Insight into the interaction of inhaled corticosteroids with human serum albumin:A spectroscopic-based study
2018-03-06CrlottPontremoliNdiBreroGuidoViscrdiSonjVisentin
Crlott Pontremoli,Ndi Brero,Guido Viscrdi,Sonj Visentin
aDepartment of Applied Science and Technology(DISAT),Politecnico of Torino,Corso Duca degli Abruzzi 24,Torino 10129,Italy
bDepartment of Chemistry and NIS Interdepartmental Centre,University of Torino,via Pietro Giuria 7,10125 Torino,Italy
cMolecular Biotechnology and Health Sciences Department,University of Torino,via Quarello 15,10135 Torino,Italy
1.Introduction
The study of protein–drug interactions plays an important role in pharmacokinetics and pharmacodynamics of drugs.They influence the distribution and elimination speed;only non-binding drug can spread and reach the target producing a biological response.One of the most important factors affecting the distribution and the free,active concentration of many administered drugs is binding affinity for human serum albumin(HSA).Drug binding to HSA increases drug half-life and lowers the free drug concentration in blood,which makes it extremely important for clinical care.In early drug discovery,the plasma protein binding is important in order to evaluate drug dosing needs and clearance from the body.
HSA is the most abundant drug carrier protein,with a well known primary structure.Its tertiary structure has been determined by X-ray crystallography[1].It has an important role in maintaining the colloidal osmotic pressure in blood and in the transport of exogenous and endogenous substances,including fatty acids,amino acids,steroids,bilirubin and drugs[2,3].Backbone of protein consists of a single polypeptide chain of 585 amino acid residues that form three homologous domains(I,II,and III),stabilized by 17 disulfide bridges due to the 34 cysteines present in the molecule;each domain contains two subdomains(A and B),respectively constituted by 6 and 4 α-helices[4–6].Crystallographic studies have revealed that HSA possesses binding sites for aromatic and heterocyclic ligands within two hydrophobic pockets:in subdomains IIA(Sudlow's site I:warfarin-binding site)and IIIA(Sudlow's site II:indole/benzodiazepine site).Both hydrophobic and electrostatic interactions play a major role in controlling the affinity towards drug binding for sites I and II.For site I,mainly hydrophobic interactions are dominant,while for site II,a combination of hydrophobic,hydrogen bonding and electrostatic interactions plays a crucial role.The tryptophan residue(Trp 214)of HSA is in subdomain IIA(site I)and plays a crucial role in spectrophotometric studies[2,3].When a ligand binds to one domain,it can induce distinct conformational changes on the other domain,as both sub domains share a common interface.For this reason,the binding of a drug to serum albumin may change considerably the binding abilities of HSA towards other molecules[7].
Fig.1.The interaction of ICSs with albumin plays an important role in governing systemic side effects.
From a pharmaceutical point of view,the interaction between inhaled corticosteroids(ICSs)and HSA is very interesting.Corticosteroids are the most potent and effective anti-inflammatory agents in many respiratory chronic diseases.In this case,the preferred way of administration of a corticosteroid is inhalation;this way permits to deliver the drug directly to the lung,where it acts locally in order to minimise the systemic side effects,compared to oral or parenteral administration.Clinical studies have shown that ICSs significantly reduce airway hy-perresponsiveness,effectively prevent acute exacerbations,improve lung function and decrease symptoms severity[8].Corticosteroids are involved in different physiological processes;in particular,they alter the production of inflammatory mediators in the airways,such as macrophages,eosinophils,lymphocytes,mast cells and dendritic cells[9].
It is well known that the safety and efficacy profile of an ICS is influenced by the pharmacokinetic properties and associated pharmacodynamic effects of the drug[10,11].If ICSs are not bound,they can circulate freely and cause systemic adverse effects.In fact,the freely circulating ICSs can bind to nonpulmonary glucocorticoids receptors and cause adverse effects such as a reduction in the function of the hypothalamic-pituitary-adrenal(HPA)axis and growth impairment[12].Extensive protein binding can,therefore,be viewed as a way to temporarily remove an ICS that is available to the tissues from the systemic circulation,thereby reducing the potential for development of adverse effects[12–14].Among other pharmacological properties,high plasma protein binding is a desirable property for any ICS,since this reduces the potential for systemic side effects[15].Therefore,adetailed investigation of drug–protein interaction assumes significance for thorough understanding of the pharmacokinetic behaviour of corticosteroids and for the design of analogues with effective pharmacological properties(Fig.1).
The purpose of this study was to evaluate the extent of protein binding of different ICSs,like betamethasone(A), flunisolide(B),prednisolone(C)and triamcinolone(D)(Fig.2)in order to develop a rapid spectroscopic method to study the interaction and eventually compare the affinity of ICSs with HSA.To our knowledge,the interaction between ICS and HSA has never been investigated by spectroscopic techniques.
In this study,UV–Vis and fluorescence spectroscopy were used to elucidate the mode of binding and probable structural alterations of HSA upon drug binding.The binding constant and the nature of binding forces were determined.Lastly,the thermodynamic and Förster's parameters associated with the binding process were also calculated.All the data obtained could clarify the type of interaction that can occur between ICS and HSA and could be fundamental to understand if and how the structural features of the drugs could modulate this interaction.Moreover,it may pave the comprehension of the bioavailability of corticosteroids,justifying the major use as inhaled administration,and facilitate the interpretation of absorption and distribution process of corticosteroids.
2.Materials and methods
2.1.Materials
Albumin from human serum(HSA)lyophilized powder,≥97%(agarose gel electrophoresis),was purchased from Sigma Aldrich(Italy).To prepare the stock solution(100μM),HSA was dissolved in 2 mM phosphate buffer solution(PBS,pH 7.4).
Betamethasone(≥98%), flunisolide(≥97%),prednisolone(≥99%)and triamcinolone were all purchased from Sigma Aldrich(Italy);the stock solutions(3 mM)were prepared by dissolving drugs in a solution of 96%ethanol and PBS(1:1,v/v).
2.2.Apparatus
All fluorescence spectra were recorded with a Horiba Jobin Yvon Fluorolog3 TCSPC spectrofluorophotometer(Bernsheim,Germany)with 1.0 cm quartz cells.UV–Vis spectra were recorded on a UH5300 Hitachi spectrophotometer(Hitachi Europe,Milan,Italy).The pH measurements were made with a Eutech Instruments pH2700(Landsmeer,The Netherlands).
2.3.Experimental conditions
UV–Vis measurements were carried out in the range of 200–400 nm.UV–Vis absorption spectra were recorded at room temperature,by using different concentrations of drugs(betametha-sone,prednisolone,and triamcinolone=2.0,4.0,6.0,8.0,10.0μM;flunisolide=5.0,10.0,15.0,20.0,25.0μM).
Fluorescence quenching spectra were measured in the range of 300–500 nm upon excitation at 280 nm.The excitation and emission slits were 6 nm and 10 nm,respectively.The fluorescence spectra were performed at three different temperatures(296 K,303 K,and 310 K).5μM HSA was titrated by successive additions of drug solutions at different concentrations.To reach protein saturation,it is necessary to use a range from 50μM to 500μM for betamethasone and from 50μM to 700μM for flunisolide,prednisolone and triamcinolone.
Fluorescence resonance energy transfer(FRET)measurements were performed at room temperature(296 K).The overlaps were obtained by using the emission spectrum of 5 μM HSA(λexcitationat 280 nm;the excitation and emission slits were 6 nm and 10 nm,respectively)and the absorption spectra of drugs(betamethasone,prednisolone,and triamcinolone=10.0μM; flunisolide=25.0μM).
3.Results and discussion
3.1.UV–Vis spectroscopy
Absorption spectroscopy is one of the techniques used to explore the structural changes of protein and to investigate proteinligand complex formation[16].HSA has two main absorption bands,and one of them is located at 280 nm,which is the absorption band of the tryptophan(Trp 214)[17,18].
The absorption spectra of the protein at room temperature in absence and in presence of different concentrations of drugs(betamethasone,prednisolone,and triamcinolone=2.0,4.0,6.0,8.0,10.0μM; flunisolide=5.0,10.0,15.0,20.0,25.0μM)were recorded and are shown in Fig.S1.As can be seen,for every sample,the absorption intensity of HSA at around 280 nm increased with the addition of increased concentrations of drugs.Moreover,the absorption spectrum of protein-drug complex was different from that of albumin and drugs alone.The maximum peak position of HSA-drug complex was slightly shifted towards lower wavelength region.These results confirmed that every studied drug can bind the protein.
3.2.Fluorescence quenching mechanism
The fluorescence spectrum of albumin was recorded in absence and in presence of drugs at different concentrations.Fig.3 shows spectra at room temperature.HSA shows a typical strong fluorescence emission peak at 350 nm,which does not shift in the presence of the drugs.Every analysed molecule causes a concentration-dependent quenching of the intrinsic fluorescence of protein that decreases gradually with the increase of drug concentration.The quenching of protein fluorescence by drugs was due to the formation of a protein-drug complex.This means that the microenvironment of HSA was changed during the binding interaction.In order to obtain thermodynamic parameters,binding studies were performed at three different temperatures(296 K,303 K and 310 K)and the obtained steady-state maximum fluorescence intensity was recorded.
Fluorescence quenching data were treated by different methods,as reported in the following paragraphs,to evaluate the equilibrium association(KA)and dissociation(KD)constants.
3.2.1.Stern-Volmer equation
First of all, fluorescence quenching of albumin was analysed by Stern-Volmer Eq.(1)[19]:

whereF0is the fluorescence intensities in the absence of quencher,Fis the steady-state fluorescence intensity in the presence of the quencher,and[Q]is the concentration of the quencher.KSVis the Stern-Volmer quenching constant and describes a collisional quenching of fluorescence.Quenching data are presented as plots ofF0/Fvs.[Q],yielding an intercept of one on the y-axis and a slope equal toKSV.Fig.4 shows Stern-Volmer plots of the fluorescence quenching of HSA by drugs at different temperatures.
A linear Stern-Volmer plot,however,does not define the quenching mechanism.In order to distinguish dynamic from static quenching,the dependence of the interaction of a drug,described byKSV,on temperature has been proposed.TheKSVvalues decrease with an increase in temperature for static quenching and the reverse effect can be observed for dynamic quenching[19].
As shown in Table 1,theKSVof the protein-drug complexes A,B,and C decreases with increased temperature.This indicates that a possible static quenching interaction between protein and drug occurs[20].TheKSVof protein-triamcinolone complex is similar to negligible variations by changing the temperature,but it is possible to observe a slight increase also in this case,so probably a static quenching interaction between protein and drug may also occur.
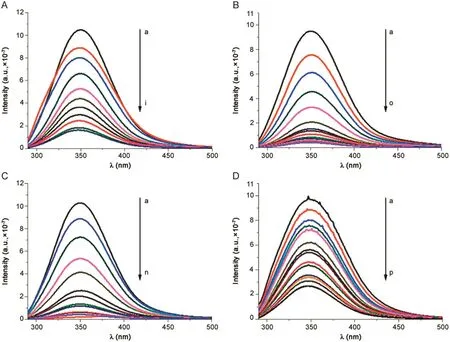
Fig.3.Fluorescence spectra of HSA–drugs interaction(T=296 K).(A)HSA–betamethasone;(B)HSA– flunisolide;(C)HSA–prednisolone and(D)HSA–triamcininolone.λex=280 nm.
3.2.2.Non-linear least squares
Non-linear least squares fit procedure is a simple method to analyse fluorescence data at different temperatures[21]based on Eq.(2):

where[Q]is the drug concentration,yis the specific binding derived by measuring fluorescence intensity,Bmaxis the maximum amount of the protein-drug complex formed at saturation,andKDis the equilibrium dissociation constant.Fig.5 shows the binding curves obtained;the percentage of bound HSA,i.e.y,derived from the fluorescence intensity emission maximum,is plotted against the drug concentration.
The correspondingKDandKA(which are reciprocals of each other)at different temperatures are shown in Table 2.The binding constant calculated for HSA-triamcinolone complex suggests the lower affinity of this drug for the protein than the other tested drugs,as reported in literature[15].TheKAof protein-triamcinolone complex is similar to negligible variations by changing the temperature,but it is also possible to observe a slight increase.In this case,affinity seems to be higher for flunisolide.By increasing temperature,for A,B and C HSA-drug complexes,the value of association constant decreases.
3.3.Binding parameters
By double logarithm regression curve(shown in Eq.(3))[22],it is possible to obtain the number of binding sites(n).Eq.(3)describes the relationship between fluorescence intensity and the quencher concentration:

whereF0is the fluorescence intensity of the protein alone,Fis the fluorescence intensity after the addition of the quencher,and[Q]is the quencher/drug concentration.The plots obtained using Eq.(3)are shown in Fig.S2.The slope of the line is the n value.If the value of n is equal to 1,it means that a strong binding exists between the protein and the drug[22].
The number of binding sites is easily calculated:for HSA–betamethasone complex is 1.50(296 K),1.30(303 K),and 1.50(310 K),for HSA– flunisolide is 1.30(296 K),0.92(303 K),and 1.10(310 K),for HSA–prednisolone complex is 1.30(296 K),1.40(303 K),and 1.30(310 K)and for HSA–triamcinolone is 1.30(296 K),1.40(303 K),and 1.30(310 K).Almost all values are approximately equal to 1,indicating that there is one independent binding site on HSA for each analysed drug[23].
3.4.Site marker competitive binding experiments
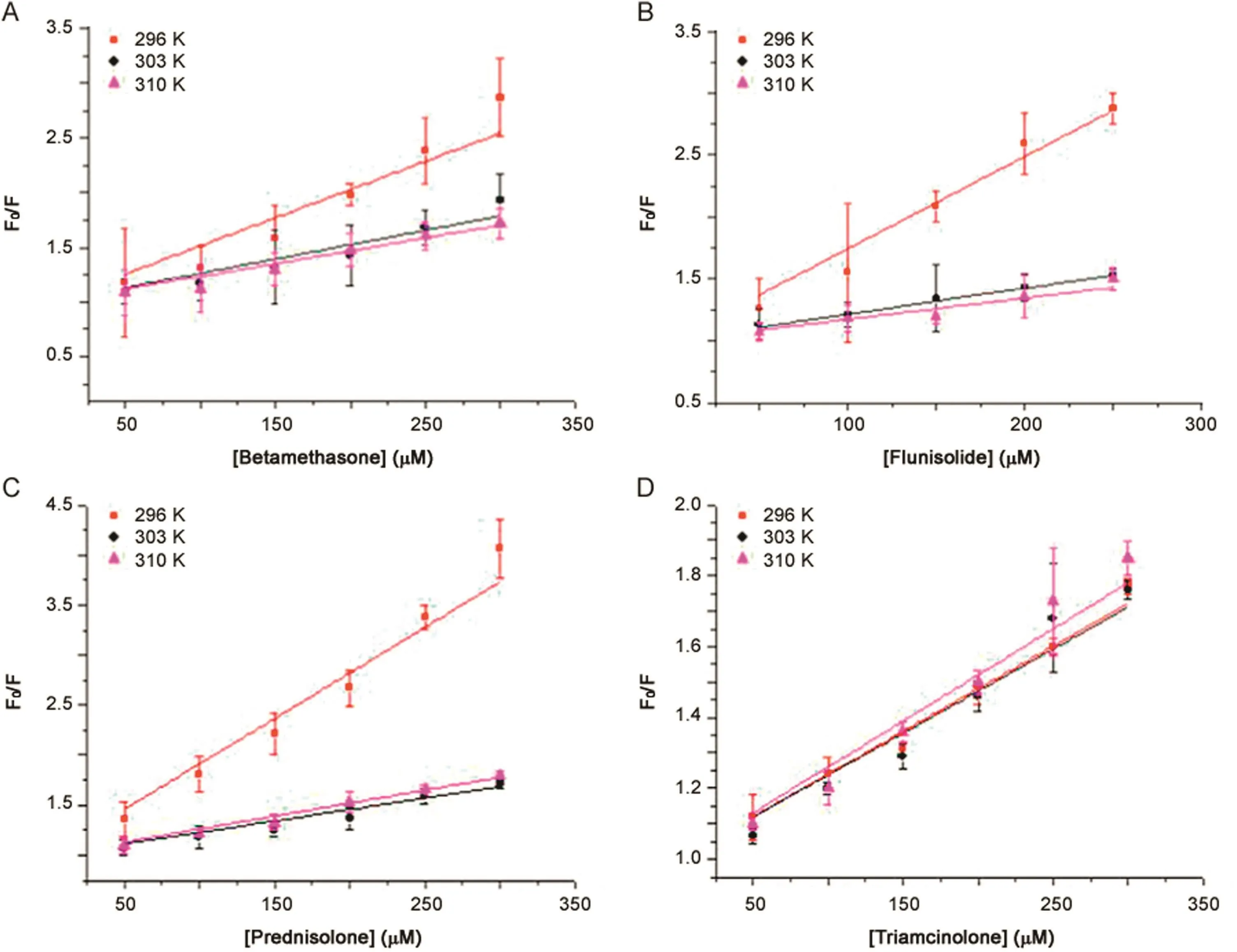
Fig.4.The Stern-Volmer plots of the fluorescence quenching of HSA by drugs at different temperatures.(A)betamethasone;(B) flunisolide;(C)prednisolone;and(D)triamcinolone.
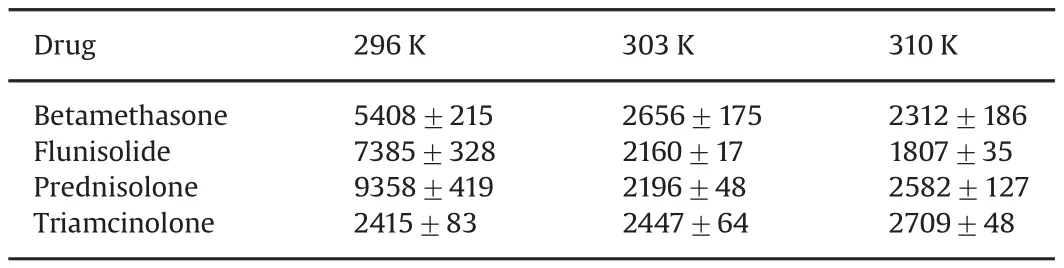
Table 1The quenching constants(KSVin M-1)of HSA and drugs at different temperatures.
In order to further investigate drug binding site and to precisely determine the location of corticosteroids on HSA,competitive binding tests were carried out.Warfarin and ibuprofen are two specific markers for HSA binding sites I and II,respectively[24].In the site marker competitive experiment,warfarin or ibuprofen was gradually added to the solution of HSA-corticosteroids complex and then fluorescence intensity of the system was recorded.As shown in Fig.S3,with the addition of warfarin in the HSA-drug solution,the fluorescence intensity was slightly higher than that without warfarin,including a red shift.Then,when increasing warfarin concentration into the solution of HSA-corticosteroids complex,the fluorescence intensity of HSA solution decreased gradually,reaching saturation at 1 mM of site marker with an effective displacement of about 98%–99%for all the tested drugs,indicating that the binding of the corticosteroids to HSA was affected by warfarin addition.On the contrary,the addition of ibuprofen to the HSA-drug complex only promoted a slight enhancement of the fluorescence intensity(Fig.S4),indicating that site II marker did not prevent the binding of corticosteroids in its usual binding location.These results suggest that corticosteroids compete with warfarin for binding to HSA to site I[24].
3.5.Thermodynamic parameters
The interaction forces between small molecules and macromolecules include four binding modes:H-bonding,Van der Waals,electrostatics and hydrophobic interactions[25].The model of interaction between drug and the protein could be obtained,according to the data of enthalpy(ΔH)and entropy change(ΔS)[26]:(1)ΔH>0 andΔS>0,hydrophobic forces;(2)ΔH<0 and ΔS<0,van der Waals interactions and hydrogen bonds;(3)ΔH<0 andΔS>0,electrostatic interactions.The thermodynamic parameters,enthalpy and entropy of the HSA-drugs complex reaction are important to confirm binding modes.The temperature-dependence of the binding constant was analysed at 296 K,303 K,and 310 K and thermodynamic parameters were calculated from the following Van’t Hoff equations[19]:

whereKAis the binding constant,R is the gas constant andTis the experimental temperature.The values ofΔHandΔSobtained for the binding sites are shown in Table S1.The negative sign forΔG means that the binding process is spontaneous for every studied interaction[23].
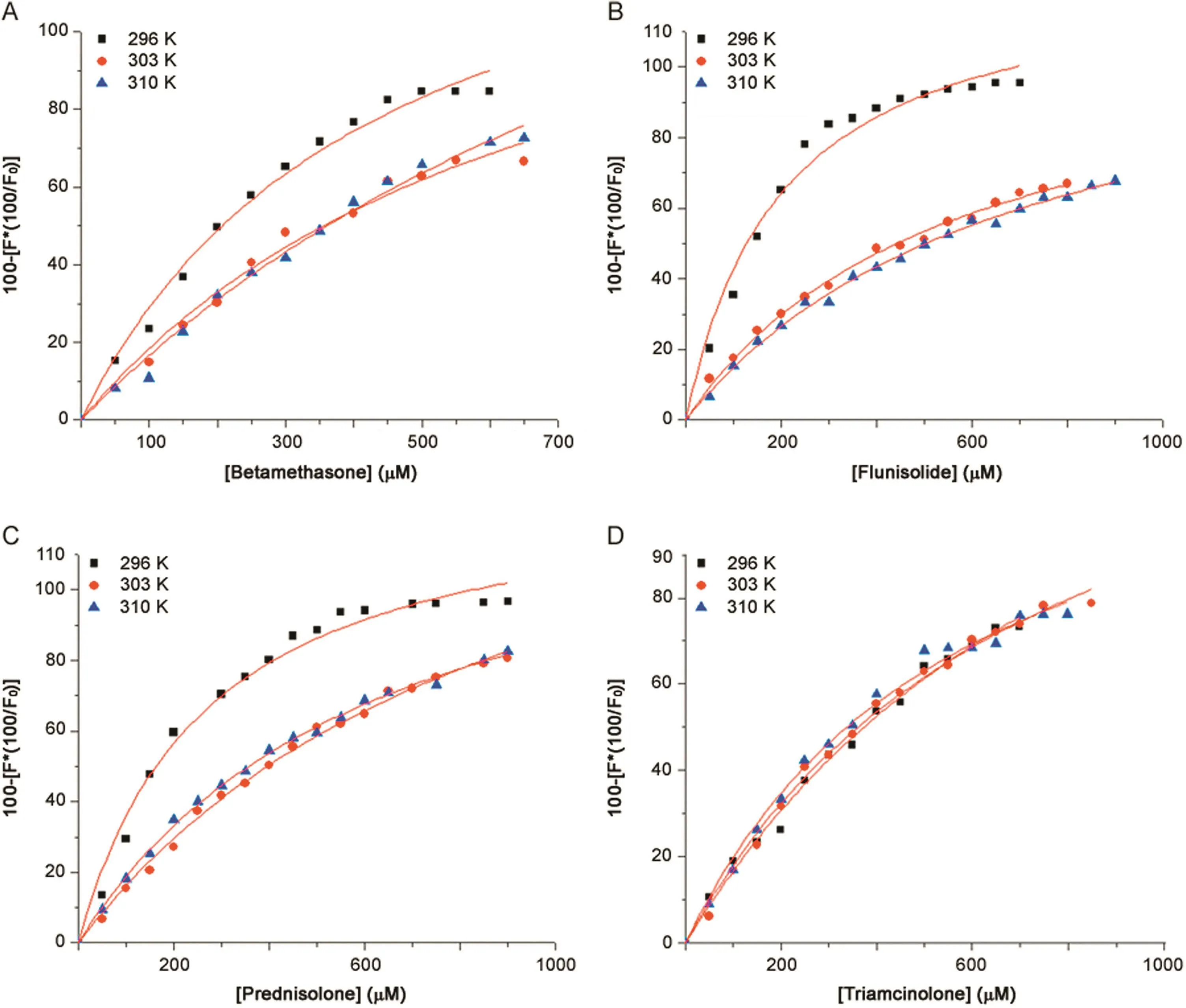
Fig.5.The binding curves of HSA–drugs complex at different temperatures.(A)betamethasone;(B) flunisolide;(C)prednisolone and(D)triamcinolone.
From Table S1 it can be seen that for HSA-betamethasone,HSA-flunisolide and HSA-prednisolone complexes,bothΔHandΔShave negative values.This indicates that van der Waals interactions and hydrogen bonds may play a major role in the binding.Conversely,in the formation of HSA-triamcinolone complex,an exothermic reaction occurs,characterized by a negativeΔHvalue and a positiveΔSvalue.From the point of view of water structure,a positiveΔSvalue is frequently taken as a typical evidence for hydrophobic interaction.Furthermore,specific electrostatic interactions between ionic species in aqueous solution are characterized by a positiveΔSvalue and a negativeΔHvalue[27].In order to evaluate the thermodynamic parameters of HSA-triamcinolone complex,it is not possible to take account of a single intermolecular force model.As described in literature for another complex(HSA-dexamethasone)[28],the binding,in this case,might involve hydrophobic interaction strongly,as evidenced by the positive values of ΔS,but electrostatic interaction can not be excluded either.
3.6.Energy transfer
FRET is a simple method to measure the distance between the acceptor(ligand)and the donor(tryptophan residues in the protein)[29].According to Förster's non-radiative energy transfer theory,energy efficiency(E),critical energy-transfer distance(R0,E=50%),the energy donor and the energy acceptor distance(r)and the overlap integral between the fluorescence emission spectrum of the donor and the absorption spectrum of the acceptor(J)can be calculated by the following equations[30]:
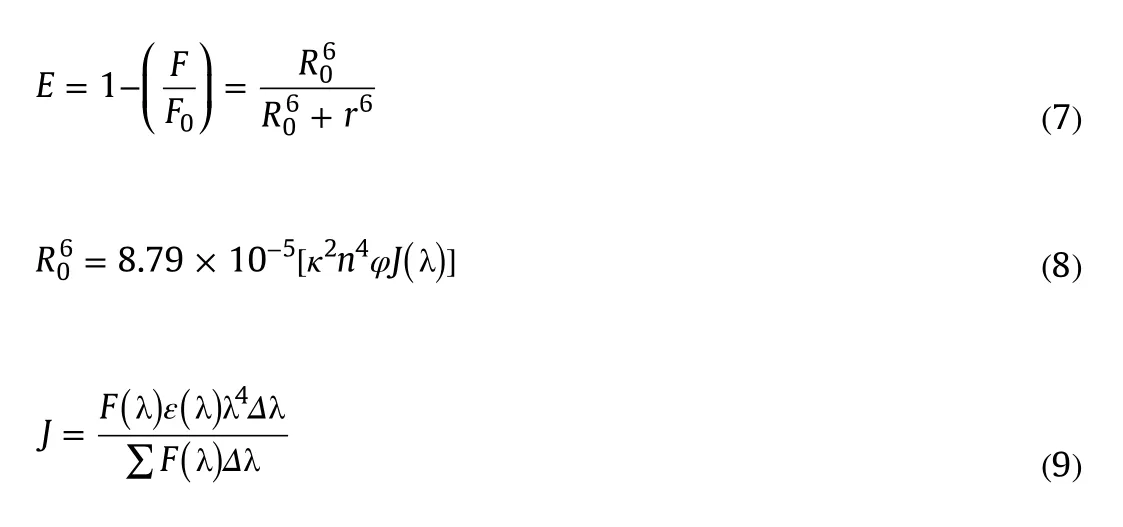
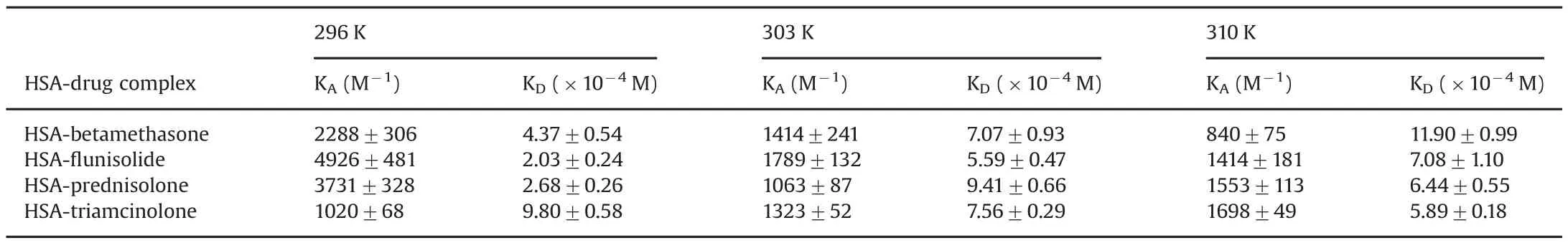
Table 2Values of the equilibrium dissociation and association constants of HSA-drug complexes at different temperatures obtained by a non linear fit equation.
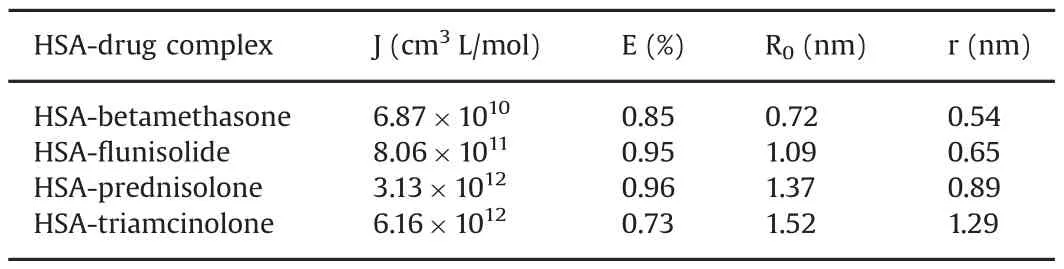
Table 3Parameters of J,E,R0and r of HSA-drug complexes at 296 K.
wherek2is the orientation factor,φis the fluorescence quantum yield of the donor,nis the refractive index of the medium,F(λ)is the fluorescence intensity of the donor at wavelength λ and ε(λ)is the molar absorption coefficient of the acceptor at wavelength λ.In this case,k2=2/3,n=1.336 andφ=0.118[16].
The overlaps of the emission spectra of the protein and the absorption spectra of drugs at room temperature were obtained(Fig.S5).Using these equations,it is possible to calculate J,E,R0and r for every interaction.Data are reported in Table 3.
The distance r<7 nm indicates that the energy transfer between protein and drugs occurred with a high possibility[16,23].This is in agreement with conditions of Föster's non-radiative energy transfer theory[31],indicating again the static quenching interaction between protein and drugs.According to Stern-Volmer plots,data obtained with different methods are comparable with each other.
3.7.Conformation investigation
Synchronous fluorescence spectroscopy introduced by Lloyd[32]has been used to investigate the conformational change of proteins.The synchronous fluorescence spectrum could be obtained by synchronously scanning the excitation and emission monochromators with a wavelength difference between excitation and emission as a constant.The intrinsic fluorescence of HSA is manifested by emission of Trp and Tyr residues present in the protein[33].
The synchronous fluorescence spectra obtained with Δλ=60 nm exclusively characterize the fluorescence of tryptophan residue.After complex formation,the local environment could change and induce a red or blue shift of the tryptophan emission spectra.The shift in the position of fluorescence emission maximum corresponds to changes of the polarity around the chromophore molecule.A blue shift of λmaxmeans that the aminoacid residues are located in a more hydrophobic environment and are less exposed to the solvent,while a red shift of λmaximplies that the amino acid residues are in a polar environment and are more exposed to the solvent[34].By synchronous fluorescence spectral changes of aminoacid residues,the conformational changes of protein can be predicted.We can also obtain the information about the location of corticosteroids binding site from the synchronous fluorescence data.
The synchronous fluorescence spectra of HSA in presence of betamethasone, flunisolide,prednisolone and triamcinolone were recorded and are shown in Fig.6.For every analysed complex,the emission maximum of tryptophan residues showed significant red shift of tryptophan residue fluorescence,confirming that every analysed drugs reached subdomain IIA,where only one Trp residue(Trp 214)is located.High concentration of drugs makes protein molecules extend,thus reducing energy transfer between aminoacids and reducing the fluorescence intensity[35].Since Trp 214 is at site I,these results indicated that corticosteroids can bind to HSA in the hydrophobic cavity on subdomain IIA,which is in full agreement with quenching and competitive binding experiments.
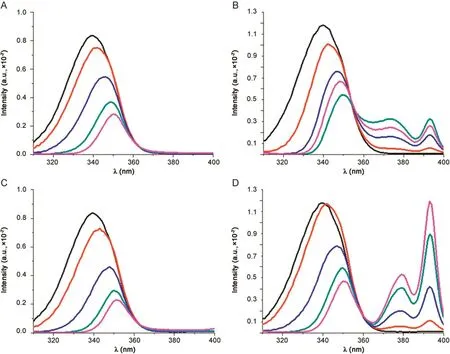
Fig.6.Synchronous fluorescence spectra of HSA-drugs at 296 K,Δλ=60 nm.[HSA]:black line;[Drugs]:50.0–800.0 μM.(A)betamethasone;(B) flunisolide;(C)prednisolone;and(D)triamcinolone.
4.Conclusions
Drug binding to HSA is a major problem in pharmaceutical research because the binding to albumin influences the effective drug concentration that can reach the target site.In this work,the interaction of albumin with four different corticosteroids was investigated at different temperatures by different spectroscopic approaches.UV–Vis spectroscopy confirmed that all the investigated drugs can bind to HSA to form a protein–drug complex.Quenching fluorescence data revealed that the protein can be bound by the studied corticosteroids,around the Trp 214(as confirmed by competitive binding experiments and synchronous fluorescence)and that the quenching is governed by a static quenching(data are comparable with results obtained by FRET).According to thermodynamic parameters(negativeΔHandΔSvalues),the hydrogen bonds and van der Waals forces play a major role in the binding process between albumin and betamethasone, flunisolide and prednisolone,while hydrophobic forces may play a major role in stabilizing albumin-triamcinolone complex.The evaluation of the equilibrium association(KA)and dissociation(KD)constants was obtained by a non-linear method at different temperatures.The data showed that temperature does not influence the formation of HSA-triamcinolone complex,while it can influence the interaction between albumin and betamethasone, flunisolide and prednisolone.This means that the drug structure may play a crucial role in the binding with the protein.Usually,drugs bind to HSA high-affinity sites with typical association constants in the range of 104–106M-1[36].As reported in literature[8],serum analysis revealed that the present corticosteroids can bind the HSA in a range between 70%and 80%thus with a low affinity.These data are in agreement with the equilibrium association constants obtained in this study.Usually a binder with high affinity,such as warfarin,showsKAaround 105M-1[37]while the studied ICSs haveKAin the order of 103M-1.
The present spectroscopic approach offers a fast screening method to eventually investigate the structure-activity relationship(SAR)of new therapeutic molecules since it can discriminate the binding affinity with simple and reliable experiments.
Conflicts of interest
The authors declare that there are no Conflicts of interest.
This work was supported by a grant from the University of Torino(Ricerca Locale ex-60%,Bando 2015).
Appendix A.Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2017.07.003.
[1]X.M.He,D.C.Carter,Atomic structure and chemistry of human serum albumin,Nature 358(1992)209–215.
[2]T.K.Maiti,K.S.Ghosh,J.Debnath,et al.,Binding of all-trans retinoic acid to human serum albumin: fluorescence,FT-IR and circular dichroism studies,Int.J.Biol.Macromol.38(2006)197–202.
[3]M.X.Xie,M.Long,Y.Liu,et al.,Characterization of the interaction between human serum albumin and morin,Biochim.Biophys.Acta 2006(1760)1184–1191.
[4]Y.Wang,H.Yu,X.Shi,et al.,Structural mechanism of ring opening reaction of glucose by human serum albumin,J.Biol.Chem.288(2013)15980–15987.
[5]F.Keshavarz,M.M.Alavianmehr,R.Youse fi,Molecular interaction of benzalkoniumibuprofenate and its discrete ingredients with human serum albumin,Phys.Chem.Res.2(2013)111–116.
[6]C.D.Kanakis,P.A.Tarantilis,M.G.Polissiou,et al.,Antioxidant flavonoids bind human serum albumin,J.Mol.Struct.798(2006)69–74.
[7]L.Trynda-Lemiesz,Paclitaxel–HSA interaction.Binding sites on HSA molecule,Bioorg.Med.Chem.12(2004)3269–3275.
[8]J.W.Georgitis,The 1997 asthma management guidelines and therapeutic issues relating to the treatment of asthma,Chest 155(1999)210–217.
[9]P.J.Barnes,Effect of corticosteroids on airway hyperresponsiveness,Am.Rev.Respir.Dis.141(1990)70–76.
[10]C.Crim,L.N.Pierre,P.T.Daley-Yates,A review of the pharmacology and pharmacokinetics of inhaled fluticasone propionate and mometasone furoate,Clin.Ther.231(2001)1339–1354.
[11]H.Derendorf,Pharmacokinetic and pharmacodynamics properties of inhaled corticosteroids in relation to efficacy and safety,Respir.Med.91(1997)22–28.
[12]O.D.Wolthers,J.W.Honour,Measures of hypothalamic-pituitary-adrenal function in patients with asthma treated with inhaled glucocorticoids:clinical and research implications,J.Asthma 36(1999)477–486.
[13]S.Rohatagi,S.Appajosyula,H.Derendorf,et al.,Risk-benefit value of inhaled glucocorticoids:a pharmacokinetic/pharmacodynamic perspective,J.Clin.Pharmacol.44(2004)37–47.
[14]H.Derendorf,G.Hochhaus,B.Meibohm,et al.,Pharmacokinetics and pharmacodynamics of inhaled corticosteroids,J.Allergy Clin.Immunol.101(1998)440–446.
[15]H.Derendorf,R.Nave,A.Drollmann,et al.,Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma,Eur.Respir.J.28(2006)1042–1050.
[16]H.M.Ma,X.Chen,N.Zhang,et al.,Spectroscopic studies on the interaction of a water-soluble cationic porphyrin with proteins,Spectrochim.Acta Part A:Mol.Biomol.Spectrosc.72(2009)465–469.
[17]J.Liu,J.N.Tian,J.Zhang,et al.,Interaction of magnolol with bovine serum albumin:a fluorescence-quenching study,Anal.Bioanal.Chem.376(2003)864–867.
[18]Y.Yue,X.Chen,J.Qin,et al.,Characterization of the mangiferin-human serum albumin complex by spectroscopic and molecular modeling approaches,J.Pharm.Biomed.49(2009)753–759.
[19]J.R.Lakowicz,Principles of Fluorescence Spectroscopy,Springer-Verlag,New York,2006.
[20]Y.J.Hu,Y.Liu,W.Jiang,et al.,Fluorometric investigation of the interaction of bovine serum albumin with surfactants and 6-mercaptopurine,J.Photochem.Photobiol.B 80(2005)235–242.
[21]N.Barbero,E.Barni,C.Barolo,et al.,A study of the interaction between fluorescein sodium salt and bovine serum albumin by steady-state fluorescence,Dyes Pigments 80(2009)307–313.
[22]K.H.Ulrich,Molecular aspects of ligand binding to serum albumin,Pharmacol.Rev.33(1981)17–53.
[23]B.Valeur,J.C.Brochon,New Trends in Fluorescence Spectroscopy,Springer Press,Berlin,1999.
[24]F.Ding,N.Li,B.Han,et al.,The binding of C.I.Acid Red 2 to human serum albumin:determination of binding mechanism and binding site using fluorescence spectroscopy,Dyes Pigments 83(2009)249–257.
[25]J.B.Madsen,K.I.Pakkanen,S.Lee,Investigation of the thermostability of Bovine Submaxillary Mucin(BSM)and its impact on lubrication,APCBEE Proc.7(2013)21–26.
[26]Y.N.Ni,G.L.Liu,S.Kokot,Fluorescence spectrometric study on the interactions of isoprocarb and sodium 2-isopropylphenate with bovine serum albumin,Talanta 76(2008)513–521.
[27]D.P.Ross,S.Subramanian,Thermodynamics of protein association reactions:forces contributing to stability,Biochemistry 20(1981)3096–3102.
[28]P.N.Naik,S.A.Chimatadar,S.T.Nandibewoor,Interaction between a potent corticosteroid drug–dexamethasone with bovine serum albumin and human serum albumin:a fluorescence quenching and fourier transformation infrared spectroscopy study,J.Photochem.Photobiol.B 100(2010)147–159.
[29]T.Förster,Transfer mechanisms of electronic excitation,Discuss.Faraday Soc.27(1959)7–17.
[30]C.Pontremoli,N.Barbero,G.Viscardi,S.Visentin,Mucin–drugs interaction:the case of theophylline,prednisolone and cephalexin,Bioorg.Med.Chem.23(2015)6581–6586.
[31]F.L.Cui,J.Fan,J.P.Li,et al.,Interactions between 1-benzoyl-4-p-chlorophenyl thiosemicarbazide and serum albumin:investigation by fluorescence spectroscopy,Bioorg.Med.Chem.12(2004)151–157.
[32]P.Qu,H.Lu,X.Y.Ding,et al.,Study on the interaction of 6-thioguanine with bovine serum albumin by spectroscopic techniques,J.Mol.Struct.920(2009)172–177.
[33]B.Tang,M.Du,Z.Z.Chen,et al.,Studies on luminescence of Trp and Tyr residues in protein denaturation by three-dimensional-synchronous-polarized spectrofluorimetry,Acta Chim.Sin.62(2004)1153–1157.
[34]T.C.O’Haver,A.F.Fell,G.Smith,Derivative spectroscopy and its applications in analysis,Anal.Proc.19(1982)22–46.
[35]X.H.Wu,J.H.Zhou,X.T.Gu,et al.,Study on interaction between hypocrellin A and hemoglobin or myoglobin using synchronous fluorescence spectra,Spectrosc.Spect.Anal.26(2006)2287–2290.
[36]L.Trynda-Lemiesz,K.Wiglusz,Interactions of human serum albumin with meloxicam.Characterization of binding site,J.Pharm.Biomed.52(2010)300–304.
[37]S.Baroni,M.Mattu,A.Vannini,R,et al.,Effect of ibuprofen and warfarin on the allosteric properties of haem–human serum albumin.A spectroscopic study,Eur.J.Biochem.268(2001)6214–6220.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- S-Nitroso-N-acetyl-L-cysteine ethyl ester(SNACET)and N-acetyl-L-cysteine ethyl ester(NACET)–Cysteine-based drug candidates with unique pharmacological profiles for oral use as NO,H2S and GSH suppliers and as antioxidants:Results and overview
- Tissue-based metabolite profiling and qualitative comparison of two species of Achyranthes roots by use of UHPLC-QTOF MS and laser micro-dissection
- A liquid chromatography with tandem mass spectrometry method for quantitating total and unbound ceritinib in patient plasma and brain tumor
- Denaturation studies on bovine serum albumin–bile salt system:Bile salt stabilizes bovine serum albumin through hydrophobicity
- Effect of nonionic surfactants in release media on accelerated in-vitro release profile of sirolimus eluting stents with biodegradable polymeric coating
- Electrooxidation of sulfanilamide and its voltammetric determination in pharmaceutical formulation,human urine and serum on glassy carbon electrode
