.S.Hildebert Wagner,R.Bauer,D.Melchart,P.Xiao(Eds.),Chromatographic Fingerprint Analysis of Herbal Medicines:Thin-layer and High Performance Liquid Chromatography of Chinese Drugs,Springer Publications,New York,2014:119–129.
[21]P.K.Hasmukh,R.Jadav,R.Galib,Pharmaceutical standardization of Apamarga kshara,J.Ayurveda Integr.Med.6(2015)290–294.
[22]J.S.Kumar,H.Raj,Ethnobotanical explorations in the Balh valley region of North Western Himalaya,Int.J.Sci.Res.2(2013)40–44.
[23]R.C.Kumar,Ethno-medicinal uses of some plants of lower foot hills of Himachal Pradesh for the treatment of oral health problems and other mouth disorders,Int.J.Adv.Res.1(2013)1–7.
[24]D.S.Rao,T.Penmatsa,A.K.Kumar,et al.,Antibacterial activity of aqueous extracts of Indian chewing sticks on dental plaque:an in vitro study,J.Pharm.Bioallied Sci.6(2014)S140–S145.
[25]M.S.Gundeti,R.G.Reddy,J.V.Muralidhar,Subcutaneous intralesional Ksharodaka injection:a novel treatment for the management of Warts:a case series,J.Ayurveda Integr.Med.5(2014)236–240.
[26]D.Y.Gawande,D.Druzhilovsky,R.C.Gupta,et al.,Anticonvulsant activity and acute neurotoxic profile of Achyranthes aspera Linn,J.Ethnopharmacol.18(2017)97–102.
[27]M.Thakur,R.Asrani,S.Thakur,et al.,Observations on traditional usage of ethnomedicinal plants in humans and animals of Kangra and Chamba districts of Himachal Pradesh in North-Western Himalaya,India,J.Ethnopharmacol.191(2016)280–300.
[28]X.He,X.Wang,X.Fang,et al.,The genus Achyranthes:a review on traditional uses,phytochemistry,and pharmacological activities,J.Ethnopharmacol.203(2017)260–278.
[29]Z.K.Li,D.D.Li,The immunomodulatory effect of Achyranthes bidentata polysaccharides,Yaoxue Xuebao.32(1997)881–887.
[30]J.I.Jing-zhang,H.U.Jing-yi,L.V.Jian-xin,Effect of Achyranthes bidentata polysaccharides on induction and differentiation of CD4+T cells,Chin.J.Pathophysiol.22(2006)228–233.
[31]Y.K.M.Fukumura,H.Ando,Y.Hirai,et al.,Achyranthoside H methyl ester,a novel oleanolic acid saponin derivative from Achyranthes fauriei roots,induces apoptosis in human breast cancer MCF-7 and MDA-MB-453 cells via a caspase activation pathway,J.Nat.Med.63(2009)181–188.
[32]L.T.Virginia,M.Tyler,Understanding Alternative Medicine:New Health Paths in America,2014.
[33]D.K.Ved,G.S.Goraya,Demand and Supply of Medicinal Plants in India,NMPB,New Delhi&FRLHT,Bangalore,India,2007.
[34]M.H.Wu,X.L.Li,M.Wang,et al.,Determination of oleanolic acid in Achyranthes bidentata BLume and its preparations by supercritical fluid chromatography,Acta Pharm.Sin.27(1992)690–694.
[35]M.Zhang,Z.Y.Zhou,J.Wang,et al.,Phytoecdysteroids from the roots of Achyranthes bidentata Blume,Molecules 17(2012)3324–3332.
[36]G.Michl,D.Abebe,F.Bucar,et al.,New triterpenoid saponins from Achyranthes aspera LINN,Sect.Title Plant Biochem.83(2000)359–363.
[37]K.S.Laddha,D.Ghosh,Isolation of 20-hydroxyecdysone from Indian medicinal plant Achyranthes aspera and development of simple HPLC analysis,Nat.Prod.Indian J.1(2005)1–4.
[38]D.Meng,S.Ji,Y.Zhang,et al.,Isolation and identification of terpenoids and saccharides from root of Achyranthes bidentata B1,Sect.Title Plant Biochem.26(2009)348–352,392.
[39]Y.Jaiswal,Z.Liang,A.Ho,et al.,Distribution of toxic alkaloids in tissues from three herbal medicine Aconitum species using laser micro-dissection,UHPLCQTOF MS and LC-MS/MS techniques,Phytochemistry 107(2014)155–174.
[40]Y.Jaiswal,Z.Liang,A.Ho,et al.,Metabolite profiling of tissues of Acorus calamus and Acorus tatarinowii rhizomes by using LMD,UHPLC-QTOF MS,and GC-MS,Planta Med.81(2015)333–341.
[41]W.E.Gnanaraj,M.Johnson,R.B.Mohanamathi,et al.,In vitro clonal propagation of Achyranthes aspera L.and Achyranthes bidentata Blume using nodal explants,Asian Pac.J.Trop.Biomed.2(2012)1–5.
[42]A.Ilczuk,E.Jacygrad,K.Jagiełło-Kubiec,A.Pacholczak,In vitro propagation of woody plants—prospects and problems,in:J.Rabiza-Świder,E.Skutnik(eds.)Ornamental horticulture as sector of the national economy,Department of Ornamental Plants,SGGW,Warszawa,2013:41–48.
[43]Y.S.Jaiswal,Z.Liang,P.Guo,et al.,Tissue-specific metabolite profiling of Cyperus rotundus L.Rhizomes and(+)-Nootkatone quantitation by using laser micro dissection,UHPLC-QTOF MS and GC-MS techniques,J.Agric.Food Chem.62(2014)7302–7316.
[44]Y.Jaiswal,Z.Liang,A.Ho,et al.,A comparative tissue-specific metabolite analysis and determination of protodioscin content in asparagus species used in traditional Chinese medicine and ayurveda by use of laser microdissection,UHPLC-QTOF/MS and LC-MS/MS,Phytochem.Anal.25(2014)514–528.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- S-Nitroso-N-acetyl-L-cysteine ethyl ester(SNACET)and N-acetyl-L-cysteine ethyl ester(NACET)–Cysteine-based drug candidates with unique pharmacological profiles for oral use as NO,H2S and GSH suppliers and as antioxidants:Results and overview
- A liquid chromatography with tandem mass spectrometry method for quantitating total and unbound ceritinib in patient plasma and brain tumor
- Denaturation studies on bovine serum albumin–bile salt system:Bile salt stabilizes bovine serum albumin through hydrophobicity
- Insight into the interaction of inhaled corticosteroids with human serum albumin:A spectroscopic-based study
- Effect of nonionic surfactants in release media on accelerated in-vitro release profile of sirolimus eluting stents with biodegradable polymeric coating
- Electrooxidation of sulfanilamide and its voltammetric determination in pharmaceutical formulation,human urine and serum on glassy carbon electrode
