MAVS介导的抗病毒天然免疫信号通路的调控
2018-03-01孙英杰
郑 航,孙英杰,张 频,丁 铲
(1.吉林农业大学动物科学技术学院,长春 130118;2.中国农业科学院上海兽医研究所,上海 200241;3. 山东农业大学动物科技学院,泰安 271018)
MAVS介导的抗病毒天然免疫信号通路的调控
郑 航1,孙英杰2,张 频1,丁 铲2
(1.吉林农业大学动物科学技术学院,长春 130118;2.中国农业科学院上海兽医研究所,上海 200241;3. 山东农业大学动物科技学院,泰安 271018)
先天性免疫通路作为抵御入侵的病原微生物第一道屏障,在宿主抗病毒反应中发挥重要的作用。细胞质中最重要的识别病毒RNA的模式识别受体是维甲酸诱导基因蛋白I和黑色素瘤分化相关基因5,它们有着相同的下游信号接头分子线粒体抗病毒信号蛋白(mitochondrial antiviral-signaling protein, MAVS),MAVS在介导的先天性免疫中起中枢作用。MAVS介导的信号通路的激活是重要的抗病毒反应,但在长期的共存过程中,病毒进化出一系列拮抗MAVS的机制。同时,在静息状态下,为了防止过度免疫反应,细胞还具备一系列调控MAVS的机制。MAVS的精细调控对于行使细胞功能和发挥抗病毒反应至关重要。本文简单介绍了MAVS的结构和功能,总结了细胞对MAVS的转录和翻译后调控,最后阐述了病毒如何通过调控MAVS拮抗宿主先天性免疫,为细胞的免疫调节和控制病毒感染提供新的思路。
天然免疫;线粒体抗病毒信号蛋白;病原体;宿主
病毒感染细胞能够激活一系列先天性抗病毒反应,其中最重要的一种先天性抗病毒机制是通过模式识别受体(pattern recognition recetproreceptor,PRRs)识别病毒从而诱导产生干扰素(interferon,IFN)和促炎性因子(proinflammatory cytokines),抑制病毒复制。而在所有保守的模式识别受体中,维甲酸诱导基因蛋白I(retinoic acid-inducible gene 1,RIG-I)和黑色素瘤分化相关基因5(melanoma differentiation-associated protein 5,MDA5)是最重要的细胞质模式识别受体,在病毒感染时识别病毒RNA,激活下游一系列抗病毒信号通路,诱导表达I型干扰素和其他的促炎因子[1,2]。RIG-I和MDA5是相似的两种受体,识别不同种类的病毒RNA,它们有着相同的下游信号接头分子线粒体抗病毒信号蛋白(Mitochondrial antiviral-signaling protein,MAVS,又称IPS-1、VISA、CARDIF)[3,4]。RIG-I、MDA5通过N端串联的caspase招募结构域(amino-terminal caspase recruitment domain,CARD)与MAVS的N端 CARD结构域互作,激活下游NF-кB(nuclear factor κB)和IRF3/7(interferon regulatory factors 3/7)相关信号通路,并诱导干扰素的表达,参与先天性抗病毒反应[5,6]。MAVS在介导的先天性免疫中起中枢作用,因此MAVS介导的信号通路的激活是重要的抗病毒反应,但在长期的共存过程中,病毒有了一系列拮抗MAVS的机制。同时,在静息状态下,为了防止过度免疫反应,细胞还具备一系列调控MAVS的机制。MAVS的精细调控对于行使细胞功能和发挥抗病毒反应至关重要[7]。
1 MAVS的结构和功能
M A V S是由细胞核基因组编码的,并在不同组织和细胞中均有表达的蛋白[5]。MAVS由N端的C A R D结构域,富含脯氨酸结构域(proline-rich domain,PRD)和C端的跨膜结构域 (transmembrane domain,TM)组成[8]。MAVS在所介导的先天性抗病毒反应中起中枢性的作用,以RIG-I信号通路为例,E3泛素连接酶Riplet和TRIM25在其识别RNA和激活的过程中发挥关键作用,RIG-I被Riplet和TRIM25(tripartite motifcontaining protein 25)介导的K63泛素化修饰后发生活化,RIG-I多聚化后和TRIM25及分子伴侣14-3-3ε形成复合物,这种复合物被称之为“转位子(translocon)”,转位子从细胞质转运至细胞内膜,例如线粒体相关膜(mitochondrion-associated membrane, MAM)和MAVS结合[9]。MAVS的N端的CARD区域与胞质中与RIG-I的2CARD结构域结合,随后激活下游的两种细胞质蛋白激酶复合物,一种包括“非经典”IKK-相关激酶TBK1(TANK-binding kinase 1)或IKK-i/ε(inducible I B kinase)和一系列接头蛋白例如TANK(TRAF family member associated NF- B activator)、NAP1(NAK-associated protein 1)和NEMO(NF- B Essential Modulator)。这种TBK1复合物负责激活转录因子IRF3和IRF7的磷酸化和二聚化,IRF3和IRF7转位至细胞核中,与干扰素刺激反应元件(IFN-stimulated response elements,ISREs)结合,诱导I型IFN基因和一系列干扰素诱导基因(ISG)的表达。另一种激酶复合物包括IKKa、IKKb和NEMO,这种IKK复合物激活NF-κB,促进下游促炎性细胞因子的表达,参与先天性抗病毒反应[5,10]。
MAVS是经典的“尾部锚定”膜蛋白,其C端的跨膜结构域(TM)使MAVS锚定在许多细胞器膜表面,例如线粒体、过氧化物酶体以及内质网的亚结构域(MAM)[6]。MAVS不同亚细胞定位的具体机制还未知,据推测MAVS可能通过识别膜上特定的脂质或蛋白而锚定在不同的细胞器膜上,发挥其抗病毒功能。MAVS的这种膜定位特性对其发挥抗病毒活性是必须的,去除MAVS跨膜结构域使其丧失抗病毒活性[6]。在表达只含有CARD结构域和TM结构域的MAVS突变体(miniMAVS),仍然可以引起MAVS介导的信号传导,miniMAVS仍可以保持MAVS线粒体定位、寡聚化、CARD结构域的吸附的功能特性,这种MAVS的解螺旋酶域和C末端结合域(CTD)是识别病毒RNA,激活下游信号通路的必需结构[5]。
2 MAVS的转录和翻译后调控
2.1 MAVS的转录和转录后调控 MAVS不属于干扰素刺激基因,其表达不直接受干扰素(IFN)调控,因此与RIG-I等基因不同,MAVS的表达和功能更多受到转录、转录后和翻译后的调控。在转录水平,MAVS mRNA水平受到活性氧(reactive oxygen species,ROS)介导的负反馈环的调控[6,11]。MAVS基因还编码一系列不同的剪切体发挥负调控MAVS介导的信号通路[12]。而在转录后水平,MAVS的翻译能够在两个不同的转录起始位点,包括用序列中部的甲硫氨酸起始翻译[13]。这种MAVS的选择性翻译可能由上游开放性读码框跳跃介导,导致398个氨基酸的缺失CARD结构域的MAVS的短异构体,这种短异构体被称之为短MAVS(short-MAVS,sMAVS)。尽管有报道认为sMAVS发挥负调控抗病毒先天性免疫的作用,但也有报道显示sMAVS发挥正向调控抗病毒信号通路[13]。最近的两篇报道显示RNA病毒感染后,全长MAVS(FL-MAVS)逐渐降解,但sMAVS量则保持恒定[13,14]。FL-MAVS降解的具体机制是由于其在RNA病毒感染后第7和第10位氨基酸发生K48多泛素化介导的蛋白酶体降解,但是由于sMAVS缺失FL-MAVS的N端序列,因此不发生K48泛素化降解。介导MAVS降解的E3连接酶是TRIM25,巧合的是,TRIM25正是介导RIG-I的K63活化的E3连接酶,因此推测RNA病毒感染后,MAVS的位置上与RIG-I转位子成分TRIM25接近而被泛素化。值得注意的是,由于FL-MAVS的降解先于IRF3的磷酸化,说明这种降解正向调控抗病毒信号[14]。
2.2 MAVS的蛋白水平调控
2.2.1 MAVS翻译后负调控 MAVS通过翻译后调控先天性抗病毒反应,其中最重要的负调控机制是对MAVS的赖氨酸位点进行K48泛素化修饰,通过蛋白酶体途径降解MAVS。例如在病毒感染时,E3泛素连接酶RNF5(ring finger protein 5)与线粒体上的MAVS的C末端的跨膜结构域相结合,RNF5在MAVS的氨基酸K362和K461位点进行K48泛素化修饰,降解MAVS,负调控MAVS介导的I型干扰素生成与细胞抗病毒应答[15];另一种E3泛素连接酶MARCH5的RING结构域与MAVS的CARD域结合,阻止MAVS聚集,促进其通过蛋白酶体途径降解;MAVS的Lys7和Lys500氨基酸位点是E3连接酶MARCH5泛素化关键位点,MARCH5负调控MAVS介导的抗病毒信号通路,防止过度的免疫反应[16]。除此之外,还有许多其他E3泛素连接酶介导了MAVS的K48泛素化降解,例如AIP4[17]、Smurf1[18]等。
除了泛素化降解之外,另一种重要的负调控机制是通过与MAVS互作阻断其介导的抗病毒信号通路,例如TSPAN6(Tetraspanin-6)在病毒感染时自身发生赖氨酸K63的泛素化修饰,促进了其与MAVS的结合,TSPAN6 和MAVS的互作干扰了RLR下游分子TRAF3, STING(stimulator of interferon genes)和IRF3招募至MAVS,阻断了信号转导分子的装配[19];E3泛素连接酶RNF125通过泛素调控MDA-5和MAVS的结合,来抑制其下游的信号通路传导;E3泛素连接酶Triad3A与MAVS的TRAF相互作用结构域(TIM)(氨基酸第143-147位)互作,竞争性结合TRAF3位点,负调控机体天然抗病毒反应[20];MUL1(Mitochondrial E3 ubiquitin protein ligase 1)定位在线粒体外膜上,与MAVS相互作用,并催化RIG-I的SUMO化修饰,抑制RIG-I介导的细胞信号传导[21]。NOD样受体NLRX1也位于线粒体上,能够通过扣留MAVS使其远离RIG-I来发挥负调控作用[22]。自噬相关蛋白ATG12-ATG5复合物能通过影响MAVS与RIG-I的结合,来负调控I型干扰素信号通路[23]。而COX5B(Cytochrome c oxidase subunit 5B)也能通过与ATG5的互作负调控MAVS介导的信号通路[24]。
除了以上两种最重要的负调控途径之外,还有其他一些其他的非经典的负调控机制,例如蛋白酶体PSMA7(α4)亚基与细胞内的MAVS相互作用介导MAVS被蛋白酶体降解[25]。胰岛素受体酪氨酸激酶底物(IRTKS)在细胞核中募集E2连接酶UBC9,它在病毒感染期间易位到细胞质,引起SUMO化的PCBP2蛋白介导MAVS降解[26]。蛋白磷酸激酶PLK1(polo-like kinase 1)磷酸化修饰MAVS,从而抑制MAVS招募信号分子。线粒体蛋白也能调控RLR信号通路[27]。两个协同的线粒体蛋白MFN1和MFN2(mitofusin 1 and 2)都可与MAVS相互作用,但是其作用不同。MFN2负调控MAVS介导的通路,MFN1通过影响线粒体动态变化正调控RLR介导的抗病毒信号通路[28]。除了细胞蛋白之外,miRNA也被报道影响MAVS介导的抗病毒反应,水泡性口炎病毒感染后,内源性的miR-576-3p 通过和STING、MAVS和TRAF3结合,抑制IRF3入核,干扰素水平下降[29]。
2.2.2 MAVS翻译后正调控 有很多蛋白被发现通过对MAVS的翻译后修饰正调控MAVS介导信号传导通路[30]。其中最重要的正调控机制是对MAVS的赖氨酸位点进行K63泛素化修饰,活化MAVS。例如线粒体接头蛋白TRIM14与MAVS相互作用,促进了MAVS信号小体(signalosome)组装。当病毒感染时,TRIM14的赖氨酸Lys365位点发生K63多聚泛素化修饰,招募NEMO到MAVS信号小体,促使IRF3和NF-κB信号通路激活,正调控RIG-I介导的抗病毒免疫。I型干扰素能促进TRIM14的表达,从而增强先天免疫反应,对抗病毒感染[31]。E3泛素连接酶MIB2(mindbomb2)与MAVS上高度保守的DLAIS基序结合,促使TBK1的K63连接的泛素化修饰,并磷酸化激活IRF3/7,激活下游转录因子和诱导更多的IFN-β的生成[32];E3泛素连接酶RNF135,能促进RIG-I的C端结构域K63聚泛素化,从而增强病毒感染早期I型干扰素的生成[33]。E3泛素连接酶TRIM25能K63泛素化修饰RIG-I、MDA5与MAVS的复合物,并正调控抗病毒信号通路,RIG-I第172位赖氨酸是TRIM25介导的K63泛素化的关键位点,增强RIG-I与MAVS相互结合[34]。有趣的是,TRIM25还对全长MAVS的在第7和第10位赖氨酸位点发生K48泛素化介导的蛋白酶体降解,而这种降解正向调控抗病毒信号[14]。
除了对MAVS的泛素化修饰之外,磷酸化修饰也是MAVS正调控的主要方式。例如MAVS的酪氨酸Tyr9位点磷酸化激活下游IFN-β的信号传导[35];酪氨酸激酶c-Abl直接与MAVS相互作用磷酸化MAVS,正调控MAVS介导的信号通路[36](图1)。
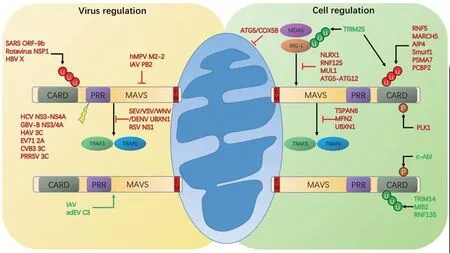
图1 MAVS介导的先天性免疫的调控Fig.1 The regulation of MAVS-mediated innate immunity
3 病毒感染调控MAVS信号通路
3.1 病毒蛋白裂解MAVS 许多病毒的蛋白具有蛋白酶功能,能够通过切割MAVS来负调控MAVS介导的信号通路。例如丙肝病毒NS3-NS4A蛋白酶特异性识别位于MAVS的跨膜结构域半胱氨酸C508位点来切割MAVS,MAVS从线粒体膜上释放,从而抑制下游I型干扰素和III型干扰素抗病毒信号通路[37]。与NS3/NS4A同源的犬肝炎病毒NS3、GB病毒B型NS3/4A,也能裂解MAVS,促使病毒免疫逃避[38,39]。除此以外,甲肝病毒的半胱氨酸蛋白酶的前体3C、乙肝病毒的HBX蛋白和肠道病毒71型的蛋白酶2A前体均可以裂解MAVS,从而阻断下游信号转导[40,41]。柯萨奇病毒B3半胱氨酸蛋白酶前体3C介导MAVS,在富含脯氨酸的区域的谷氨酰胺Q148位点裂解,导致了MAVS在线粒体膜的重新定位并抑制下游信号通路[42]。猪繁殖与呼吸综合征病毒的3C样蛋白酶通过在蛋白酶体和独立的含半胱氨酸的天冬氨酸蛋白水解酶(caspase)方式在谷氨酸Glu268位点切割MAVS,抑制病毒诱导IFN-β的产生[43]。
3.2 蛋白酶体降解MAVS 除了直接裂解MAVS之外,有些病毒能利用蛋白酶体降解MAVS途径直接抑制MAVS。冠状病毒编码的开放阅读框9B(ORF-9B)通过PCBP2介导的E3泛素连接酶AIP4降解MAVS、TRAF3和TRAF6的复合物。冠状病毒SARS的ORF-9B能操纵宿主细胞的线粒体功能,以帮助其逃避宿主天然免疫[44]。轮状病毒非结构蛋白1(NSP1)在感染后,介导MAVS通过蛋白酶体途径被泛素化降解,抑制线粒体外膜上MAVS聚集体的形成,从而抑制了抗病毒信号级联反应[45]。乙型肝炎病毒X蛋白对MAVS的赖氨酸Lys136位点进行泛素化修饰后,被蛋白酶体降解[46]。鲤春病毒血症病毒(SVCV)的N蛋白可以通过经由泛素-蛋白酶体途径降解斑马鱼的MAVS[47]。肠道病毒(CVB)感染细胞后,E3泛素连接酶Gp78通过蛋白酶体途径和内质网关联降解通路(ER-associated degradation)直接降解MAVS,Gp78也能与MAVS的N端、C端的结构域相结合,负调控MAVS介导的抗病毒信号转导[48]。
3.3 与MAVS互作影响调控 病毒感染后,细胞内某些蛋白能够与MAVS直接相互作用,阻碍下游接头分子与MAVS结合。例如UBXN1(domaincontaining protein 1)能负调控RNA病毒诱导的I型干扰素反应。水泡口炎病毒、仙台病毒、西尼罗河病毒、登革热病毒等RNA病毒感染时,诱导生成的N端具有UBA结构域的泛素结合蛋白UBXN1特异性与MAVS结合,竞争TRAF3/6的结合位点(氨基酸第455-460位),阻碍MAVS招募TRAF3/6(TNF receptor-associated factor 3/6),干扰胞内的MAVS寡聚化,负调控MAVS下游信号通路[49]。
病毒蛋白与MAVS的互作也会通过影响线粒体上MAVS的集聚或改变MAVS的空间定位和构象,从而调控MAVS介导的通路。呼吸道合胞病毒(RSV)感染细胞后,非结构蛋白NS1与MAVS相结合,阻碍招募RIG-I的下游干扰素活化因子,如TRAF3、TRAF6和RIP1(receptor interacting protein-1)[50]。人类偏肺病毒(hMPV)的毒力因子M2-2蛋白通过与MAVS的互作抑制MAVS介导的细胞抗病毒反应,帮助偏肺病毒逃避先天性免疫[51]。甲型禽流感病毒(IAVAIV)H5N1的RNA聚合酶复合体PB2亚基通过直接相互作用于MAVS第1-37位氨基酸位点,阻碍MAVS的寡聚化和分子间构象变化,导致MAVS复合物的失活[52]。禽甲型流感病毒H5N1 RNA编码的开放阅读框(ORF)变化形成的毒力因子PB1-F2蛋白和PB2Δ蛋白都能用与MAVS在线粒体的相互作用,PB1-F2蛋白抑制宿主的先天免疫应答,PB2Δ蛋白增加甲型流感病毒诱导的I型干扰素表达,降低病毒复制水平[53,54]。Caspase募集域和膜相关鸟苷酸激酶样结构域蛋白(CARD recruited membrane associated protein 3,CARMA3)作为宿主因子,在RNA病毒感染后,能抑制线粒体上MAVS的集聚,负调控RIG-I与MAVS介导的TBK1和IRF3激活[55]。
3.4 病毒感染后正调控 抗病毒反应与正调控MAVS介导的信号通路并不矛盾,病毒感染也可以正调控MAVS介导的信号通路。流感病毒感染时,鸭的TRIM27.1基因和TRIM27-L基因表达上调。TRIM家族蛋白是常见的E3泛素连接酶, TRIM27-L基因表达能强烈激活RIG介导的MAVS先天免疫信号,诱导抗病毒基因MX1和IFN-β的转录水平上调[56]。猪干扰素诱导蛋白三十四肽重复3(poIFIT3)是猪流感病毒(SIV)诱导出的基因之一,poIFIT3通过靶向MAVS ,诱导IFN-β水平上调,而且poIFIT3的过度表达可有效抑制SIV的复制[57]。腺病毒能共价结合补体C3进入细胞内,在细胞质内C3能增强激活线粒体抗病毒信号(MAVS)依赖的信号级联和诱导促炎细胞因子的分泌[58](图1)。
4 展望
先天性抗病毒信号通路,作为第一道屏障消灭入侵的病原微生物,还避免了过度炎症细胞损伤。作为先天性免疫的中枢蛋白,MAVS的精细调控,对于MAVS发挥功能和防止过度免疫反应至关重要。如上所述,细胞和病毒使用许多不同的机制来调控MAVS信号通路,包括转录和翻译后修饰,与MAVS关联的蛋白质-蛋白质相互作用。作为先天性免疫反应的中枢分子,越来越多的研究结果显示:MAVS除了参与先天性免疫之外还参与了很多其他重要的细胞通路,例如细胞凋亡、细胞自噬等。反之其他通路的成分也被发现能够通过调控MAVS来影响先天性免疫,例如自噬相关蛋白ATG5-ATG12等[7]。MAVS作为RLR信号通路中保守的接头蛋白,在I型干扰素通路中起重要作用。动物病毒通过干扰MAVS,影响宿主干扰素反应,来逃避机体的抗病毒天然免疫。尽管近年来研究进展显著,目前关于动物病毒抑制MAVS介导的干扰素表达机制的研究很少,尤其是禽类和水生动物类,大部分动物体内MAVS介导的抗病毒信号通路的调控机制不明确。通过对动物的MAVS介导的抗病毒天然免疫信号通路的调控机制还有待探索,有希望从该通路入手寻找到新的治疗靶点来防治动物传染病。
[1]Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors[J]. Trends Mol Med, 2007, 13(11): 460-469.
[2]Loo Y M, Gale M Jr. Immune signaling by RIG-I-like receptors[J]. Immunity, 2011, 34(5): 680-692.
[3]Kato H, Takeuchi O, Sato S,et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses[J]. Nature, 2006, 441(7089): 101-105.
[4]Loo Y-M, Fornek J, Crochet N,et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity[J]. J Virol, 2008, 82(1): 335-345.
[5]Seth R B, Sun L, Ea C-K,et al. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3[J].Cell, 2005, 122(5): 669-682.
[6]Vazquez C, Horner S M. MAVS Coordination of Antiviral Innate Immunity[J]. J Virol, 2015, 89(14): 6974-6977.
[7]Belgnaoui S M, Paz S, Hiscott J. Orchestrating the interferon antiviral response through the mitochondrial antiviral signaling (MAVS) adapter[J]. Curr Opin Immunol, 2011, 23(5): 564-572.
[8]Ye J, Maniatis T. A prion-like trigger of antiviral signaling[J]. Cell, 2011, 146(3): 348-350.
[9]Liu H M, Loo Y M, Horner S M,et al. The mitochondrial targeting chaperone 14-3-3epsilon regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity[J]. Cell Host Microbe, 2012, 11(5):528-537.
[10]Takeuchi O, Akira S. Pattern recognition receptors and inflammation[J]. Cell, 2010, 140(6): 805-820.
[11]Koshiba T. Mitochondrial-mediated antiviral immunity[J]. Biochim Biophys Acta, 2013, 1833(1): 225-232.
[12]Lad S P, Yang G, Scott D A,et al. Identification of MAVS splicing variants that interfere with RIGI/MAVS pathway signaling[J]. Mol Immunol, 2008, 45(8): 2277-2287.
[13]Brubaker S W, Gauthier A E, Mills E W,et al. A bicistronic MAVS transcript highlights a class of truncated variants in antiviral immunity[J]. Cell, 2014,156(4): 800-811.
[14]Castanier C, Zemirli N, Portier A,et al. MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors[J]. BMC Biol, 2012, 10: 44.
[15]Zhong B, Zhang Y, Tan B,et al. The E3 ubiquitin ligase RNF5 targets virus-induced signaling adaptor for ubiquitination and degradation[J]. J Immunol, 2010,184(11): 6249-6255.
[16]Yoo Y S, Park Y Y, Kim J H,et al. The mitochondrial ubiquitin ligase MARCH5 resolves MAVS aggregates during antiviral signalling[J]. Nat Commun, 2015, 6:7910.
[17]You F, Sun H, Zhou X,et al. PCBP2 mediates degradation of the adaptor MAVS via the HECT ubiquitin ligase AIP4[J]. Nat Immunol, 2009, 10(12): 1300-1308.
[18]Wang Y, Tong X, Ye X. Ndfip1 negatively regulates RIG-I-dependent immune signaling by enhancing E3 ligase Smurf1-mediated MAVS degradation[J]. J Immunol, 2012, 189(11): 5304-5313.
[19]Wang Y, Tong X, Omoregie E S,et al. Tetraspanin 6(TSPAN6) negatively regulates retinoic acid-inducible gene I-like receptor-mediated immune signaling in a ubiquitination-dependent manner[J]. J Biol Chem, 2012,287(41): 34626-3434.
[20]Nakhaei P, Mesplede T, Solis M,et al. The E3 ubiquitin ligase Triad3A negatively regulates the RIG-I/MAVS signaling pathway by targeting TRAF3 for degradation[J].PLoS Pathog, 2009, 5(11): e1000650.
[21]Jenkins K, Khoo J J, Sadler A,et al. Mitochondrially localised MUL1 is a novel modulator of antiviral signaling[J]. Immunol Cell Biol, 2013, 91(4): 321-330.
[22]Moore C B, Bergstralh D T, Duncan J A,et al. NLRX1 is a regulator of mitochondrial antiviral immunity[J].Nature, 2008, 451(7178): 573-577.
[23]Huang X, Yue Y, Li D,et al. Antibody-dependent enhancement of dengue virus infection inhibits RLR-mediated Type-I IFN-independent signalling through upregulation of cellular autophagy[J]. Sci Rep, 2016, 6:22303.
[24]Zhao Y, Sun X, Nie X,et al. COX5B regulates MAVS-mediated antiviral signaling through interaction with ATG5 and repressing ROS production[J]. PLoS Pathog,2012, 8(12): e1003086.
[25]Jia Y, Song T, Wei C,et al. Negative regulation of MAVS-mediated innate immune response by PSMA7[J].J Immunol, 2009, 183(7): 4241-4248.
[26]Xia P, Wang S, Xiong Z,et al. IRTKS negatively regulates antiviral immunity through PCBP2 sumoylation-mediated MAVS degradation[J]. Nat Commun, 2015, 6: 8132.
[27]Vitour D, Dabo S, Ahmadi Pour M,et al. Polo-like kinase 1 (PLK1) regulates interferon (IFN) induction by MAVS[J]. J Biol Chem, 2009, 284(33): 21797-21809.
[28]Arnoult D, Soares F, Tattoli I,et al. Mitochondria in innate immunity[J]. EMBO Rep, 2011, 12(9): 901-910.
[29]Yarbrough M L, Zhang K, Sakthivel R,et al. Primatespecific miR-576-3p sets host defense signalling threshold[J]. Nat Commun, 2014, 5: 4963.
[30]Eisenacher K, Krug A. Regulation of RLR-mediated innate immune signaling--it is all about keeping the balance[J]. Eur J Cell Biol, 2012, 91(1): 36-47.
[31]Zhou Z, Jia X, Xue Q,et al. TRIM14 is a mitochondrial adaptor that facilitates retinoic acid-inducible gene-I-like receptor-mediated innate immune response[J]. Proc Natl Acad Sci U S A, 2014, 111(2): E245-254.
[32]Ye J S, Kim N, Lee K J,et al. Lysine 63-linked TANK-binding kinase 1 ubiquitination by mindbomb E3 ubiquitin protein ligase 2 is mediated by the mitochondrial antiviral signaling protein[J]. J Virol, 2014, 88(21): 12765-12776.
[33]Oshiumi H, Matsumoto M, Hatakeyama S,et al. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection[J]. J Biol Chem, 2009, 284(2): 807-817.
[34]Gack M U, Shin Y C, Joo C H,et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity[J]. Nature, 2007, 446(7138): 916-920.
[35]Wen C, Yan Z, Yang X,et al. Identification of tyrosine-9 of MAVS as critical target for inducible phosphorylation that determines activation[J]. PLoS One, 2012, 7(7):e41687.
[36]Song T, Wei C, Zheng Z,et al. c-Abl tyrosine kinase interacts with MAVS and regulates innate immune response[J]. FEBS Lett, 2010, 584(1): 33-38.
[37]Ferreira A R, Magalhaes A C, Camoes F,et al. Hepatitis C virus NS3-4A inhibits the peroxisomal MAVS-dependent antiviral signalling response[J]. J Cell Mol Med, 2016, 20(4): 750-757.
[38]Chen Z, Benureau Y, Rijnbrand R,et al. GB virus B disrupts RIG-I signaling by NS3/4A-mediated cleavage of the adaptor protein MAVS[J]. J Virol, 2007, 81(2):964-976.
[39]Parera M, Martrus G, Franco S,et al. Canine hepacivirus NS3 serine protease can cleave the human adaptor proteins MAVS and TRIF[J]. PLoS One, 2012, 7(8):e42481.
[40]Paulmann D, Magulski T, Schwarz R,et al. Hepatitis A virus protein 2B suppresses beta interferon (IFN) gene transcription by interfering with IFN regulatory factor 3 activation[J]. J Gen Virol, 2008, 89(Pt 7): 1593-1604.
[41]Wang B, Xi X, Lei X,et al. Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses[J]. PLoS Pathog, 2013, 9(3): e1003231.
[42]Mukherjee A, Morosky S A, Delorme-Axford E,et al.The coxsackievirus B 3C protease cleaves MAVS and TRIF to attenuate host type I interferon and apoptotic signaling[J]. PLoS Pathog, 2011, 7(3): e1001311.
[43]Dong J, Xu S, Wang J,et al. Porcine reproductive and respiratory syndrome virus 3C protease cleaves the mitochondrial antiviral signalling complex to antagonize IFN-beta expression[J]. J Gen Virol, 2015, 96(10): 3049-3058.
[44]Shi C S, Qi H Y, Boularan C,et al. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome[J]. J Immunol, 2014, 193(6): 3080-3089.
[45]Nandi S, Chanda S, Bagchi P,et al. MAVS protein is attenuated by rotavirus nonstructural protein 1[J]. PLoS One, 2014, 9(3): e92126.
[46]Wei C, Ni C, Song T,et al. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein[J]. J Immunol,2010, 185(2): 1158-1168.
[47]Lu L F, Li S, Lu X B,et al. Spring Viremia of Carp Virus N Protein Suppresses Fish IFNphi1 Production by Targeting the Mitochondrial Antiviral Signaling Protein[J]. J Immunol, 2016, 196(9): 3744-3753.
[48]Jacobs J L, Zhu J, Sarkar S N,et al. Regulation of mitochondrial antiviral signaling (MAVS) expression and signaling by the mitochondria-associated endoplasmic reticulum membrane (MAM) protein Gp78[J]. J Biol Chem, 2014, 289(3): 1604-1616.
[49]Wang P, Yang L, Cheng G,et al. UBXN1 interferes with Rig-I-like receptor-mediated antiviral immune response by targeting MAVS[J]. Cell Rep, 2013, 3(4): 1057-1070.
[50]Webster Marketon J I, Corry J, Teng M N. The respiratory syncytial virus (RSV) nonstructural proteins mediate RSV suppression of glucocorticoid receptor transactivation[J].Virology, 2014, 449: 62-69.
[51]Ren J, Wang Q, Kolli D,et al. Human metapneumovirus M2-2 protein inhibits innate cellular signaling by targeting MAVS[J]. J Virol, 2012, 86(23): 13049-13061.
[52]Patel D, Schultz L W, Umland T C. Influenza A polymerase subunit PB2 possesses overlapping binding sites for polymerase subunit PB1 and human MAVS proteins[J]. Virus Res, 2013, 172(1-2): 75-80.
[53]Varga Z T, Grant A, Manicassamy B,et al. Influenza virus protein PB1-F2 inhibits the induction of type I interferon by binding to MAVS and decreasing mitochondrial membrane potential[J]. J Virol, 2012, 86(16): 8359-8366.
[54]Boergeling Y, Rozhdestvensky T S, Schmolke M,et al.Evidence for a Novel Mechanism of Influenza Virus-Induced Type I Interferon Expression by a Defective RNA-Encoded Protein[J]. PLoS Pathog, 2015, 11(5):e1004924.
[55]Jiang C, Zhou Z, Quan Y,et al. CARMA3 Is a Host Factor Regulating the Balance of Inflammatory and Antiviral Responses against Viral Infection[J]. Cell Rep,2016, 14(10): 2389-2401.
[56]Blaine A H, Miranzo-Navarro D, Campbell L K,et al.Duck TRIM27-L enhances MAVS signaling and is absent in chickens and turkeys[J]. Mol Immunol, 2015, 67(2 Pt B): 607-615.
[57]Li Y, Wen Z, Zhou H,et al. Porcine interferon-induced protein with tetratricopeptide repeats 3, poIFIT3, inhibits swine influenza virus replication and potentiates IFN-beta production[J]. Dev Comp Immunol, 2015, 50(1): 49-57.
[58]Tam J C, Bidgood S R, Mcewan W A,et al. Intracellular sensing of complement C3 activates cell autonomous immunity[J]. Science, 2014, 345(6201): 1256070.
THE REGULATION OF MAVS-MEDIATED ANTIVIRAL INNATE IMMUNITY
ZHENG Hang1, SUN Ying-jie2, ZHANG Pin3, DING Chan2
(1. College of Animal Science and Technology, Jilin Agricultural University, Changchun 130118, China; 2. Shanghai Veterinary Research Institute, CAAS, Shanghai 200241, China; 3. College of Animal Science and Technology, Shandong Agricultural University, Taian 271018,China)
As the fi rst barrier of immune defense towards invading pathogens, the signaling pathway of the innate immunity plays an important role in antiviral response. The most important cytoplasmic pathogen recognition receptors are retinoic acid-inducible gene 1 and differentiation-associated protein 5. They possess the same downstream adaptor mitochondrial antiviral-signaling protein (MAVS).MAVS functions as the central molecular tool in innate immunity signaling pathway. MAVS-mediated signaling pathway is the important antiviral mechanism. However, viruses obtain a series of anti-MAVS mechanisms in the long-term coexistence status. Furthermore, cells in the resting state possess a number of MAVS regulation mechanisms to avoid excessive immune response. The delicate regulation of MAVS is critical for cell function and antiviral response. This review brie fl y introduces the structure and function as well as transcriptional and translational regulation mechanisms of MAVS. Furthermore, the mechanisms how viruses “fight back” MAVS-mediate innate immunity are elaborated. Understanding the regulation mechanism of MAVS may provide new insights into therapeutic strategies for the immunity regulation and virus infection.
Innate immunity; mitochondrial antiviral-signaling protein; pathogen; host
S852.42
A
1674-6422(2018)01-0081-08
2016-08-29
国家自然科学基金重点项目(31530074);国家自然科学基金面上项目(31372421);国家自然科学基金青年基金(31400144)
郑航,男,硕士研究生,预防兽医学专业
丁铲,E-mail: shoveldeen @ shvri.ac.cn
