Denaturation studies on bovine serum albumin–bile salt system:Bile salt stabilizes bovine serum albumin through hydrophobicity
2018-03-06KrpgrjMlrkniIvySrkrSusithrSelvm
Krpgrj Mlrkni,Ivy Srkr,Susithr Selvm,c,✽
aDepartment of Chemistry,Vel Tech University,Avadi,Chennai 600062,India
bDepartment of Chemistry,Indian Institute of Technology Madras,Chennai 600036,India
cDepartment of Chemistry,PSG College of Technology,Peelamedu,Coimbatore 641004,India
1.Introduction
Protein therapeutics is a highly advantageous group of drugs which can be used in enzymatic or regulatory activity,specific targeting and protein vaccines.Proteins have a highly specific and complex set of functions,which are beneficial and difficult for a simple drug molecule to mimic[1].However,there are quite a few challenges in formulating proteins as drugs viz.protein solubility,route of administration,distribution and stability.During formulation of protein as drug,there arise a few negative effects such as protein instability,misfolding,unfolding and aggregation[2],are collectively called protein denaturation.Denaturation of protein can lead to amyloidoes,which further leads to neuro-degenerative pathologies like Alzheimer and Parkinson's diseases[3].Hence,it is important to stabilize the proteins in their native state during formulation.To this aim,many studies have been carried out using pharmaceutical excipients as stabilizers for proteins.
Serum albumin serves as a depot or transport protein,present as the most abundant soluble protein in the blood plasma.It binds to the ligands and contributes to 80%of osmotic blood pressure[4,5].Bovine serum albumin(BSA)is one of the albumins,which has similar structure,properties and functionalities to human serum albumin(HSA),containing a single polypeptide chain,with 583 amino acids.The tertiary structure of BSA is composed of three domains I,II and III,with each domain further divided into two sub domains A and B.As domains II and III share the common interface,binding of probe to domain III leads to conformational changes affecting the binding affinities of domain II.Hence,the conformations of BSA are destroyed leading to protein denaturation.As a rule,the denaturation is accompanied by loss of functional properties of protein[6–9].The interaction of BSA with different types of surfactants,drug molecules and denaturants(urea and glutamine hydrochloric acid)is well documented and has been a topic of burgeoning interest[7].BSA has two tryptophan residues,Trp-134 and Trp-213,found in subdomain IB and IIA,respectively.Both the Trp moieties are highly sensitive to local environment and the degree of exposure of tryptophan moieties is depicted by the position of fluorescence emission peak[10,11].A substantial spectral shift towards blue or red region is observed by addition of denaturants.Few reports show that SDS aggregates stabilize BSA or HSA structure at low concentration of urea and thermal induced denaturation[12–14].
Bile salts(BSs)are naturally occurring amphiphiles synthesized in and also released by the liver,as well as stored in the gallbladder.BS structure leads to a unique aggregation pattern,accounting for their solubilizing effect on both hydrophobic and hydrophilic solutes.Consequently BSs have received much attention as drug delivery media/pharmaceutical excipient[15–18].The aggregation pattern of BSs follows a two-step process;initially dimeric primary aggregates are formed through the hydrophobic interaction between steroidal domains and the increasing concentration of BSs leads to the formation of larger secondary aggregates,formed primarily due to the hydrogen bonding between hydroxyl and carboxyl groups of different dimeric BS units[19–21].Owing to the structural association of BS aggregates,it is claimed that these systems do not have a sharp critical micellar concentration(cmc),whereas a cmc range is estimated,viz.[NaC]=6–16 mM and[NaDC]=4–8 mM.
The association of BSA with BS media is already studied and it is proposed to be associated with hydrophobicity of both molecules[22].BS as a well-known drug delivery medium/pharmaceutical excipient,can be adopted to stabilize BSA in its native state,during formulation.Here we propose a study of denaturation on BSA-BS,through chemical and physical denaturants,so as to understand the stabilization effect of BS media on BSA.Sodium deoxycholate(NaDC)and sodium cholate(NaC)were the BSs,chosen for the study.NaDC and NaC are hydrophobic and hydrophilic in nature,respectively.Urea was used to induce chemical denaturation on pre-for med BSA-BS media and a condition of varying temperature was applied on the BSA-BS system to study the effect of physical denaturation.
2.Materials and methods
2.1.Materials
BSA was purchased from Sigma Chemicals Limited,USA.Sodium cholate(NaC)and sodium deoxycholate(NaDC)were purchased from Sisco Research Laboratories Private Limited(India)and used without further purification.A sodium phosphate buffer of pH 7.4 was used for all the experiments.Triply distilled water was used to prepare the buffer solution.
2.2.Instrumentation and methods
Absorption studies were performed on a Shimadzu UV 1800 double beam spectrophotometer,using 10 mm path length of quartz cuvettes.A Horiba Jobin Yvon FluoroMax-4 spectro-fluorometer was used to measure emission and excitation spectra with 5 nm in band width.The steady state fluorescence anisotropy(rss)is defined as[23]
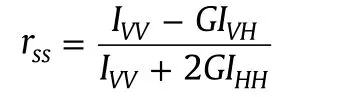
where Ivvand IVHare the fluorescence intensities and the subscript indicates the vertical(V)and horizontal(H)orientation of the excitation and emission polarizer.G is the instrumental correction factor.
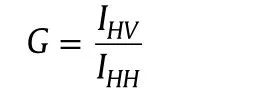
The time-resolved fluorescence intensity decay profiles were collected using Horiba JobinYvon TCSPC Life Time System with FluoroHub single photon counting arrangement.Nano-LED of 280 nm(slit–4)was used as excitation source.The pulse repetition rate was set at 1 MHz.The instrument response function was collected by using scattered medium,LUDOX AS40 colloidal silica.IBH software was used for the decay analysis.Decays were fitted to get a symmetrical distribution keepingχ2value at 0.99≤ χ2≤1.2.The average lifetime was determined by using the following equation[23],
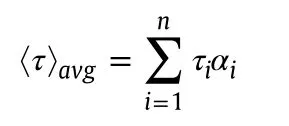
where τiis the lifetime of a component having amplitudeαi.
2.3.Preparation of BSA-BS solutions
The stock solution of BS was prepared with phosphate buffer of pH 7.4 with a concentration which is higher than cmc range of both the BSs.A range of different concentrations of BS solutions were prepared by diluting these stock solutions using buffer solution.BSA concentration was maintained at 5μM for all the experiments.The concentration range of BS was varied at[NaC]=0–43.2 mM and[NaDC]=0–18 mM.The solutions were incubated for 2 h to achieve equilibrium.
2.4.Chemical denaturation studies
The stock solutions of BS and BSA were prepared with pH 7.4 phosphate buffer and the concentration for BS was maintained at[NaC]=20 mM,[NaDC]=12 mM and the concentration of BSA at 5μM for all the experiments.The concentrations of urea were varied from 0 M to 9.6 M.The solutions were incubated for 2 h to achieve equilibrium.The temperature was maintained at 25°C.
2.5.Thermal denaturation studies
Temperatures for absorption studies were adjusted by using the thermostat,Bio-Laab Private Limited,India.For both steady state and time-resolved fluorescence measurements,temperature control was achieved by circulating water through the jacketed cuvette holder from refrigerated bath(Julabo,Germany).The thermal denaturation studies of BSA-BS system were carried out by varying the temperature from 15 °C to 75 °C.
3.Results and discussion
3.1.Association of BSA-BS system
BSA exhibits an absorption maximum(λmax)at 278 nm due to the tryptophan moiety in BSA.With the addition of increasing concentration of both the BSs,no shift in peak was observed;only a small increase in absorbance was observed(Fig.1A and B).Fluorescence emission peak λemwas obtained at 347 nm for BSA(λex=288 nm)in the homogeneous medium.With the initial addition of BS, fluorescence emission spectra were significantly quenched and there was a blue shift of 17 nm(from 347 nm to 330 nm).Further addition of BS increased the fluorescence intensity to a small extent as shown in Fig.1C and D.The data suggests that the Trp 213 moiety of BSA is exposed more for quenching and lies closer to hydrophobic core of BSA aggregates.This may suggest that the binding site for BS in BSA is the hydrophobic site of sub domain IIA as observed with other surfactants[22,24,25].The steady state fluorescence anisotropy(rss)studies show that the values varied from 0.09 to 0.11 with the increasing concentration of BS(Table S1).The minimal change in the rssvalues indicates that there is a diminutive micro-environmental change surrounding the Trp moieties and the increase in the values can be indicative of hydrophobic nature of association between BSA and BSs.
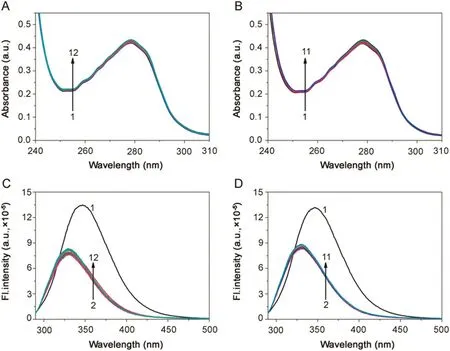
Fig.1.Absorption spectra of BSA(5 μM;λmax=278 nm)in(A)BSA-NaC and(B)BSA-NaDC.Emission spectra of BSA(5 μM;λex=288 nm)in(C)BSA-NaC and(D)BSA-NaDC.[NaC](1→12)=0–43.2 mM;[NaDC](1→11)=0–18 mM;T=25 °C;pH=7.4.
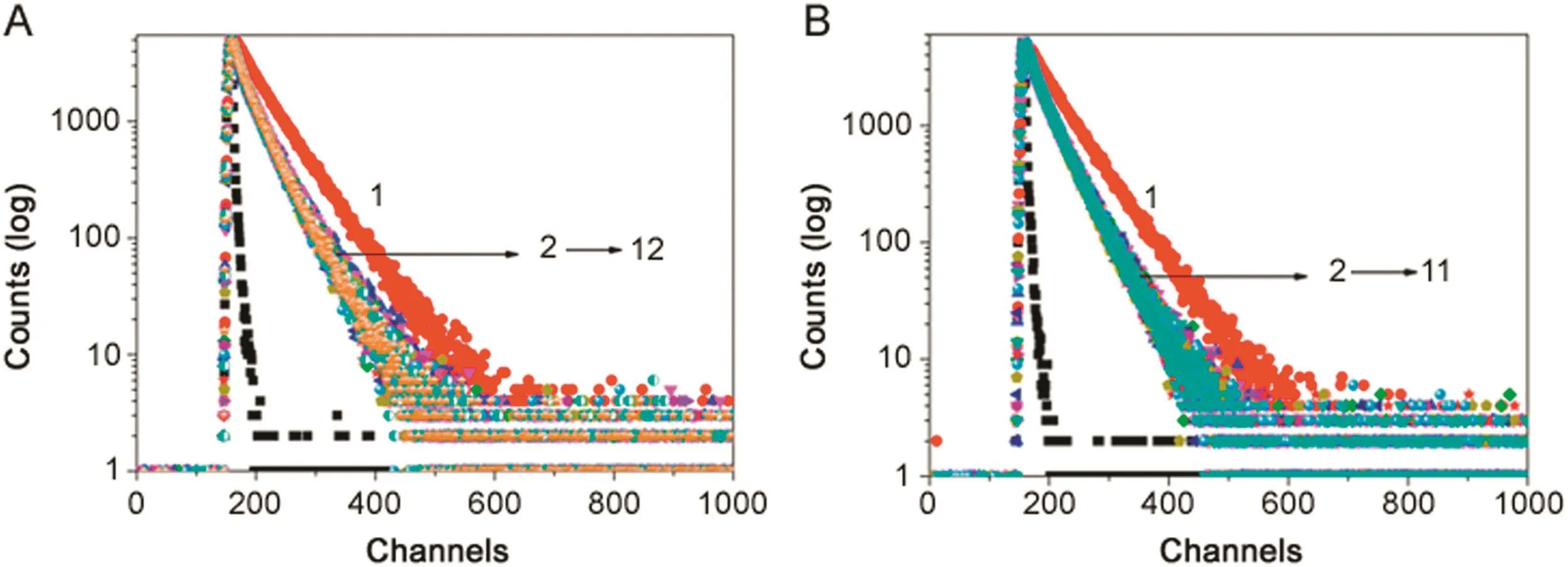
Fig.2.Fluorescence intensity decay of BSA(λex=280 nm LED,λem=347 nm)in(A)BSA-NaC and(B)BSA-NaDC.[BSA]=5 μM;[NaC](1→12)=0–43.2 mM;[NaDC](1→11)=0–18 mM;T=25 °C;pH=7.4.
Time-resolved fluorescence decay measurement of BSA in buffer with the increasing concentration of BS was carried out at λem=330 nm with excitation source of 280 nm LED.Bi-exponential decay profile was observed with the fluorescence lifetime values of τ1=3.36 ns(α1=19.48)/ τ2=6.70 ns(α2=80.52)with ‹τ›avg=6.02 ns for BSA in homogeneous medium.Generally,the excited state species is suggested to have shorter life-time if it is experiencing a more polar environment,and the longer life-time is suggested for an excited state species experiencing a non-polar environment[23].Accordingly,the shorter lifetime component is designated to Trp 134 and the longer life-time component to Trp 213,which lies in the hydrophobic core of BSA.With the initial addition of BS,the fluorescence life time values of BSA changed to τ1=1.94 ns(α1=31.82)and τ2=5.65 ns(α2=68.14)with ‹τ›avg=4.46 ns for BSA-NaC system and for BSA-NaDC system,τ1=1.84 ns(α1=25.08)and τ2=5.10 ns(α2=74.92)with ‹τ›avg=4.28 ns.On further increasing the concentrations of both the BS molecules beyond their cmc range,it was noted that the values changed to τ1=1.59 ns(α1=27.05)and τ2=4.84 ns(α2=72.95)with ‹τ›avg=3.60 ns for BSA-NaC system and for BSA-NaDC system,τ1=2.18 ns(α1=35.03)and τ2=5.09 ns(α2=64.97)with ‹τ›avg=4.07 ns.The fluorescence decay profile of BSA in presence of both the BS molecules is given in Fig.2.It shows that there is a considerable decrease in the fluorescence life-time of BSA with the initial addition of BSs.Whereas,the change is minimal with further addition of BSs.The decrease in fluorescence life-time values of Trp moieties with addition of BSs is indicative of their association and thereby a change in micro-heterogeneous environmental surrounding of the Trp residues.
The association of BSA with BS systems was analyzed with plots of variation of absorbance, fluorescence intensity and fluorescence life-time against the concentration of NaC and NaDC as shown in Fig.3.Fig.3 suggests that the association of BSA-BS systems follows a three-step model,where BSA associates with(1)primary aggregates(dimers),(2)secondary aggregates and(3)larger aggregates,of BSs,as they are formed with increasing concentration of BSs[26].Fig.4 is a schematic representation of the step-wise association of BSA-BS system showing a probable hydrophobic interaction between the hydrophobic subdomain IIA(Trp-213)of BSA with the increasing concentration of BSs.It is also put forth here that the Trp 213 moiety in BSA associates with(i)BS monomer,(ii)BS dimers,(iii)BS secondary aggregates and(iv)BS larger aggregates as they are being formed.
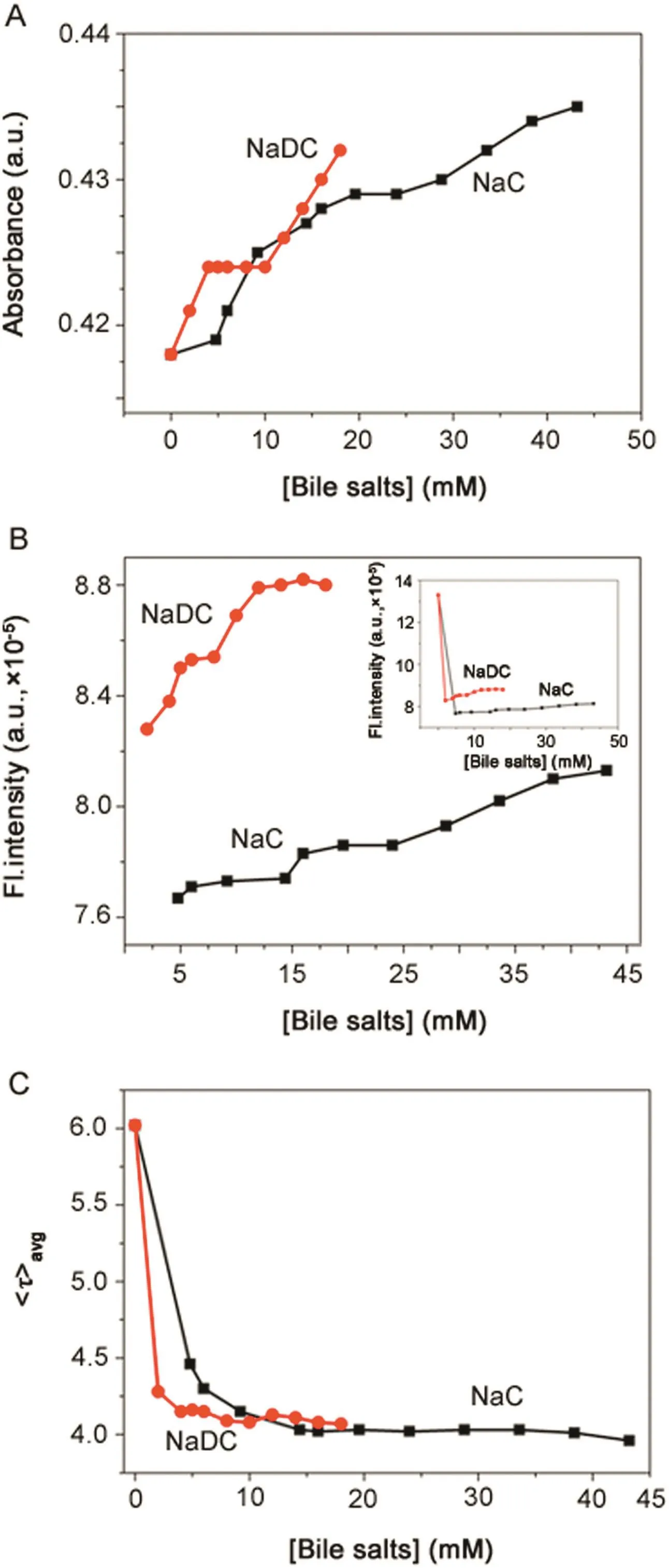
Fig.3.Variation of(A)absorbance(λmax=278 nm),(B) fluorescence intensity(λex=288 nm,λem=347 nm),and(C) fluorescence life-time decay(λex=280 nm LED,λem=347 nm)of BSA in presence of BSs.Inset of(B):variation of fluorescence intensity of BSA in presence of BSs along with BSA in buffer.[BSA]=5 μM;[NaC]=0–43.2 mM);[NaDC]=0–18 mM;T=25 °C;pH=7.4.
The spectroscopic analysis suggested that among the three subdomains of BSA,two of them viz.subdomain IIB(where Trp 134 is located)and subdomain IIA(where Trp 213 is located)are associated with BS molecules and lead to the stabilization of BSA in its native state.From the data,it can also be visualized that Trp moiety can sense the aggregation behavior of BSs,as a fluorescence probe can sense[27–29].
3.2.Denaturation studies on BSA-BS systems
3.2.1.Effect of urea—chemical denaturation
To understand the effect of urea on BSA and BSA-BS systems,urea of varying concentration(0–9.6 M)was added to BSA in buffer and pre-formed BSA-BS micellar system.The concentration range for urea was chosen with accordance to literature[12,30],in which denaturation of serum albumin was induced by the addition of urea.Addition of urea up to 9.6 M to the BSA-BS system resulted in a minimal increase in absorbance of Trp(Figs.5A and B).Figs.5C and D show the influence of urea on emission spectra of BSA and BSA-BS systems,whereas the insets of Figs.5C and D depict the shift in emission wavelength with the addition of urea to BSA and BSA-BS systems.
Addition of urea(9.6 M)to BSA in homogeneous medium resulted in fluorescence quenching accompanied with the hypsochromic shift in emission maxima(λem347 nm to 342 nm).Whereas,addition of urea up to 4.8 M and 6 M to BSA-NaC and BSA-NaDC,respectively,did not show a profound change in fluorescence intensity and shift in emission maxima.Further increasing the concentration of urea up to 9.6 M in each of the BSABSs showed a small decrease in the fluorescence intensity along with 5 nm red shift for BSA-NaC(λem330–335 nm)and 3 nm for BSA-NaDC(λem330–333 nm).The rssvalues for BSA in buffer did not change and remained constant with addition of urea.Whereas with the BSA-BS systems,it showed variation of rssvalues from 0.11 to 0.10,at all the concentration of urea used here and hence it was observed to be almost constant.The fluorescence decay profiles for BSA and BSA-BS systems in urea were obtained as bi-exponential decay with ‹τ›avgdecreasing and increasing concentration of urea shown as in Fig.6.
The corresponding fluorescence life-time data of BSA-BS systems in varying concentration of urea is presented in Tables 1 and 2.
Variation of absorbance, fluorescence intensity and fluorescence life-time of BSA and BSA-BS systems with increasing concentration of urea is shown in Fig.S1.The absorbance of BSA-BS systems with addition of urea does not show any significant change.Whereas,both fluorescence intensity and fluorescence life-time data obtained here suggest that BSA-BS media are unperturbed up to 4.8 M of urea in BSA-NaC and up to 6 M of urea in BSA-NaDC.This data effectively shows that the hydrogen bonding between urea and BSA forming an amide linkage did not occur in the presence of BS molecules,perfectly pointing out that the hydrophobic interaction of BSA-BS media is more prominent in stabilizing the protein in native form and thus restricts the denaturation effect of urea[31–34].
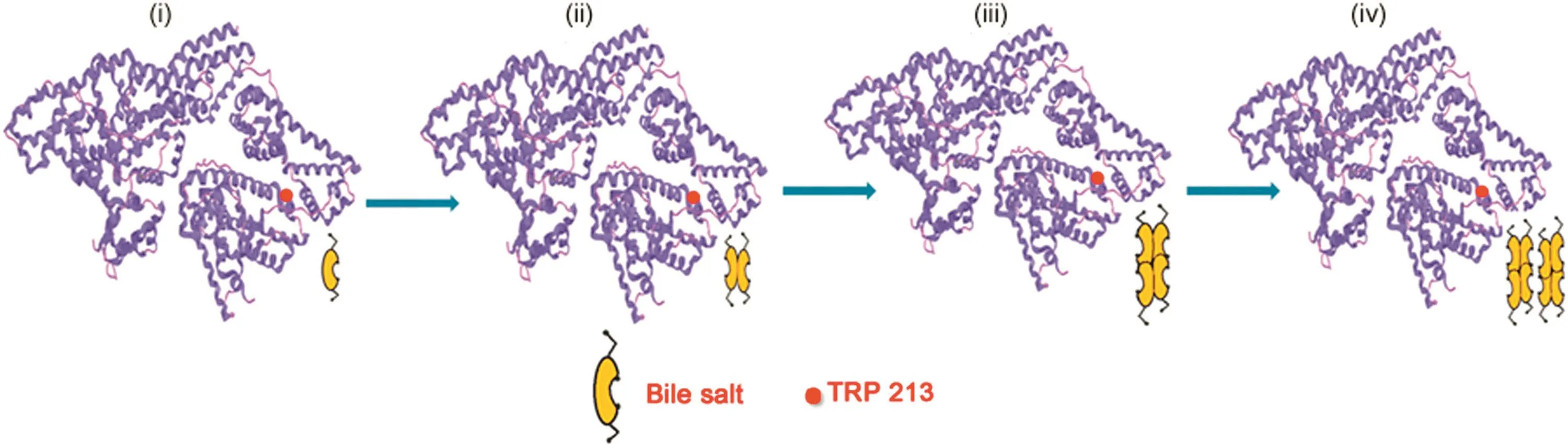
Fig.4.Schematic representation of step-wise association of Trp 213 in BSA with(i)BS monomer,(ii)BS dimers,(iii)BS secondary aggregates and(iv)BS larger aggregates corresponding to the step-wise aggregation of BSs with increasing concentration of BSs.
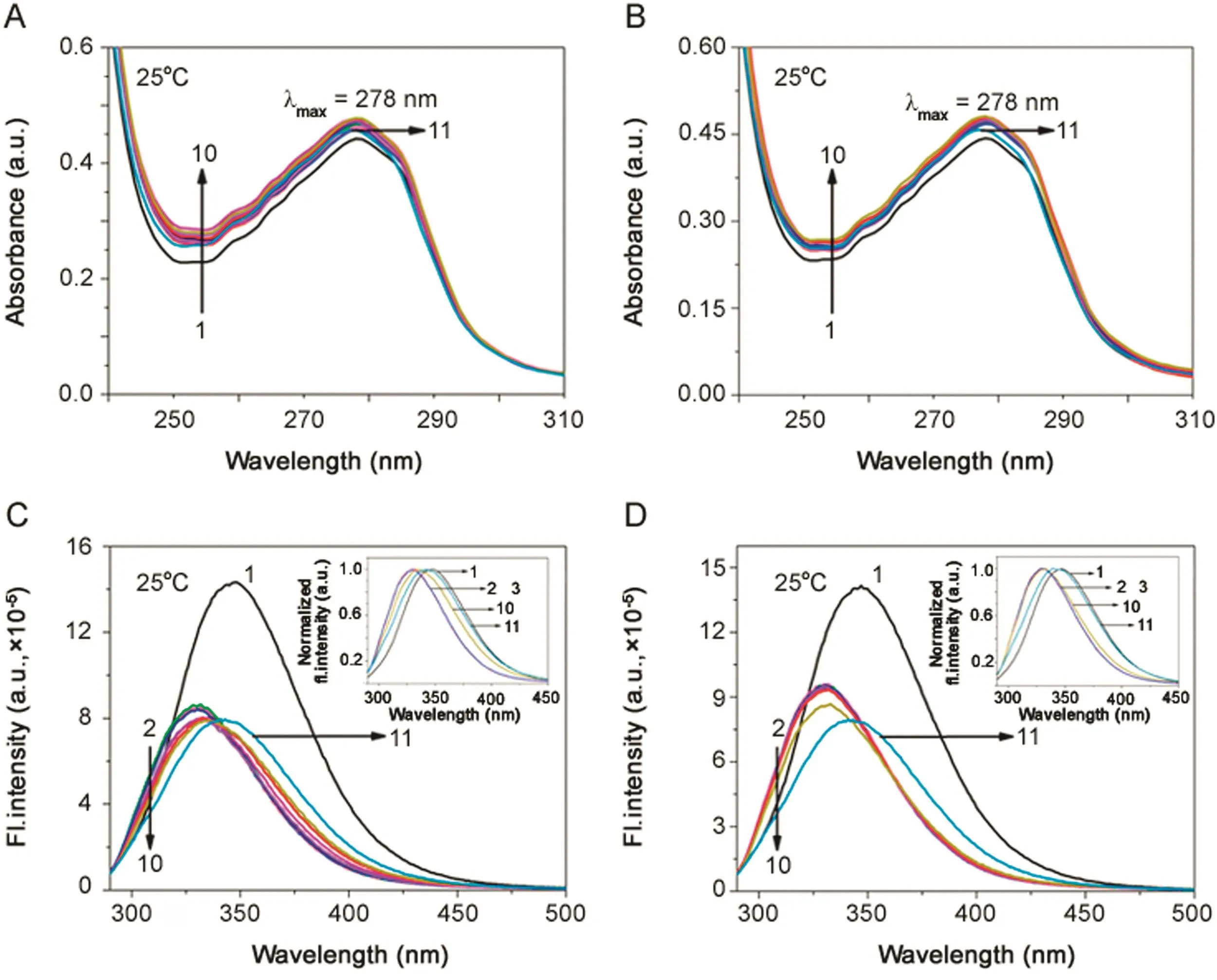
Fig.5.Absorption spectra of(A)BSA-NaC-urea and(B)BSA-NaDC-urea.Emission spectra of(C)BSA-NaC-urea and(D)BSA-NaDC-urea.Insets of(C)and(D):Normalized fluorescence spectra of BSA and BSA-BS-urea systems,showing the emission wavelength shift in each of the system.[BSA]=5 μM;[NaC]=20 mM;[NaDC]=12 mM;[urea](1 →10)=0–9.6 M;[urea](11)=9.6 M;T=25 °C;pH=7.4.
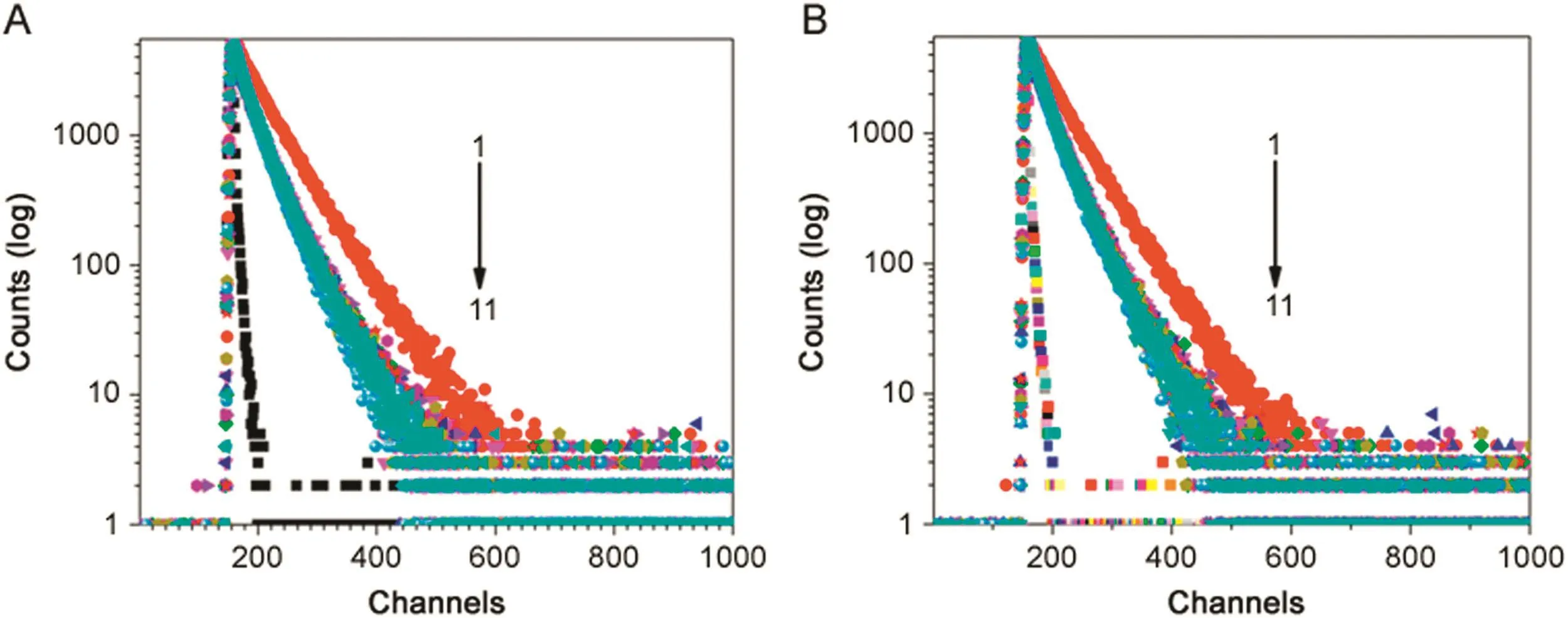
Fig.6.Fluorescence intensity decay of BSA(λex=280 nm LED,λem=347 nm)in(A)BSA-NaC-urea and(B)BSA-NaDC-urea.[BSA]=5 μM;[NaC]=20 mM;[NaDC]=12 mM;[urea](1 →10)=0–9.6 M;[urea](11)=9.6 M;T=25 °C;pH=7.4.
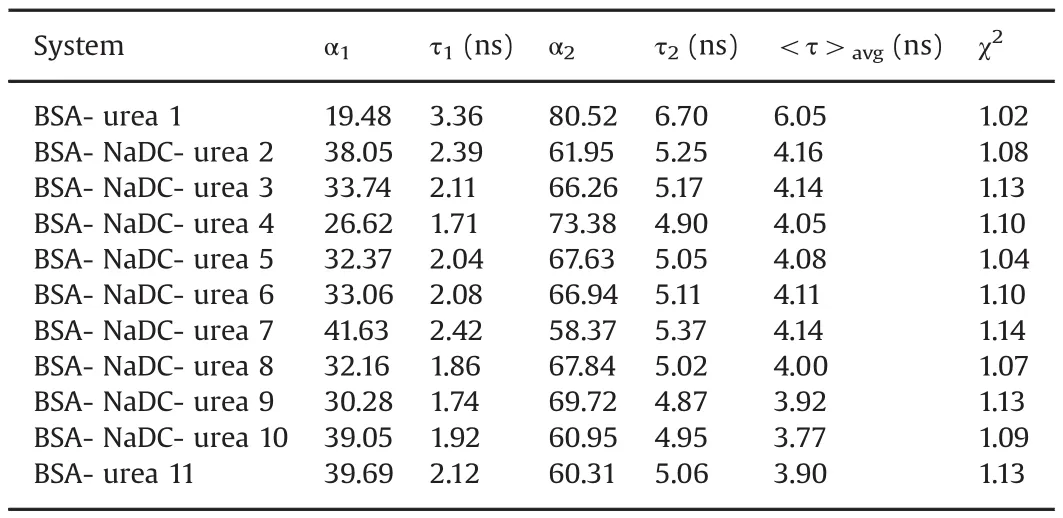
Table 2Fluorescence lifetime values of BSA and BSA-NaDC system with addition of urea.[NaDC]=12 mM;[BSA]=5 μM;[urea](1 →10)=0–9.6 M;[urea](11)=9.6 M;T=25°C;pH 7.4.
At higher concentrations of urea,the denaturation of BSA is expected due to the breaking of water structure of BSA by urea[35,36].This effect is seen even in the BSA-BS systems.The data obtained here for BSA at 9.6 M urea concentration revealed a 5 nm blue shift(347 nm to 342 nm).Whereas the BSA-BS system showed a red shift at this concentration of urea(BSA-NaC:5 nm red shift(λem330–335 nm)and BSA-NaDC:3 nm red shift(λem330–333 nm)).
3.2.2.Effect of temperature—thermal denaturation
The second denaturation study was conducted with variation of temperature;viz.thermal denaturation studies were carried out on the BSA-BS systems and the changes in the photophysical properties of Trp moieties in BSA were monitored.Figs.S2 and S3 show the change in the absorption spectra of BSA-BS systems from 15 °C to 75 °C.Fig.S4 depicts the variation of absorbance of both BSA-NaC and BSA-NaDC systems against temperature.It was observed that there was no change in the absorption spectra of BSA-BS systems over the different concentrations of BSs and temperatures.But there was a significant difference with the homogeneous solution of BSA,which showed an increase with increasing temperature.Even though the change in absorbance of BSA in buffer with increasing temperature was minimal,the effect of temperature on BSA-BS systems and BSA in buffer was relatively different.
The effects of temperature on the emission properties of Trp in BSA-NaC and BSA-NaDC systems are provided in Figs.7 and 8,respectively.The data obtained here reflects certain salient changes from that of BSA in homogeneous medium.
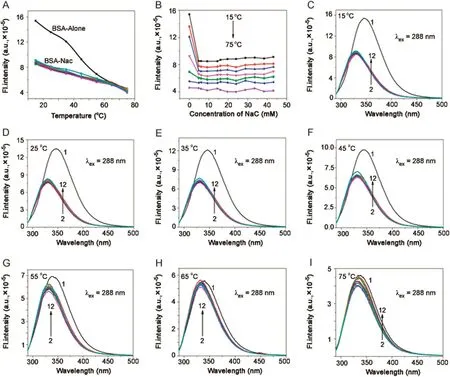
Fig.7.Plot of variation of fluorescence intensity of BSA(5 μM; λex=288 nm, λem=347 nm)in NaC against(A)temperature and(B)concentration of NaC.(C)→(I)Fluorescence emission spectra of BSA-NaC system at 15,25,35,45,55,65,and 75°C,respectively.[NaC](1→11)=0–43.2 mM;pH=7.4.
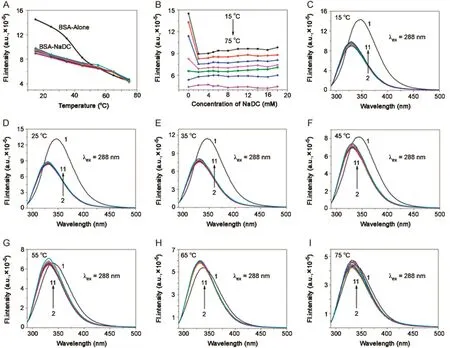
Fig.8.Plot of variation of fluorescence intensity of BSA(5 μM; λex=288 nm, λem=347 nm)in NaDC against(A)temperature and(B)concentration of NaDC.(C)→(I)Fluorescence emission spectra of BSA-NaDC system at 15,25,35,45,55,65,and 75°C,respectively.[NaDC](1→11)=0–18.0 mM;pH=7.4.
With increasing temperature,there was blue shift for BSA in the absence of BSs(λem347–337 nm).In both BSA-BS systems,it was seen that there was no emission shift up to 55°C.Fluorescence intensity decreased with increasing temperature for BSA in buffer and BSA-BS systems.However,the magnitude of decrease in case of BSA was drastic compared to that of BSA-BSs.In the same way,the step-wise association of BSA and BS molecules was unperturbed with temperature,favouring a hydrophobic nature of association.From 55–75 °C,there was red shift for both BSA-BS systems and a decrease in fluorescence intensity was observed.Here the effect of temperature on BSA-BS systems corresponded to the disruption of the aggregation pattern of BSs.With initial concentration of BSs,there was red shift for BSA-NaC:5 nm(λem330–335 nm)and for BSA-NaDC:4 nm red shift(λem330–334 nm).Between the cmc range for BSA-NaC([NaC]=6–16 mM),the shift is 4 nm(λem330–334 nm)and for BSA-NaDC([NaDC]=4 –8 mM),the shift is 3 nm(λem330–333 nm).Beyond the cmc range,for BSA-NaC,the shift is 3 nm(λem330–333 nm)and for BSANaDC,it is 2 nm(λem330–332 nm).The rssvalues of BSA and BSABS systems remain almost constant over the different concentrations of BSs and various temperatures(Tables.S2 and S3).The thermal denaturation study on BSA-BSs was also carried out using time-resolved fluorescence.The decay profiles of BSA-NaC and BSA-NaDC with increasing temperature are given in Figs.9 and 10,respectively.The decay profiles were found to exhibit bi-exponential decay as similarly observed in association of BSA-BS and also collaborate with that of BSA-BS-urea systems.With increasing temperature,‹τ›avgvalues for both BSA-BS systems showed a decrease over all the temperatures used(Tables S4 and S5).However,the magnitude of decrease in these values was found to be more beyond 55 °C.The analysis of data also revealed thatτ2,assigned to Trp 213,changed drastically compared with τ1,assigned to Trp 134.The close proximity of Trp 213 to the BS aggregates was seen,supporting the schematic representation of the association of BSABS systems in Fig.4.
The aggregates of BSs associating with Trp 213 at the sub-domain IIA of BSA were observed to be more affected with the effect of temperature[37–40].Since Trp 213 is located at the hydrophobic pocket of BSA,it can be proposed that the stabilization of BSA by BS molecules is through probable hydrophobic association of BSA and BSs.
Conversely,with increasing temperature,the decrease in fluorescence intensity and fluorescence life-time is indicative of disaggregation of BS molecules.The increasing temperature decreases the hydrophobicity of the BS aggregation media.As an outcome,BSA experiences a lesser hydrophobic site of interaction with BSs.This again reflects the nature of association of BSA with BSs to be hydrophobic in nature as it can be seen with other fluorescent molecules associating with BSs media[15–17].
3.3.Discussion
The present study deals with the association of BSA and BSs,followed by understanding the protective nature of BSs towards BSA denaturation and stabilizing BSA in their native state.The intrinsic fluorescence property of BSA,contributed by Trp 134 and Trp 213,was utilized for the study.Accordingly it was observed that the association of BSA and BS molecules occured through hydrophobic site of BSA,viz.sub-domain IIA,where the Trp 213 is reflective of the micro-environmental change in presence of BSs.It is proposed from the study that the association of BSA and BS takes place at the sub-domain IIA,close proximity toTrp 213,and a three-step model of association is proposed,in collaboration with the step-wise aggregation pattern authentic for any BS media.The data obtained here also reports the cmc range for each of the BSs,viz.NaC:6–16 mM and NaDC:4–8 mM.Hence the association of BSA and BSs is essentially through hydrophobicity[22,31].
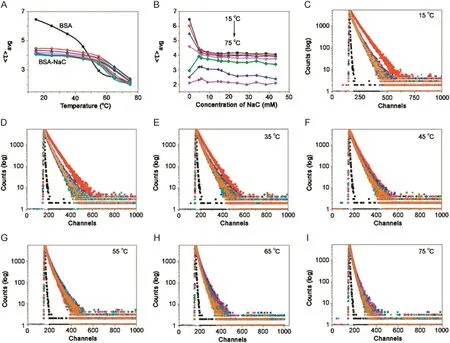
Fig.9.Plot of variation of fluorescence life-time of BSA(5 μM; λex=288 nm, λem=347 nm)in NaC against(A)temperature and(B)concentration of NaC.(C)→(I)Fluorescence intensity decay of BSA-NaC system at 15,25,35,45,55,65,and 75°C,respectively.[NaC](1→11)=0–43.2 mM;pH=7.4.
The study on denaturation of BSA-BS systems was carried out with urea as the chemical denaturant and variation of temperature as thermal(physical)denaturant.Up to a particular concentration of urea,viz.4.8 M for BSA-NaC and 6 M for BSA-NaDC,the effect of urea on the respective systems was minimal.When concentration of urea was increased further to 9.6 M,there was a red shift for each of the BSA-BS systems along with decrease in fluorescence intensity and fluorescence life-time.This change in photophysical properties of BSA in homogeneous medium was observed to be prominent under the influence of urea and led to denaturation of BSA.It showed that the hydrogen bonding between BSA and urea was controlled due to the hydrophobic association of BSA with BSs and change in water structure of BSA with higher concentrations of urea was avoided by BSs to a certain extent.Hence the data obtained indicate BSs can effectively avoid the conformational changes of BSA in presence of urea.
Similarly,the effect of temperature on the pre-formed BSA-BS systems is studied by varying the temperature from 15 to 75°C.The thermal denaturation studies revealed that up to 55°C,there was no significant effect on BSA-BS systems.However,red shift in fluorescence emission and decrease in fluorescence intensity and fluorescence life-time for the temperatures from 55 to 75°C are indicative of temperature effect on BSA-BS system.However,the effect of decrease in the fluorescence parameters with temperature can be visualized as the disaggregation pattern of BSs and hence the subsequent effect on BSA-BS systems.The rssvalues were almost constant for the BSA-BS systems in presence of urea and also varying temperatures.The data indicate the fact that there is no micro-environmental change effect on the BSA-BS systems,once the hydrophobic association between BSA and BSs is established.The fluorescence life-time values decreased under the influence of both denaturants with the addition of BS media.However,they did not change drastically with the pre-formed BSA-BS systems.Hence,the association of BSA with BSs was unperturbed with the chemical and thermal denaturants.In both the denaturation studies,the effect of the denaturant was found to be minimal on the BSA-BS systems in comparison to BSA in homogeneous medium.
4.Conclusions
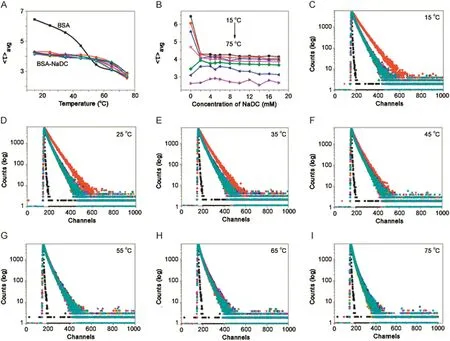
Fig.10.Plot of variation of fluorescence life-time of BSA(5 μM; λex=288 nm, λem=347 nm)in NaDC against(A)temperature and(B)concentration of NaDC.(C)→(I)Fluorescence intensity decay of BSA-NaDC system at 15,25,35,45,55,65,and 75°C,respectively.[NaDC](1→11)=0–18.0 mM;pH=7.4.
The current study is an attempt to propose the BSs,well-known pharmaceutical excipients to be used as stabilizers for proteins.BSA was used as a model protein and two BSs,viz.NaC and NaDC,differing in their hydrophobicity,are used.The study was carried out methodologically using the intrinsic Trp fluorescence property of BSA.The Trp absorption studies along with steady state fluorescence and time-resolved fluorescence studies indicate the association of BSA-BS as hydrophobic in nature.They also relate the BSA-BSs systems’association to the three-step aggregation model of BSs and thereby follow a three-step model of association between BSA and BSs.The pre-formed BSA-BSs systems were subjected to addition of chemical denaturant,urea and physical denaturant by varying temperature.It was also found from the experiments that the hydrophobic nature of association of BSA-BS plays a key role in stabilizing BSA against urea and thermal denaturation and protects the protein from conformational damage to certain extent.Hence,from the present study it can be put forth that the BS aggregates offer a protective effect on BSA to the two denaturants and can stabilize them at their native state.
Conflicts of interest
The authors declare that there are no Conflicts of interest.
One of the authors,Dr.Susithra Selvam,is thankful to DSTSERB,India(SB/FT/CS-032/2012),for the financial support and Prof.Ashok Kumar Mishra,Department of Chemistry,Indian Institute of Technology Madras,for unrestricted usage of spectro fluorometer.
Appendix A.Supplementary material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jpha.2017.06.007.
[1]B.Leader,Q.J.Baca,D.E.Golan,Protein therapeutics:A summary and pharmacological classification,Nat.Rev.Drug Discov.7(2008)21–39.
[2]T.Chakraborthy,I.Chakraborthy,S.P.Moulik,et al.,Physicochemical and conformation studies on BSA-surfactant interaction in aqueous medium,Langmuir 25(2009)3062–3074.
[3]V.Militello,V.Vetri,M.Leone,Conformational changes involved in thermal aggregation processes of bovine serum albumin,Biophys.Chem.105(2003)133–141.
[4]D.L.Carter,J.X.Ho,Structure of serum albumin,Adv.Protein Chem.45(1994)153–203.
[5]T.Peter,Serum albumin,Adv.Protein Chem.37(1985)161–245.
[6]I.M.Vlasova,V.V.Zhuraveva,A.A.Vlasov,et al.,Interaction of cationic surfactants cethyltrimethylammonium bromide with bovine serum albumin in dependence on pH:A study of tryptophan fluorescence,J.Mol.Struct.1034(2013)89–94.
[7]S.Ghosh,S.Chakrabarty,D.Bhowmik,et al.,Stepwise unfolding of bovine and human serum albumin by an anionic aurfactant:an investigation using the proton transfer probe norharmane,J.Phys.Chem.B 119(2015)2090–2102.
[8]G.G.Glenner,C.W.Wong,Alzheimer's disease:initial report of the purification and characterization of a novel cerebrovascular amyloid protein,Biochem.Biophys.Res.Commun.425(2012)534–539.
[9]J.C.Cheung,C.M.Deber,Misfolding of the cystic fibrosis transmembrane conductance regulator and disease,Biochemistry 47(2008)1465–1473.
[10]J.Steinhardt,J.Krijn,J.G.Leidy,Differences between bovine and human serum albumins.Binding isotherms,optical rotatory dispersion,viscosity,hydrogen ion titration,and fluorescence effects,Biochemistry 10(1971)4005–4015.
[11]C.A.Royer,C.J.Mann,C.R.Matthews,Resolution of the fluorescence equilibrium unfolding profile of trpaporepressor using single tryptophan mutants,Protein Sci.11(1993)1844–1852.
[12]Y.Moriyama,K.Takeda,Re-formation of helical structure of human serum albumin by the addition of small amounts of sodium dodecyl sulfate after the disruption of the structure by urea.A comparision with bovine serum albumin,Langmuir 15(1999)2003–2008.
[13]Y.Moriyama,Y.Kawasaka,K.Takeda,Protective effect of small amounts of sodium dodecyl sulfate on helical structure of bovine serum albumin in thermal denaturation,J.Colloid Interface Sci.257(2003)41–46.
[14]C.A.Royer,Probing protein folding and conformational transitions with fluorescence,Chem.Rev.106(2006)1769–1784.
[15]S.Selvam,A.K.Mishra,Multiple prototropism of fisetin in sodium cholate and related bile salt media,Photochem.Photobiol.Sci.10(2011)66–75.
[16]S.Selvam,A.K.Mishra,Disaggergation of amphotericin B by sodium deoxycholate micellar aggregates,J.Photochem.Photobiol.B 93(2008)66–70.
[17]S.Selvam,A fluorescence parameter based analysis on the solubilization of carvediol by bile salt media,J.Photochem.Photobiol.B 116(2012)105–113.
[18]J.Rohacova,M.L.Lusia,M.A.Miranda,Complex between fluorescent cholic acid derivatives and human serum albumin,J.Phys.Chem.B 114(2010)4710–4716.
[19]C.J.O’Connor,R.G.Wallace,Physico-chemical behavior of bile salts,Adv.Colloid Interface Sci.22(1985)1–111.
[20]S.Mukhopadhyay,U.Maitra,Chemistry and biology of bile acids,Curr.Sci.87(2004)1666–1683.
[21]S.Reis,C.G.Moutinho,C.Matosa,et al.,Noninvasive methods to determine the critical micelle concentration of some bile acid salts,Anal.Biochem.334(2004)117–126.
[22]N.Ghosh,R.Mondal,S.Mukherjee,Hydrophobicity is the governing factor in the interaction of human serum albumin with bile salts,Langmuir 31(2015)1095–1104.
[23]J.R.Lakowicz,Principles of Fluorescence Spectroscopy,3rd ed.,Springer,New York,2006.
[24]U.Anand,C.Jash,S.Mukherjee,Spectroscopic probing of the microenvironment in a protein-surfactant assembly,J.Phys.Chem.B 114(2010)15839–15845.
[25]S.De,S.Das,A.Girigowami,Spectroscopic probing of bile salt-albumin interaction,Colloids Surf.Biointerfaces 54(2007)74–81.
[26]S.Ghosh,J.Dey,Binding of fatty acid amide amphiphiles to bovine serum albumin:role of amide hydrogen bonding,J.Phys.Chem.B 119(2015)7804–7815.
[27]N.R.Syme,C.Dennis,A.Bronowska,et al.,Comparison of entropic contributions to binding in a “hydrophilic”versus “hydrophobic”ligand-protein interaction,J.Am.Chem.Soc.132(2010)8682–8689.
[28]K.A.Majorek,P.J.Porebski,A.Dayal,et al.,Structural and immunologic characterization of bovine,horse and rabbit serum albumins,Mol.Immunol.52(2012)174–182.
[29]B.Ojha,G.Das,Role of hydrophobic and polar interaction for BSA-amphiphile composites,Chem.Phys.Lipids 164(2011)144–150.
[30]R.Kumaran,P.Ramamurthy,Denaturation mechanism of BSA by urea derivatives:evidence for hydrogen-bonding mode from fluorescence tools,J.Fluoresc.21(2011)1499–1508.
[31]A.Das,C.Mukhopadhyay,Urea-mediated protein denaturation:a consensus view,J.Phys.Chem.B 113(2009)12816–12824.
[32]B.K.Paul,A.Samanta,N.Guchhait,Exploring hydrophobic subdomain IIA of the protein bovine serum albumin in the native,intermediate,unfold,and refold states by a small fluorescence molecular reporter,J.Phys.Chem.B 114(2010)6183–6196.
[33]F.Vanzi,B.Madan,K.Sharp,Effect of the protein denaturants urea and guanidinium on water structure:a structural and thermodynamic study,J.Am.Chem.Soc.120(1998)10748–10754.
[34]E.P.O’Brin,R.I.Dima,B.Brooks,Interaction between hydrophobic,ionic solutes in aqueous guanidinium chloride and urea solution:lessons for protein denaturation mechanism,J.Am.Chem.Soc.129(2007)7346–7353.
[35]E.L.Duggan,J.M.Luck,The combination of organic anions with serum albumin:IV.Stabilization against denaturation,J.Biol.Chem.172(1948)205–220.
[36]R.Kumaran,P.Ramamurthy,Photophysical studies on the interaction of amide with bovine serum albumin(BSA)in aqueous solution: fluorescence quenching and protein unfolding,Luminescence 148(2014)277–284.
[37]C.Y.Ma,V.R.Harwalker,Study of thermal denaturation of oat gluobulin by ultraviolet and fluorescence spectrophotometry,J.Agric.Food Chem.36(1988)155–160.
[38]Y.Moriyama,E.Watanabe,K.Kobayashi,et al.,Secondary structural change of bovine serum albumin in thermal denaturation up to 130°C and productive effect of sodium dodecyl sulfate on the change,J.Phys.Chem.B 112(2008)16585–16589.
[39]Y.Moriyama,K.Takeda,Productive effect of small amounts of bis(2ethylhexyl)sulfosuccinate on the helical structures of human and bovine serum albumins in their thermal denaturation,Langmuir 21(2005)5524–5528.
[40]S.Xia,Y.Li,Q.Zhao,et al.,Probing conformational change of bovine serum albumin-dextran conjugates under controlled dry heating,J.Agric.Food Chem.63(2015)4080–4086.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- S-Nitroso-N-acetyl-L-cysteine ethyl ester(SNACET)and N-acetyl-L-cysteine ethyl ester(NACET)–Cysteine-based drug candidates with unique pharmacological profiles for oral use as NO,H2S and GSH suppliers and as antioxidants:Results and overview
- Tissue-based metabolite profiling and qualitative comparison of two species of Achyranthes roots by use of UHPLC-QTOF MS and laser micro-dissection
- A liquid chromatography with tandem mass spectrometry method for quantitating total and unbound ceritinib in patient plasma and brain tumor
- Insight into the interaction of inhaled corticosteroids with human serum albumin:A spectroscopic-based study
- Effect of nonionic surfactants in release media on accelerated in-vitro release profile of sirolimus eluting stents with biodegradable polymeric coating
- Electrooxidation of sulfanilamide and its voltammetric determination in pharmaceutical formulation,human urine and serum on glassy carbon electrode
