Photoelectrochemical Reduction of CO2 over Graphene-Based Composites: Basic Principle, Recent Progress, and Future Perspective
2018-01-15QUANQuanXIEShunJiWANGYeXUYiJun
QUAN Quan XIE Shun-Ji WANG Ye,* XU Yi-Jun,*
Photoelectrochemical Reduction of CO2over Graphene-Based Composites: Basic Principle, Recent Progress, and Future Perspective
QUAN Quan1XIE Shun-Ji2WANG Ye2,*XU Yi-Jun1,*
(12)
In response to aggravated fossil resources consuming and greenhouse effect, CO2reduction has become a globally important scientific issue because this method can be used to produce value-added feedstock for application in alternative energy supply. Photoelectrocatalysis, achieved by combining optical energy and external electrical bias, is a feasible and promising system for CO2reduction. In particular, applying graphene in tuning photoelectrochemical CO2reduction has aroused considerable attention because graphene is advantageous for enhancing CO2adsorption, facilitating electrons transfer, and thus optimizing the performance of graphene-based composite electrodes. In this review, we elaborate the fundamental principle, basic preparation methods, and recent progress in developing a variety of graphene-based composite electrodes for photoelectrochemical reduction of CO2into solar fuels and chemicals. We also present a perspective on the opportunities and challenges for future research in this booming area and highlight the potential evolution strategies for advancing the research on photoelectrochemical CO2reduction.
Photoelectrochemical; CO2reduction; Graphene-based composite
1 Introduction
With human society developed rapidly, the depletion of fossil resources and the deterioration of greenhouse effect have become global issues1,2. CO2as one of key components of greenhouse gas is potential to be utilized producing solar fuels3–6. Therefore, the demand for the future energy supply and global environment improvement has stimulated research activities towards the efficient reduction of CO2to value added products. However, because CO2is a stable molecule with high thermodynamic stability and kinetic inertness, the reduction of CO2is required to break a high activation barrier and regarded as a challenging research theme in chemical science7,8.

The plants photosynthesis, an important medium for nature carbon cycle, is to transform CO2with H2O into carbohydrates and O2under sunlight illumination at room temperature. Enlightened by this natural process, numerous research efforts have been devoted to developing artificial or synthetic photocatalysts for converting CO2into useful chemicals including carbonic oxide, formic acid, formaldehyde, methanol, ethanol, methane, higher hydrocarbons, etc.9–14. While multitudinous encouraging achievements have been gained toward artificial photocatalytic reduction of CO2, further effort is still required to increase the solar-to-fuel efficiencies, promote the selectivity of products and reinforce the stability of photocatalysts13,15. Besides, the electrochemical CO2reduction is another significant route for the transformation of CO2to value added feedstocks16. Because the electrochemical devices are not limited by traditional thermochemical cycles, their achievable efficiency is often significantly higher than their chemical counterpart. Meanwhile, the separated non-direct reactions permit researchers to tailor the properties needed for each redox process independently17. However, generally a high overpotential for electrocatalysis is needed to overcome the energy barrier of CO2reduction18. At standard pressure, the solubility of CO2in aqueous solution is low. Thus, higher pressures are necessary to increase the CO2concentration in the liquid phase, which, in turn, limit the electrode stability19.
Nowadays, with combination of heterogeneous photo- catalysis and electrochemical techniques, photoelectrocatalysis is regarded as an alternative and powerful approach for CO2reduction20.On one hand, introducing solar energy can markedly lower the applied voltage in comparison with the electrocatalysis. On the other hand, the imposition of an external electrical bias can facilitate the separation of electrons and holes, which is of significant importance to promote the photocatalytic efficiency. Furthermore, the photoelectro- chemical, physically separating the stages of oxidation and reduction in dual-chamber, can avoid the re-oxidation of obtained products as well as other negative reacting competitions21. In 1978, Halmann22earliest reported the photoelectrocatalytic reduction of CO2, using-type gallium phosphide as a photocathode to reduce aqueous carbon dioxide, getting formic acid, formaldehyde and methanol as products. Henceforth, this research direction has been extensively studied.
In fact, just in regard to an experimental setup, photo- electrochemical reaction is similar to electrochemical reaction23. Instead of conductor electrodes applied in electrochemistry, photoelectrochemical devices use semi- conductor as one or two electrodes to be light harvester. When activity at semiconductor surface for CO2reduction is poor, the corresponding photoelectrocatalytic reaction rate is definitely slow, irrespective of a level of applied external potential. Therefore, selecting the appropriate electrode materials and optimizing their performance are significantly crucial for realizing the high-efficiency of photoelectrocatalytic reaction. In recent years, graphene has rocketed as a shining star material, which not only is now available at large quantities, but also possesses many unique properties such as high surface area, excellent conductivity and mechanical strength24,25. Therefore, many research efforts have been paid to combine graphene with semiconductors as synergistic heterogeneous composites to improve the efficiency of either photocatalytic or electrochemical CO2reduction25–28. Notably, graphene and graphene-based composites have also stepped into the photoelectrochemical fields and been applied to optimize the composition and the performance of photoelectrodes29–31.
The goal of this review is to describe the current status of the use of graphene in tuning the efficiency of photoelectro- chemical CO2reduction to solar fuels and chemicals over graphene-based composites, and demonstrate how to prepare graphene-based composite electrodes and utilize the key property of graphene to facilitate photoelectrochemical reduction of CO2by selecting typical examples in this research field. Beyond that, we will discuss the potential strategies that can be evolved to optimize the performance of photoelectro- chemical CO2reduction, the possible opportunities and key challenges in future development of this research area.
2 Basic principle of photoelectrochemical CO2 reduction over graphene-based composites
When CO2reduction is conducted in the cathodic chamber of photoelectrochemical cell, the H2O oxidation is typically adopted as electron donor and proton source in the anodic chamber32,33, which has been reported in many researches and reviews9,10,21,34–40. Therefore, the coupling of CO2reduction with H2O oxidation to form closing of the complete photo- electrochemical cycle is the premise of following elaboration.
2.1 Thermodynamics and pathways of CO2reduction
Photoelectrochemistry is regarded as a multidisciplinary field involving photochemistry, electrochemistry, surface science and solid-state physics41. Before discussion about the rationales of photoelectrochemical reduction of CO2, the thermodynamics of CO2reduction and the pathways of CO2electroreduction are first elaborated to provide a preliminary comprehension. Because of high stability of linear structure and low energy grade of CO2molecule, the chemical transforma- tions of CO2are thermodynamically highly unfavorable42. The standard Gibbs free energies (Δ0) and the standard redox potential (Δ0) of the multi-electron CO2reduction are listed in Table 1. Obviously, the Δ0values of the reduction of CO2with water are all highly positive, indicating that a large input of external energy is required to drive the desired transformations. Therefore, the reaction of CO2reduction is quite challenging.
So far, various hypothesizes concerning mechanism of CO2reduction have been explored and proposed by different research groups43–48, which are indicated as complex multistep reactions involving shared intermediates and multiple reaction pathways. The electron paramagnetic resonance (EPR) spectroscopy,diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), transient absorption spectroscopy (TAS) and scanning tunneling microscope (STM) are employed as exploratory techniques to investigate the reaction mechanism and pathways49. The typical two-electron reduced reaction pathways for electroreduction of CO2in aqueous medium are illustrated in Fig.1A. The initial step is that CO2molecule accepts one electron to form the carbon dioxide anion radical (CO2•–)50, which is more likely to be broken due to the bending structure in contrast to linear geometry structure of CO251. When CO2•–is adsorbed on the electrode, H+in aqueous media may easily react with the O atom, because C atom is bonded to the surface preventing reaction with H+50. The CO2H(ads), formed in this way, will be further reduced by another electron into adsorbed CO, or may participate in subsequent complex reaction with H+and electrons turning into CH4, CH3OH or other value-added hydrocarbons52. When CO2•–is not adsorbed on the electrode in aqueous solution, the nucleophilic carbon atom acts as the Lewis base and the formate ion is formed with intrusion of second electron. Interestingly, CO2molecule contains delocalized-conjugated binding, while graphene manifests a large 2D-conjugated structure, which results in a strong–conjugation interaction established between graphene and CO2molecule (Fig.1B). The reaction intermediates such as CO2•–with a delocalized electronic structure can also be attached onto graphene through–non-covalent bonds53. When graphene- based composites act as electrodes for CO2reduction, the strong–conjugation can facilitate CO2adsorption and also contribute to the destabilization and activation of CO2molecules54,55. Moreover, the theoretical surface area of a single graphene sheet is 2630 m2·g−156, providing a large number of adsorption sites for the CO2molecules. Therefore, the large surface area and strong–conjugation provide synergistic effects on promoting CO2adsorption, thus resulting in tuning the efficiency of reaction for CO2reduction.
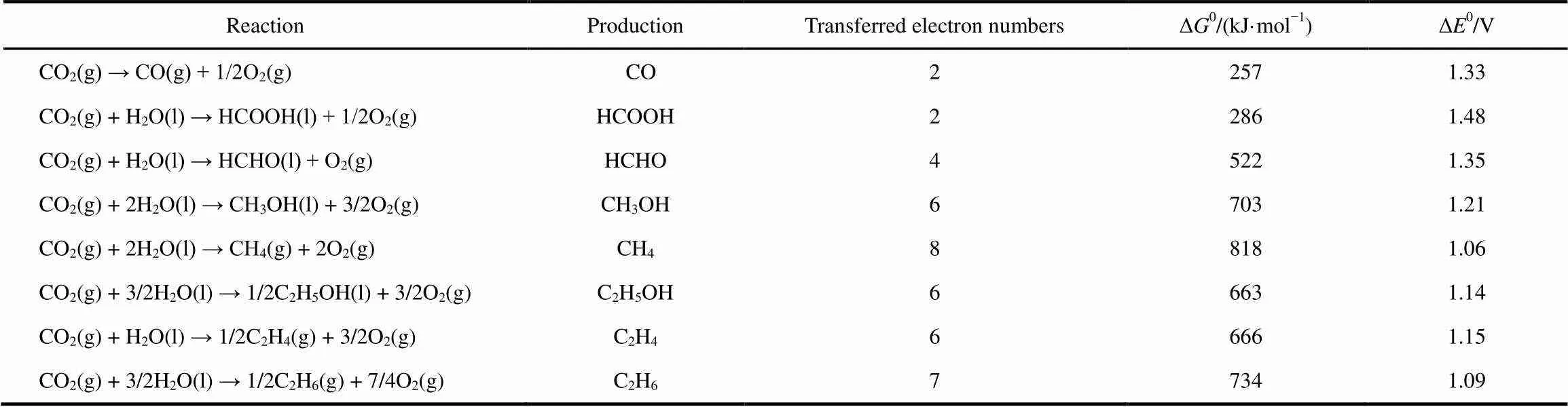
Table 1 Thermodynamics of CO2 reduction.
The process of CO2reduction is highly sensitive to the reaction conditions, such as pH, external bias, electrode surface structure, temperature, pressure and so on57. Thereinto, the pH value of the electrolyte has critical impact on the preferred reaction pathway and the final products. At a low pH value, the competing hydrogen evolution reaction (HER) dominates while the reaction rates for CO2reduction improve with the increase in pH values. The external bias on the cathode plays an important role in the product distribution of CO2reduction. Furthermore, the negative external bias should be controlled applicably in order to avoid the competitive HER and promote CO2conversion. As for graphene-based composites, different weight ratio of graphene could also influence the selectivity of products for CO2reduction. For instance, Han53have found that with the increase of the weight ratio of graphene in the graphene-TiO2composites, the production rate of CH4slowly decreases while the production rate of C2H6increases to some degree. Actually, the pathway of CO2reduction is more complicated in specific reaction than the aforementioned theories depending on the practical conditions. The conclusive evidence about the reaction mechanism for CO2reaction is still not available42,52, so that the pathways of CO2reduction and the mechanism of graphene-based composites for CO2reduction need further exploration.
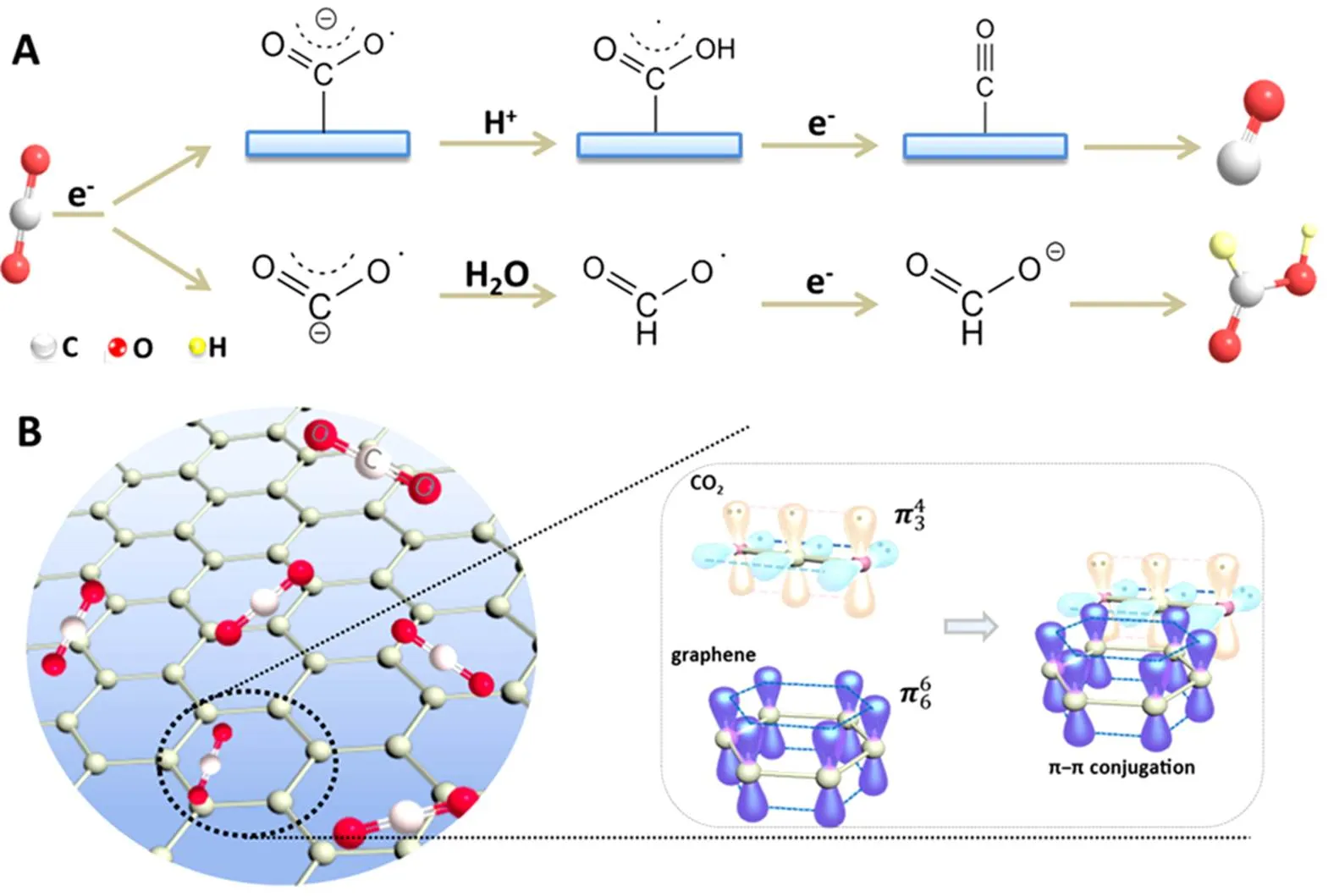
Fig.1 (A) Two-electron reaction mechanism of CO2 reduction in aqueous solutions, (B) The formation of π-π conjugation interaction between graphene and CO2 molecule.
2.2 Categories and processes of photoelectrochemical CO2 reduction
A photoelectrochemical setup typically contains four components. Firstly, the electrolyte is an essential component. The common used electrolytes for photoelectrochemical reduction of CO2include carbonates, sulfates, phosphates, perchlorates of alkali salts, and tetraalkylammonium salts, etc., all of which can affect the distribution of products by influencing the reaction pathways. Generally, the carbonates favor formic acid production while the other electrolytes favor the formation of carbon monoxide58. Secondly, the conductive substrate is a basic component. Traditionally, it is fluorine doped tin oxide (FTO) or indium tin oxide (ITO) glass. Nowadays, metal foil (e.g., Cu foil59, Ti foil60) and metal foam (e.g., Ni foam61, Cu foam62) are also developed as substrates. Thirdly, the semiconductor film anchored on substrate as working electrode is the most important component for the efficiency of photoelectrochemical setup. Fourthly, a counter electrode (e.g., platinum electrode) is applied to conduct the half reaction cooperating with working electrode to form the complete photoelectrochemical cycle. There are other components applied in a photoelectrochemical setup depending on specific demand. For example, the reference electrode (RE) (e.g., Ag/AgCl electrode, saturated calomel electrode) is used in the three-electrode system and the proton exchange membrane is needed in a dual-chamber system to delivery proton (H+). In contrast, the two-electrode and single-chamber photoelectrochemical setups do not employ reference electrodes and proton exchange membranes, respectively.
As for the semiconductor film in the photoelectrochemical system, it generates electron-hole pairs after absorbing equal or higher photon energy than its intrinsic band gap energy (g). Depending on the charge densities of electrons and holes, semiconductors can be typically categorized into two types. Simply speaking, semiconductors providing electrons as the majority charge carrier are defined as-type semiconductors, while-type semiconductors use positively charged holes as the majority charge carrier63. When-type or-type semiconductors are placed into an electrolyte solution, if the Fermi level (F) of a semiconductor does not match the redox potential (R) of the electrolyte, a charge transfer between the semiconductor and liquid phase will take place and thus a band bending will be induced to render changedFmatching theR64,65. Specifically, as for a-type semiconductor, theFis located above the valence band but below theR. The interfacial electrons flow towards the semiconductor to attain equilibrium, resulting in an increase in the Fermi level energy to match theR. Thus, a downward band bending is established, which facilitates conduction band electrons moving to semiconductor-electrolyte (S-E) interface66, as depicted in Fig.2A. Conversely, the-type semiconductor occurs an upward band bending that moves valence band holes toward the S-E interface (Fig.2B). Therefore,-type semiconductors are generally used in the reduction compartment as photocathodes because the electrons can flow toward the S-E interface to accelerate the CO2reduction. The anodic chamber usually utilizes-type semiconductors considering that upward band bending enables holes to shift toward the S-E interface facilitating the H2O oxidation.
Generally, the photoelectrochemical setups can be classified into three typical types: (I) the-type semiconductors as photocathodes with counter electrodes; (II) the-type semiconductors as photoanodes with counter electrodes. The type III is a peculiar category combining the-type semiconductors as photocathodes and-type semiconductors as photoanodes, whose working mechanism is same to type I and type II and will be discussed in Section 5.1. Fig.2(A, B) respectively illustrate the type I and type II in a three-electrode dual-chamber photoelectrochemical setup, in which type I is taken as an example to elaborate the process and mechanism of photoelectrochemical CO2reduction. The-type semiconductor adsorbs photons under light illumination to create electron-hole pairs, with the conductive band electrons moving to the S-E interface and the holes migrating toward conductive substrate. The external circuit is conducted by a potentiostat (i.e, electron pump)67, which drives electrons flow to the photocathode and then deplete with the generated holes on the cathode. This process efficiently suppresses the recombination of electrons and holes on the semiconductor. The counter electrode remains holes reacting with H2O into O2and H+. The H+is transmitted from anolyte to catholyte by membrane and then cooperates with electrons at the S-E interface to react with CO2into CO, CH4, CH3OH, HCOOH, etc. Under cooperation with light illumination and external bias, the complete photoelectro- chemical cycle with CO2reduction and H2O oxidation can keep continuous running.

Fig.2 Schematic illustrations of (A) type I and (B) type II for photoelectrochemical setup.
Viewing the elaboration for the whole process of photoelectrochemical CO2reduction, minimizing electron-hole pairs recombination and accelerating electrons transfer are significantly pivotal for enhancing photoelectrochemical activity68. Because of their good electrical conductivity coupled with a large interface area, carbon materials are often the desirable choice of the electrode. Furthermore, graphene is an excellent candidate for optimizing the electron transfer when acting as electrode materials69,70. In photocatalysis fields, the most-widely recognized role of graphene is an electron reservoir to accept, transport, and shuttle electrons photogenerated from the excitation of photoactive components in the composites71,72. The electrons rapidly transfer from the photoactive catalyst to graphene while photoinduced holes remain in the photocatalyst, which contributes to suppressing electron-hole pairs recombination73,74. Therefore, in the photoelectrochemical system, graphene can have a synergetic effect with external bias, which could further facilitate electron transfer and suppress the electron-hole pairs recombination. More interestingly, graphene can alter the conduction band potential of semiconductor to form graphene-based composite, which induces the occurrence of reduction of CO275. Bai76have reported that graphene/WO3nanobelt composites are able to reduce CO2into CH4, in which graphene elevates the conduction band of WO3in the composites while the single WO3is inherently limited to reduce CO2. On the other hand, it is should be noted that when a graphene-based composite is applied for CO2reduction, the carbonaceous products are possible to be obtained from the hydrogenation of the acid and alcoholic groups on graphene to lower the veracity of results. For dispelling the suspicion, relevant test experiments are necessary to confirm the original sources of products. For example, the experiment in the inert gases (e.g, N2, Ar) rather than CO2atmosphere could be conducted under same condition to detect the product77. Besides, the more precise method is to conduct an isotope tracing experiment, for example, isotope tracing experiment using13CO2gas78.
3 Fabrication of graphene-based composite electrodes
The preparation methods of (photo)electrodes on substrates can remarkably influence the physicochemical properties of assembled electrodes (e.g., thickness, homogeneity)79and thus affect their photoelectrochemical performances. It is crucial to utilize suitable preparation methods of electrodes for constructing high-performance photoelectrochemical system. The basic methods of (photo)electrodes preparation includes dip-coating, drop-casting, spray-coating, spin-coating, electrostatic layer-by-layer self-assembly, electrodeposition and electrophoretic deposition, etc.79–82As for graphene-based composite electrodes, the conventional fabrication methods are chemical vapor deposition (CVD) of graphene sheets on substrates with finely tuned temperature and pressure conditions83,84, or electrophoretic and electrodeposition of graphene or its precursor (e.g., graphene oxide) onto target films71. The other general methods can also be applied to prepared graphene and graphene-based composites on substrates as electrodes for desirable applications. There are many reviews focusing on the synthetic methods for graphene and its derivatives but rarely paying attention to the preparation methods for graphene or graphene-based electrodes85–88. Therefore, we will summarize and elaborate the basic preparation methods for graphene or graphene-based composites on target substrates as (photo)electrodes in this section.
3.1 Physical coating methods
The physical preparation methods are to adhere available graphene or graphene-based composites on target substrates without varying their physicochemical properties. The dip-coating and drop-casting are two of the simplest approaches to conduct because of their easily available apparatus and mild operation conditions. Generally, the target material suspension is deposited onto the substrate surface by a dropping or dip-lift method and then dried for evaporation of solvents, leaving the composite films formed on the substrate72, as illustrated in Fig.3(A, C), respectively. For instance, Amal89have drop-casted ethanol solution containing BiVO4-reduced graphene oxide (BiVO4-RGO) composites on the FTO electrodes with the aid of a micro-syringe and then dried the electrodes under flowing air for evaporation of the ethanol, leaving the BiVO4-RGO homogeneously deposited on FTO surface (Fig.3B). Because single dip-coating method has some inevitable problems, e.g., the nonuniform coverage and the weak interfacial contact between materials and substrates, the strategies combining dip-coating and electrostatic layer-by-layer self-assembly are usually applied to fabricate desirable graphene-based composites electrodes90,91. As a typical example, Liu28have fabricated RGO-CdS quantum dots (QDs) composites on FTO substrate. Firstly, the cleaned FTO substrate is rapidly dipped into poly(diallyldi- methylammoniumchloride) (PDDA) aqueous solution to have a positively charged surface; Secondly, the FTO is immersed in negatively charged CdS QDs aqueous solution; Finally, the resultant substrate is dipped into positively charged RGO-poly(allylamine hydrochloride) (PAH) aqueous solution. The multilayered films with desired film thickness can be fabricated by repeating the number of dipping cycles (Fig.3D).
Besides, the spin-coating method is also an alternative for fabricating graphene or graphene-based composite electrodes. A small amount of coating material is put on the center of the substrate and then rotated at high speed to spread the coating material by centrifugal force. After drying, the film material is formed (Fig.4A). The spin coating speeds can vary the orientation of the graphene sheets in the composite thin films. Chhowalla92have found that at high spin-coating speeds (2000 r·min−1), the graphene sheets are sparsely distributed and oriented almost parallel to the substrate surface (Fig.4B). At lower spin-coating speeds (600 r·min−1), graphene sheets are densely distributed over the substrate (Fig.4C). The shear force is small thus to form the random orientation of graphene sheets. Jang93have fabricated graphene oxide/hematite (GO/α-Fe2O3) photoanode on FTO, in which GO is coated by spin-coating and serves as a sacrificial underlayer. They have also discovered that varying the spin speed from 3000 to 6000 r·min−1leads to an improved photocurrent (30% increase) from 1 to 1.3 mA∙cm−2at 1.4 Vreversible hydrogen electrode (RHE), representing a strong dependence and influence on the spin speed.

Fig.3 (A) Schematic illustration of a drop-casting process, (B) SEM image of BiVO4-RGO (the inset is photographs of BiVO4 and BiVO4-RGO electrodes), (C) Schematic illustration of a dip-coating process, (D) FESEM image of RGO-CdS QDs composite films self-assembled on FTO substrate with the same five deposition cycles. (B) is reprinted with permission from Ref.89, Copyright 2010 American Chemical Society. (D) is reprinted with permission from Ref.28, Copyright 2010
American Chemical Society.

Fig.4 (A) Schematic illustration of a spin-coating process, (B) AFM image of thin films prepared at 2000 r·min−1 (the inset is the schematic of the spin coating process as 2000 r·min−1), (C) AFM image of thin films prepared at 600 r·min−1 (the inset is the schematic of the spin coating process as 600 r·min−1).
(B, C) are reprinted with permission from Ref.92, Copyright 2008 American Institute of Physics.
3.2 CVD graphene transferred or grown on target substrates
Until now, metal-catalyzed CVD method has been widely employed for the scalable production of high-quality graphene, which makes use of the pyrolysis of hydrocarbon compounds on the surface of a metal catalyst (e.g., Ni94and Cu95) at high temperatures86. However, the as-prepared graphene growing on the metals should be transferred onto target substrates to constitute desirable electrodes for electrochemical or photoelectrochemical applications. The common used method for transferring CVD graphene is to etch away the metal substrate using a suitable etchant. For instance, Qiu96have removed CVD graphene from Cu foil with FeCl3as an etching agent, obtaining the graphene film on the surface of FeCl3solution. After that, the graphene film is carefully transferred to deionized water to remove the adsorbed ions. Lastly, the graphene is transferred onto pre-cleaned FTO glass and dried at 120°C for 3 h under vacuum. Seo97have grown single-layer graphene on Cu foil by CVD and then spin-coated Poly(-methyl methacrylate) (PMMA) on to graphene. Cu foil is etched away in ammonium persulfate (APS) solution and graphene/PMMA stacks are transferred onto SiO2/Si substrate after cleaning in deionized water. Finally, PMMA is removed by acetone. Furthermore, a novel electrochemical bubbling method for transferring single graphene grains and graphene films to arbitrary substrates has been reported by Cheng’s group, as illustrated in Fig.598. The specific processes are that: (a) the graphene grown on Pt substrate is spin-coated with PMMA and cured at 180°C for 30 min; (b) the PMMA/graphene/Pt is used as a cathode and a Pt foil is used as an anode in a 1 mol∙L−1NaOH aqueous solution under a constant current of 1 A; (c) the PMMA/graphene is gradually separated from the Pt substrate driven by the H2bubbles produced at the cathode after applying a constant current. These complicated and skilled transfer processes, either chemical etching or electrochemical bubbling, face up with some problems, e.g., high cost, residual contamination, surface wrinkles and breakage of graphene samples, greatly compromising the material performance86,99.

Fig.5 Schematic illustration of the graphene transfer process by electrochemical bubbling method.
(A) A Pt foil with grown graphene covered by a PMMA layer, (B) The PMMA/graphene/Pt in (A) is used as a cathode and a Pt foil is used as an anode, (C) The PMMA/graphene is gradually separated from the Pt substrate driven by the H2bubbles produced at the cathode after applying a constant current, (D) The completely separated PMMA/graphene layer and Pt foil after bubbling for tens of seconds. The PMMA/graphene layer is denoted by a red arrow in (C) and (B). (A–D) are reprinted with permission from Ref.98, Copyright 2012 Nature Publishing group.
Nowadays, the advanced strategies for direct CVD graphene growth on target semiconductors and dielectrics for transfer-free fabrication of electrodes are investigated and developed. Chiu100have grown graphene on silicon oxides (SiO2) by remote catalyzation using floating Cu and H atoms for the decomposition of hydrocarbons in the CVD process, rather than using Cu foils as substrates (Fig.6A). The defect density of the resulting graphene layers can be significantly reduced by tuning growth parameters such as the gas ratios, Cu surface areas, and substrate-to-Cu distance. Wee101have developed a plasma-enhanced CVD (PECVD) growth method for high-quality graphene at low temperature, in which a H2plasma is introduced to etch graphene from the edges and C2H4or CH4is used as the carbon source during graphene growth (Fig.6B). As illustrated in Fig.6(C–F), graphene nanoclusters are used as the growth seeds and hexagonal graphene crystals (HGCs) are produced on SiO2/Si. Apart from growth of graphene on conventional silicon-based substrates, Zhang102have realized that epitaxial growth of graphene on hexagonal boron nitride (h-BN) at a low temperature (~500°C) through a remote plasma-enhanced CVD (R-PECVD) process (Fig.6G). These novel methods can directly produce high-quality graphene electrodes but the condition is too strict and complicated to be generally applied upon various and normal martials as substrates, which deserves ongoing efforts for further exploration.
3.3 Electrophoretic and electrodeposition methods
The electrophoretic deposition (EPD) is a versatile processing technique applied to deposit graphene with controllable thickness and homogeneous structure on a wide range of substrates103. Generally, an anode and a cathode are vertically oriented in the stable and uniform suspension containing the charged particles or precursors for synthesis of intended electrodes. Upon applying a potential or current, target materials can be driven to migrate and deposit on the substrates by an electric filed between two electrodes.As displayed in Fig.7A, Ruoff104have applied a typical EPD process to anchor overlapped and stacked RGO on the mesh stainless steel and various other electrically conductive substrates. When a direct current (DC) voltage is applied, the GO platelets migrate toward the positive electrode with the oxygen functional groups significantly removed to obtain deposited RGO on the substrate. In addition to preparing the individual RGO electrodes, the EPD method can also be used to synthesize graphene-based composites on substrates. Zhao105have developed a facile EPD route to obtain the Fe3O4/carbon nanotubes (CNTs)/RGO composite electrode, simultaneously achieving material synthesis and electrode assembling (Fig.7B). In a specific experiment process, the pre-prepared GO, Fe2O3nanoparticles, CNTs and I2are dispersed in acetone. The deposition substrate (working electrode) and the counter electrode are Cu foil and Pt plate, respectively, which are placed 1.5 cm apart. After applying a DC voltage of 100 V for 15 s, the Fe2O3/CNTs/GO film deposits on the Cu foil. Finally, the Fe2O3/CNTs/GO is transformed into final Fe3O4/CNTs/ RGO composite electrode by heat treatment. It is noted that the success of multi-components EPD method is dependent on the same ionic charge around each component in the suspension. Herein, I2reacts with acetone to create protons which adsorb onto the surface of the suspended particles to make them all positively charged.
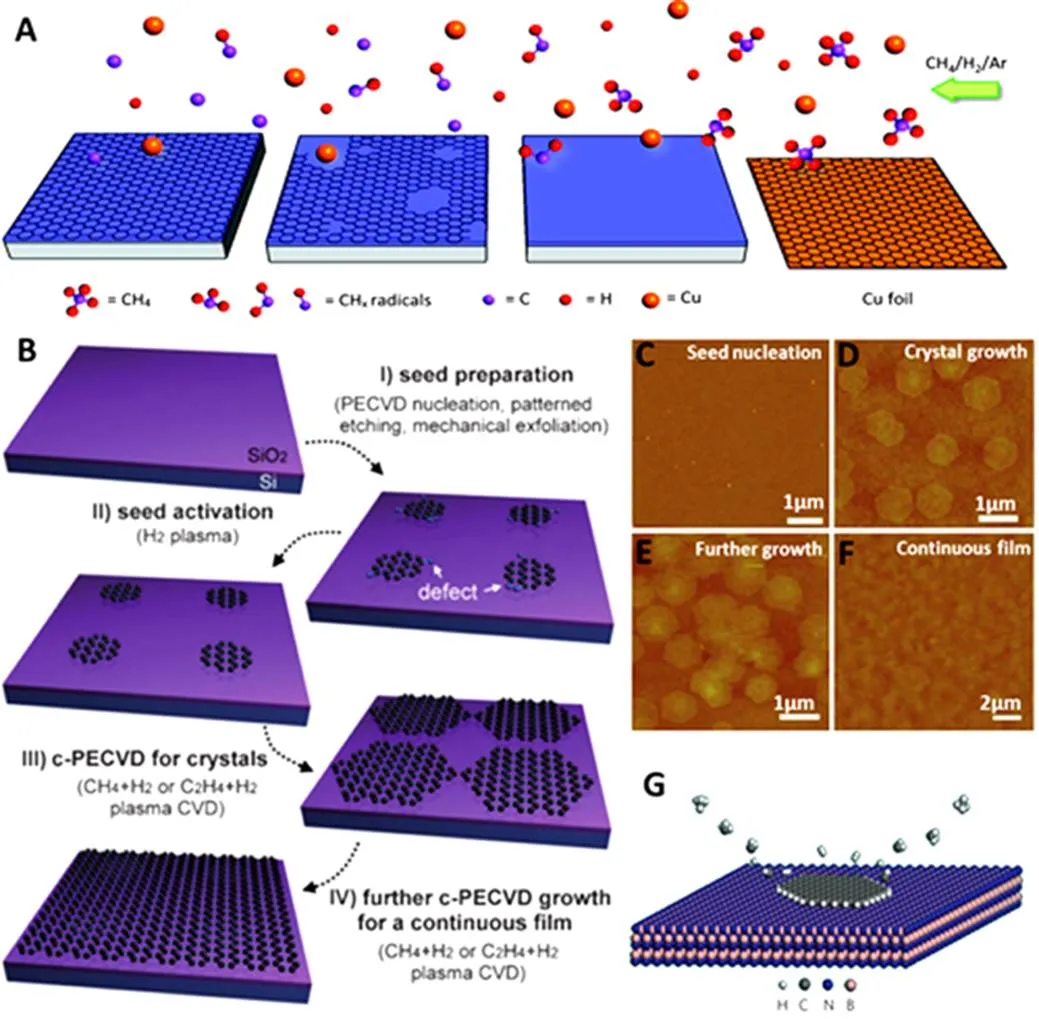
Fig.6 (A) Schematic illustration of graphene growth mechanism involving decomposition of CH4 by floating Cu and H, sublimation of Cu particles from the Cu foil at 1000 °C, and growth of graphene on SiO2 substrates after obtaining a certain distance from the Cu foil, (B) Schematic illustration of the PECVD procedure, (C) AFM image of the graphitic clusters after nucleation at 650 °C. AFM images of the HGCs on SiO2 /Si after PECVD (CH4 + H2) growth at 600 °C for(D) 90 min and (E) for 120 min, (F) AFM image of a graphene membrane on SiO2/Si, (G) Schematic illustration of the growth mechanism on h-BN.
(A) is reprinted with permission from Ref.100, Copyright 2012 American Chemical Society. (B–F) are reprinted with permission from Ref.101, Copyright 2013 John Wiley & Sons, Inc. (G) is reprinted with permission from Ref.102, Copyright 2013 Nature Publishing group.

Fig.7 (A) Schematic illustration of the EPD process and cross-sectional SEM image of RGO film, (B) Schematic illustration of fabrication process for the Fe3O4/CNTs/RGO composite electrode.
(A) is modified with permission from Ref.104, Copyright 2010 American Chemical Society. (B) is reprinted with permission from Ref.105, Copyright 2016 American Chemical Society.
The EPD method is generally applied with high potential which in turn could influence the stability of the electrodes during processing. Applied with much lower potential than EPD method, the electrodeposition method conducts electrochemical reduction-induced formation of target materials on various substrates, in which cyclic voltammetry method is usually employed for preparation of graphene or graphene-based composite electrode106. Cao107have performed cyclic voltammetric reduction to obtain a thin and merged graphene film on TiO2nanotube arrays (TNAs) (Fig.8A). Specifically, a three-electrode system is immersed into the GO dispersion. The working electrode is annealed TNAs/Ti, the counter electrode is Pt sheet, and the reference electrode is saturated calomel electrode (SCE). The scan is from –1.5 to 1 V at a rate of 50 mV·s−1and operates with 20 cycles. The cycle numbers have a tunable effect on the surface coverage of graphene, with increased cycle numbers obtaining larger surface coverage108. Not only that GO in solution can be direct electrochemically reduced to yield graphene on an electrode surface by cyclic voltammetry, one-step coelectrodeposition of graphene-metal-composite films can also be obtained when both GO- and metal-reduction reactions can occur under cathodic conditions. Luo109have synthesized graphene-Au composite on a glassy carbon electrode (GCE), which exhibits layered nanostructures consisting of alternating layers of Au nanoparticles (NPs) and graphene sheets, as illustrated in Fig.8B. The cyclic voltammetric reduction is performed in the deposition solutions containing 1.0 mol·L−1GO and 100 µmol·L−1tetrachloroauric acid (HAuCl4) with bubbling N2and using a three-electrode system: a GCE as the working electrode, Pt foil as the counter electrode, and an SCE as the reference electrode. The scan is performed between −1.5 and 0.6 V at a rate of 25 mV∙s−1. The metal NPs intercalation between graphene sheets not only prevents graphene agglomeration but also improves the conductivity of the graphene film.

Fig.8 (A) SEM image of graphene/TNAs, (B) Cross-sectional SEM image of graphene-Au composite.
(A) is reprinted with permission from Ref.107, Copyright 2016 Elsevier. (B) is reprinted with permission from Ref.109, Copyright 2011 John Wiley & Sons, Inc.
4 Fundamental roles of graphene-based composite electrodes
4.1 Acting as dark cathodes
The graphene-metal composite is usually used as a dark cathode without demand of optical excitation while n-type semiconductor is applied as a photoanode to form a photoelectrochemical setup19,110,111. As a typical example, Cheng77,112have conducted Pt-modified reduced graphene oxide (Pt-RGO) as cathode electrocatalyst and Pt-modified TiO2nanotubes (Pt-TNTs) as anode photocatalyst to establish a novel photoelectrochemical cell for converting CO2into C2H5OH, CH3COOH, etc. (Fig.9A). The Pt-RGO composite is prepared by hydrothermal reaction with GO and H2PtCl6·6H2O as precursors and then the obtained catalyst is applied onto a nickel foam. The Pt nanoparticles with a uniform size are both homogeneously dispersed on the surface of RGO and the wall of TNT, as revealed in Fig.9(B, C), respectively. In this research, the performance of photoelectrochemical reactor in the absence of CO2is explored and hydrogen is found to be the only product, indicating that graphene cannot produce carbonaceous products mixing with actually reduced products from CO2. The highest carbon atom conversion rate reaches 1130 nmol·h−1·cm−2with Pt-RGO catalyst, which is 6-fold and 3-fold higher than that with Pt-CNT and Pt-C catalyst, respectively (Fig.9D). A combined acid and alcohol generation rate of 600 nmol·h−1·cm−2is obtained with the Pt-RGO catalyst, which is significantly higher than those with Pt-CNT [82 nmol·h−1·cm−2] and Pt-C [220 nmol·h−1·cm−2] (Fig.9E). The outstanding catalytic activity of Pt-RGO is ascribed to the fact that RGO possesses high reactant absorptivity and efficient charge transportation. On the other hand, it is noted that, as reflected in Fig.9F, the selectivity of single-carbon products (e.g., CH3OH, HCOOH) for CO2reduction by Pt-RGO is still low and needs improvement in future studies.
In a subsequent work of Cheng, they have further investigated to optimize CO2reduction conditions to increase carbon atom conversion, using the same Pt-RGO||Pt-TNT photoelectrochemical cell113. A maximum carbon atom conversion rate of 1500 nmol·h−1·cm−2is obtained by Pt-RGO reduction for 24 h when a 2 V voltage is applied, the catholyte pH is 8.8, and nickel foam with an average pore size of 160 μm is used as a support. Under optimum conditions, the liquid product selectivity of CO2reduction can reach up to 99%. And the major products of CO2reduction are liquid because the high specific surface area of RGO provides plenty of adsorption sites for the reactants and intermediates to favor consecutive reduction to higher-order products, such as acetic acid and ethanol. Therefore, RGO-based catalysts have potential utilization as blueprints for photoelectrochemical CO2reduction.
4.2 Acting as photocathode
Nanostructured hybrid assemblies that combine-type inorganic semiconductors with high-conductive large surface area materials such as graphene acting as photocathode exhibit multiple favorable synergistic properties for intended applications, one of which is photoelectrochemical CO2reduction. Furthermore, the metal-free CO2reduction system is low cost, easy accessibility and environmental friendliness, compared with the systems applying rare-earth metals such as Cu, Au, Re and Rh, as well as their complexes to reduce CO2molecules. Nam78have developed nitrogen-doped graphene quantum sheets (N-GQSs) on-type silicon nanowire as heterogeneous photocathode for selective CO production in acetonitrile. The N-GQSs are synthesized by hydrothermal method and then transferred to-type Si by drop casting. The current density of the N-GQSs on-type Si nanowire increases gradually from the onset potential of −1.53 VAg/Ag+and reaches 7 mA·cm−2at −2.5 VAg/Ag+, which is approximately 5-fold higher than that of-type Si nanowire without N-GQSs (Fig.10A). Based on the fact that approximately 75% of the electrons can be consumed for reducing CO2into CO on bare planar-type Si electrodes, as revealed in Fig.10B, the chemical selectivity for CO is dramatically enhanced by 15%decorating N-GQSs on planar-type Si. And when the Si substrate is fabricated into nanowires, CO is more exclusively evolved with a selectivity of up to 95%. An isotope tracing experiment using13CO2gas is conducted to verify the source of the evolved CO from13CO2gas and not from the reduction of carbon residue or the decomposition of N-GQSs. Therefore, the N-GQSs can enhance the efficiency for reduction of CO2to CO, which evolves very stably on-type Si nanowire with N-GQSs in wide ranges of the applied potential once the CO2molecule is activated.

Fig.9 (A) Schematic illustration of the photoelectrochemical system, (B) TEM image of Pt-RGO, (C) SEM image of Pt-TNT, (D) Carbon atom conversion rate of CO2 reduction under varying cathode catalysts, (E)Chemical generation rates and (F) current efficiency under varying cathodes.
(A–F) are reprinted with permission from Ref.77, Copyright 2014 American Chemical Society.

Fig.10 (A) TEM image of N-GQSs on monolayer graphene, (B) Photocurrent density-potential (J-E) curves, (C) Band alignment of p-type Si and calculated band edge positions of different sized GQS, (D) Proposed photoelectrocatalytic cycle of CO2 to CO reduction on pyridinic N doped coronene (C24H12).
(A−D) are reprinted with permission from Ref.78, Copyright 2016 John Wiley & Sons, Inc.
Based on the density functional theory (DFT) calculations, the calculated band edge positions of various sizes of GQS ranging in diameter from 0.96 nm (C24H12) to 3.4 nm (C294H42) with respect to the experimental conduction band of-type Si are shown in Fig.10C. The position of the conduction band minimum of GQS decreases as the diameter is increased until ~3 nm and is eventually located more negative than that of-type Si when the diameter becomes larger than 3 nm. Therefore, the photoexcited electrons in-type Si can be transferred to the GQS and participate in the catalytic reduction of CO2when the diameter is larger than 3 nm. The N dopants is also turned out to have minor effect on the band edge positions and serve as an active center for CO2reduction. Moreover, based on result of DFT calculations, the overall photoelectro- catalytic reduction mechanism of CO2to CO is summarized in Fig.10D. The specific steps are (1) the first N-GQS reduction, (2) adsorption of CO2to the pyridinic N site, (3) the first protonation, (4) the second N-GQS reduction, and (5) the second protonation to yield CO and H2O.
4.3 Acting as photoanodes
Subramanian114have prepared the 3D tree-like architectures as candidates of photoanodes for photoelectro- chemical performance. As visually displayed in Fig.11A, a nature-inspired “tree”-like 3D hierarchical branch titania (bTiO2) architecture strategically assembles reduced graphene oxide (RGO) and cadmium sulfide (CdS). An optimum loading (1 mg·mL−1) of RGO boosts the photoresponse by an additional 150% compared to “CdS-only” photoanodes (Fig.11B), which is ascribed to the effective electron shuttling capability of the RGO interlayer. The on/off cycles (Fig.11C) and the/data (Fig.11D) synergistically manifest that the presence of RGO significantly enhances the photoelectrochemical performance of the 3D bTiO2/CdS. Therefore, successful integration of RGO with other materials can facilitate more efficient charge separation leading to further increase in the photoelectro- chemical performance.

Fig.11 (A) Schematic illustration of strategic integration of the RGO with CdS over 3D TiO2 architectures, (B) Comparative values of the stabilized peak current obtained in the photoelectrochemical measurements of panel with CdS (140% increment), RGO/CdS (150% increment) and combined effect of RGO and CdS (500% increment), Photoelectrochemical responses showing (C) the multiple “On-Off” cycles and (D) J-V characteristics (rTiO2 represents TiO2 nanorod).
(A−D) are reprinted with permission from Ref.114, Copyright 2015 American Chemical Society.
Additionally, graphene can also be employed as a scaffold to create a 2D conductive support path for charge transport at the electrode surface115. Kamat116have conducted fortification of CdSe quantum dots (QDs) with graphene oxide (GO) as photoanode in an open cell configuration, consisting of Cu2S/RGO films as counter electrode and S2−/S2−as the redox electrolyte (Fig.12A). Submonolayer loading of CdSe QDs on typically mono- to few-layered graphene sheets is shown in Fig.12C. Both RGO and GO serve as effective quenchers of excited CdSe (Fig.12D). And GO has greater electron- accepting capacity because GO quenches more efficiently than RGO at all concentrations (inset, Fig.12D).The significant enhancement of CdSe-(R)GO photoanodes over CdSe-only film for the incident photon to current efficiencies (IPCE) is attributed to the indirect participation of (R)GO in accepting and shuttling electrons to the electrode surface (Fig.12E). Therefore, it is schematically illustrated in Fig.12B that the random walks of electrons in CdSe QDs become sequential flow along graphene after incorporation of graphene in CdSe QDs loading. There is a new avenue opened toward the application of graphene as a scaffold to improve the charge transfer.
Inspired by aforementioned research, these superiorities of graphene can be utilized to advance photoanodes for photo- electrochemical CO2reduction. The graphene on anodic electrode directly influences the efficiency of water decomposition provided protons and electrons for cathode CO2reduction, which, in turn, affects the catalytic efficiency of anodization procedure. Therefore, although the reduction of CO2takes place in cathodic chamber, its efficiency is still indirectly affected by the cathodic reaction. Mat117have constructed copper-doped, titanium dioxide-reduced graphene oxide (Cu-RGO-TiO2) nanocomposite photoanode for photoelectrocatalytic reduction of CO2to methanol and formic acid, with a platinum (Pt) wire and a saturated calomel electrode (SCE) as the counter and reference electrode, respectively (Fig.13A). The advantages of graphene for facilitating electrons transfer and electron-hole pairs separation are indicated by the lowest emission intensity of the Cu-RGO-TiO2in the photoluminescence spectrum (Fig.13B) and lowest resistance of the Cu-RGO-TiO2in the Nyquist plots (Fig.13C) than these of RGO-TiO2and TiO2.
The above works have proven the distinct superiorities and great potential of graphene applied in photoelectrochemical CO2reduction. When graphene-based composites act as cathode, the 2D-conjugated structure and large surface area of graphene mainly provide more basic sites and thus facilitate CO2adsorption. Furthermore, the graphene mainly plays the role of electron acceptor and transporter when graphene-based composite is adopted as anode to indirectly improve the efficiency of photoelectrochemical CO2reduction. So far, there are no researches on the graphene-based composites as dark anodes combining with-type semiconductor as photocathodes. However, many researches on the graphene- based electrodes (e.g., Co3O4-graphene118, Fe3O4-graphene119) for electrochemical oxygen evolution reaction (OER) of water have been reported, indicating that graphene-based composites have promising potential of acting as dark anodes with proper photocathodes in photoelectrochemical system.

Fig.12 Schematic illustrations of (A) an open cell configuration in which CdSe QDs with graphene oxide as photoanode and (B) electron transfer pathways, (C) TEM micrograph of GO after illumination (> 420 nm) of CdSe-GO dispersion, (D) Quenching of CdSe QDs photoluminescence by GO and RGO (the inset is effective quenching even at low graphene concentration), (E) Incident photon to current efficiency (IPCE) of CdSe and CdSe-(R)GO film.
(A−E) are reprinted with permission from Ref.116, Copyright 2012 American Chemical Society.
5 Potential research categories for photoelectrochemical applications
Photoelectrochemical catalysis, synergistically integrated electrochemical and photocatalytic methods, possesses two advantages:120(i) the application of certain electrode potential not only drives specific electrode reaction but also facilitates the separation of photoinduced carriers, enhancing photocatalytic process; (ii) the assistance of light irradiation lowers the electrochemical barrier and promotes the electrode kinetics for a specific reaction, favoring the electrochemical process. Therefore, with regard to the superiorities of photoelectrochemical system, we discuss the strategies about evolution of potential photocatalytic and electrocatalytic system into photoelectrochemical applications for CO2reduction on the basis of their common characteristics, which are not limited to only graphene-based composite electrode and able to extend researches on photoelectrochemical CO2reduction system.
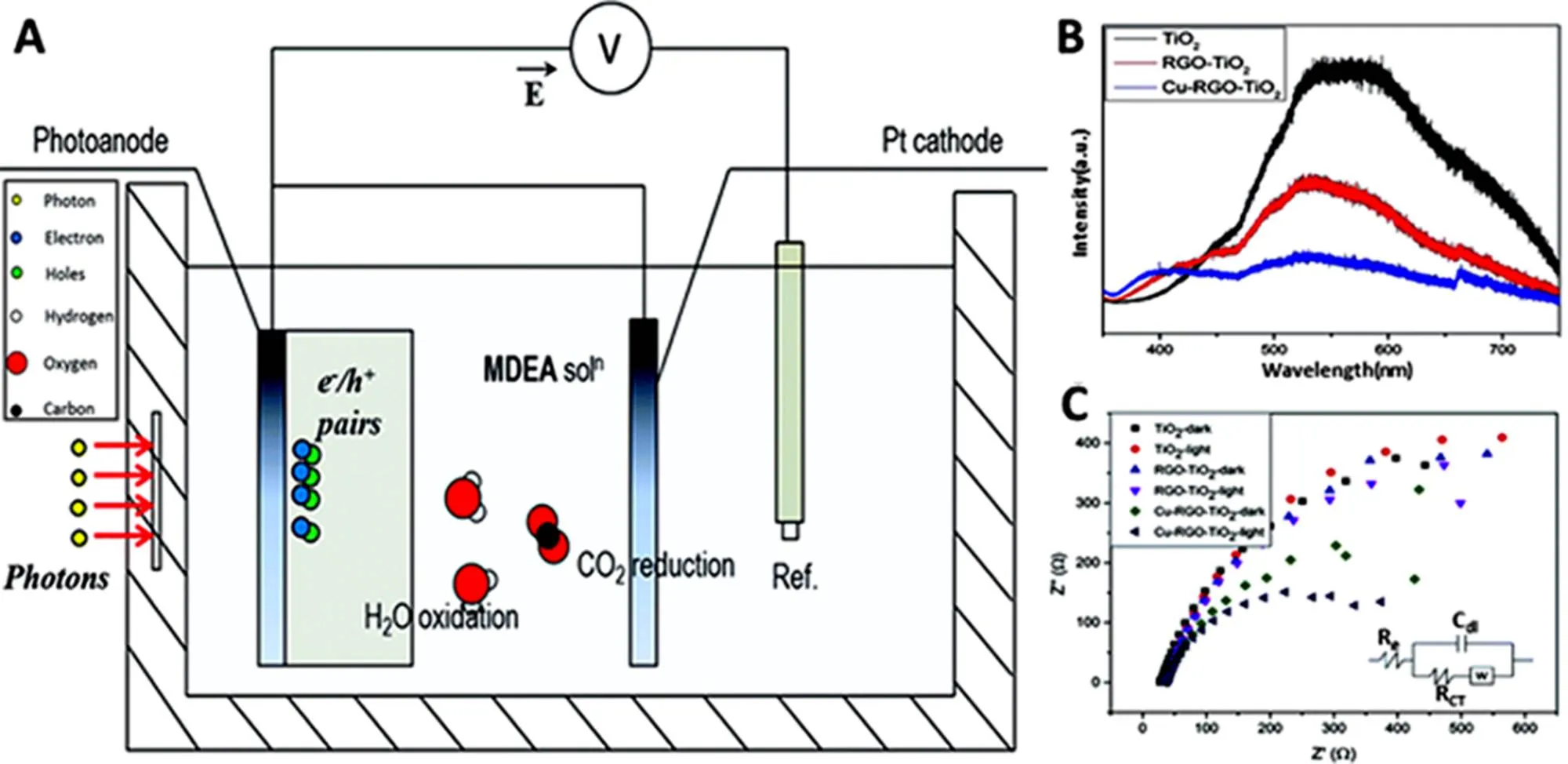
Fig.13 (A) Schematic illustration of photoelectrochemical reactor, (B) Photoluminescence spectrum of TiO2, RGO-TiO2and Cu-RGO-TiO2,
(C) Nyquist plots of the TiO2, RGO-TiO2and Cu-RGO-TiO2film electrodes at open circuit potentials both in the dark and under visible irradiation (the inset is the equivalent circuit).
(A−C) are reprinted with permission from Ref.117, Copyright 2015 Royal Society of Chemistry.
5.1 Potential Z-scheme photocatalytic systems
-scheme photocatalysis system mimicking plant photosynthesis has become a novel artificial two-step photosynthetic system, whose processes occur simultaneously in time but separately in space121,122. The-scheme system containing-type and-type semiconductors is an indispensable factor for evolution of photoelectrochemical system, possessing a similar electron flow. Specifically, after both semiconductors are stimulated by light, the photogenerated electrons in an O2-evolving side are migrated to combine with the holes in a CO2-reducting side, remaining the holes in the O2-evolving side and electrons in the CO2-reducting side to participate in following reaction123. The distinction between the two systems is the way of electrons migration. In a-scheme system, it can happen at contact interface of the two semiconductors or electron mediators (e.g., graphene124,125) between the two semiconductors while in a photoelectrochemical system, the electrons are commonly migrated through external wire connecting the two semiconductors126,127. Therefore, properly changing the way of electrons migration is feasible for the-scheme photocatalysis system to be evolved as a newly designed photoelectrochemical setup, which can be categorized as type III discussed in Section 2.2.

Fig.14 Schematic illustrations of (A) Z-scheme system for CO2 reduction and H2O oxidation, and (B) the photoelectrochemical reduction of CO2 with a two-electrode configuration and no electrical bias.
(A, B) are reprinted with permission from Ref.128, Copyright 2013 Royal Society of Chemistry.
Araihave investigated the-scheme system to be tuned as photoelectrochemical system128. The total reaction mechanism of the-scheme system is shown in Fig.14A, including the SCRED/[MCP] (Metal Complex Polymer) photocatalyst for CO2reduction and the SCOXphotocatalyst for H2O oxidation (SCREDand SCOXdenote semiconductors at the sites of the reduction and oxidation,respectively)129. Based on the-scheme photocatalysis system, herein, a wired two-compartment quartz cell is conducted for photoelectrochemical reduction of CO2into formate, combining the reduced SrTiO3(r-STO) photoanode with the InP/[RuCP] (Ruthenium Complex Polymer) photocathode (Fig.14B)78. After 3-hour irradiation of simulated solar lights, the obtained conversion efficiency from solar energy to formate is ca. 0.14%, indicating the photoelectrochemical system transferred from Z-scheme system can well work and realize the reduction of CO2. Kudo130have fabricated a-scheme system in which a visible-light-driven CoO-loaded BiVO4as an O2-evolving photocatalyst, metal sulfide as a H2-evolving and CO2-reducing photocatalyst, and RGO as an efficient electron mediator (Fig.15A). On the basis of this-scheme system and Pt/CuGaS2and CoO/BiVO4as-type and-type semiconductors, Pt/CuGaS2and CoO/BiVO4are respectively applied as cathodic and anodic photoelectrodes to form a photoelectrochemical system. The photogenerated electrons flow from the conduction band of BiVO4to external wire and finally to the valence band of CuGaS2after light irradiation. Then, the flowing electrons are combined with the holes generated form CuGaS2and the paired electrons are motivated to the conduction band of CuGaS2to participate in following reaction (Fig.15B). Because Pt-cocatalyst works as active sites for photocatalytic H2generation, the photoelectrochemical system primarily works for H2evolution without an external bias.
These results have indicated that the-scheme system containing-type and-type semiconductors is feasible and potential to be conducted as a newly-designed photoelectrochemical system. The photocatalytic system commonly utilizes a suspension of photocatalyst particles in a solvent for the reduction of dissolved CO249. In this regard, the technological barrier for this kind of transformation is to make the dispersed particle anchor on conducted electrode. The physical coating methods (i.e., dip-coating, drop-casting and spin-coating) discussed in Section 3.1 may be the most convenient and efficient approaches and still deserve further exploration.
5.2 Potential electrocatalytic systems
The electrochemical CO2reduction has triggered a large number of researches for three decades. There are three typical cells used for electrochemical CO2conversions in laboratory, which are schematically summarized in Fig.16131. Particularly, the setup in Fig.16B is similar to the configuration of photoelectrochemical CO2reduction, with anodic and cathodic chambers separated by an H+-conducting membrane. Besides, for photoelectrocatalytic applications, an artificial lamp source or a solar simulator111is installed outside of the reactor and the light passes through the quartz windowembedded in the wall. There is an exception that a UV lamp can be located in internal configuration132.
It is typical for both electrochemical and photoelectro- chemical reactors that the separated non-direct reactions in two compartments permit researchers to tailor the properties needed for each redox process independently. Furthermore, considering the similarity of their setups, electrocatalytic reactor is able to be combined with half-cell photoelectrochemical reactor in separated compartments. Centi52have successfully conducted a continuous flow electrocatalytic reactor simulating the anode part of the photoelectrochemical reactor. The Pt nanoparticles on carbon-based electrodes are used to electrochemical reduction integrating TiO2photoanode to ultimately use solar energy and water to convert CO2to long carbon-chain hydrocarbons at room temperature and atmospheric pressure.
Using the conventional liquid phase electrochemical approach, many problems exist including the solubility of CO2, the formation of products type, etc.19. To overcome these problems, gas phase electrocatalytic reduction of CO2is applied with a gas diffusion electrode (GDE), as displayed in Fig.16C.Interestingly, the anodic chamber filled with electrolyte is able to simulate the half-cell of the photoelectrochemical device. Perathoner133have proposed a novel concept based on a gas-phase photoelectrochemical device. The electrocatalyst is the Pt supported on carbon nanotubes, which is then deposited on a conductive carbon cloth to allow the electrical contact and the diffusion of gaseous CO2. Photocatalytic anode is composed of a nanostructured TiO2-based thin film, where H2O is splitted to produce O2and protons. The CO2in gas phase is reduced by protons passing through a Nafion membrane from the photocatalytic side and electrons from the wire. This device, which is different from the conventional liquid phase electrochemical and photoelectrochemical approach, is able to form long-chain hydrocarbons at room temperature. Therefore, depending on their distinguishing features for dual-chamber, it is feasible to take advantage of half-cell photoelectrochemical application combined with electrochemical setup to obtain a desirable and further tunable performance for photoelectrochemical CO2reduction, which is not limited to only graphene-based composite materials.

Fig.15 Schematic illustrations of (A) Z-scheme system for water splitting and CO2 reduction and (B) photoelectrochemical system consisting of a metal sulfide photocatalyst electrode with a p-type semiconductor character and a CoOx/BiVO4 photoelectrode.
(A, B) are reprinted with permission from Ref.130, Copyright 2016 American Chemical Society.

Fig.16 Laboratory cells used for electrochemical CO2 conversion: (A) two-compartment cell, (B) cell with electrodes separated by an H+-conducting membrane, and (C) cell with a gas diffusion electrode.
(A−C) are reprinted with permission from Ref.131, Copyright 2013 Royal Society of Chemistry.
6 Conclusions and perspectives
In this review, we elaborate the basic principle and highlight the recent progress in developing efficient graphene-based composites for photoelectrochemical CO2reduction. The basic preparation methods for graphene or graphene-based composite (photo)electrodes are summarized. The role of graphene can be excellent CO2absorber and electrons transfer media, which could significantly improve efficiency of the relevant (photo)anode or (photo)cathode. We have also demonstrated the potential photocatalytic and electrochemical systems that could be evolved to photoelectrochemical application for CO2reduction, which will further advance our graphene-based composite systems toward improving the efficiency of photoelectrochemical reduction of CO2. After all the researches in this field are at an early stage, there exist a number of challenges in the exploitation of highly activity graphene-based composites for photoelectrochemical CO2reduction.
To further develop the application of graphene-based composite electrodes in photoelectrochemical reduction of CO2, ongoing efforts could be devoted to the following aspects. (i) More explorations are needed to achieve more comprehensive understanding of the rationales of photoelectro- chemical CO2reduction over graphene and thus to better control the pathways of CO2reduction and utilize the traits of graphene more felicitously. (ii) Besides performing as CO2absorber and conductive media, graphene possesses other excellent properties widely acknowledged in photocatalytic filed, such as band gap tuning and the macromolecular photosensitizer role. To make full use of its advantages to tune photoelectrochemical CO2reduction, further investigations are imperative on the basis of achievements about fundamental roles of graphene in the graphene-based composite photocatalysts. (iii) To fabricate higher-performance graphene- based composite electrodes, a system-level engineering needs to be optimized, including controlling morphologies and structures at the nanoscale, tailoring the interface and interactions among graphene and targeted materials. Regarding the overall photoelectrochemical performance, it is essential to achieve the harmonious combination of each component to maximally optimize the functional electrodes. (iv) The existing preparation methods of graphene-based composite electrodes deserve improvement to optimize the thickness, homogeneity and stability of the materials and thus to tune the performance of photoelectrochemical CO2reduction. (v) The tailored graphene materials, such as zero-dimensional graphene quantum dots, one-dimensional graphene nanoribbons and three-dimensional graphene frameworks show a variety of fascinating features, thereby offering a fertile and flexible ground for the further development of graphene-based photoelectrochemical catalysis for CO2reduction.
With regard to the implementation of potential photocatalysts and electrocatalysts into newly-designed photoelectrochemical applications, the equilibrium between enhanced efficiency and the cost is ought to be concerned because the complicacy and costing of photoelectrochemical applications are higher than those of sole photocatalytic and electrocatalytic ones. Being cost-effective is a current key issue to further optimize the stable, efficient, safe photoelectro- chemical system. Taking advantage of the nature solar light instead of artificial lamp sources enables the energy saving, cost reduction and environmentally friendly. Therefore, a solar-driven photoelectrochemical-based CO2reduction device is a promising development trend toward a sustainable system in the future.
(1) Luthi, D.; Le Floch, M.; Bereiter, B.; Blunier, T.; Barnola, J. M.; Siegenthaler, U.; Raynaud, D.; Jouzel, J.; Fischer, H.; Kawamura, K.; Stocker, T. F.2008,, 379. doi: 10.1038/nature06949
(2) Canadell, J. G.; Le Quere, C.; Raupach, M. R.; Field, C. B.; Buitenhuis, E. T.; Ciais, P.; Conway, T. J.; Gillett, N. P.; Houghton, R. A.; Marland, G.2007,, 18866. doi: 10.1073/pnas.0702737104
(3) Somorjai, G. A.; Frei, H.; Park, J. Y.2009,, 16589. doi: 10.1021/ja9061954
(4) Aresta, M.; Dibenedetto, A.2007, 2975. doi: 10.1039/B700658F
(5) Centi, G.; Perathoner, S.2009,, 191. doi: 10.1016/j.cattod.2009.07.075
(6) Jiang, Z.; Xiao, T.; Kuznetsov, V. L.; Edwards, P. P.2010,, 3343. doi: 10.1098/rsta.2010.0119
(7) Grills, D. C.; Fujita, E.2010,, 2709. doi: 10.1021/jz1010237
(8) Bolton, J. R.1978,, 705. doi: 10.1126/science.202.4369.705
(9) Ikeue, K.; Yamashita, H.; Anpo, M.; Takewaki, T.2001,, 8350. doi: 10.1021/jp010885g
(10) Varghese, O. K.; Paulose, M.; LaTempa, T. J.; Grimes, C. A.2009,, 731. doi: 10.1021/nl803258p
(11) Dimitrijevic, N. M.; Vijayan, B. K.; Poluektov, O. G.; Rajh, T.; Gray, K. A.; He, H.; Zapol, P.2011,, 3964. doi: 10.1021/ja108791u
(12) Liu, Q.; Zhou, Y.; Kou, J.; Chen, X.; Tian, Z.; Gao, J.; Yan, S.; Zou, Z.2010,, 14385. doi: 10.1021/ja1068596
(13) Izumi, Y.2013,, 171. doi: 10.1016/j.ccr.2012.04.018
(14) Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z.2012,, 3420. doi: 10.1002/anie.201108357
(15) Roy, S. C.; Varghese, O. K.; Paulose, M.; Grimes, C. A.2010,, 1259. doi: 10.1021/nn9015423
(16) Benson, E. E.; Kubiak, C. P.; Sathrum, A. J.; Smieja, J. M.2009,, 89. doi: 10.1039/B804323J
(17) Spinner, N. S.; Vega, J. A.; Mustain, W. E.2012,, 19. doi: 10.1039/C1CY00314C
(18) Li, C. W.; Kanan, M. W.2012,, 7231. doi: 10.1021/ja3010978
(19) Ampelli, C.; Centi, G.; Passalacqua, R.; Perathoner, S.2010,, 292. doi: 10.1039/B925470F
(20) Bard, A. J.1980,, 139. doi: 10.1126/science.207.4427.139
(21) Sato, S.; Arai, T.; Morikawa, T.2015,, 5105. doi: 10.1021/ic502766g
(22) Halmann, M.1978,, 115. doi: 10.1038/275115a0
(23) Sato, S. Photoelectrochemical CO2Reduction. In; Springer New York: 2014; pp 1535.
(24) Li, D.; Kaner, R. B.2008,, 1170. doi: 10.1126/science.1158180
(25) Li, Y.; Su, H.; Chan, S. H.; Sun, Q.2015,, 6658. doi: 10.1021/acscatal.5b01165
(26) Yang, M. Q.; Xu, Y. J.2016,, 185. doi: 10.1039/C5NH00113G
(27) Zhu, D. D.; Liu, J. L.; Qiao, S. Z.2016,, 3423. doi: 10.1002/adma.201504766
(28) Xiao, F. X.; Miao, J.; Liu, B.2014,, 1559. doi: 10.1021/ja411651e
(29) Liu, Q.; Liu, Z.; Zhang, X.; Yang, L.; Zhang, N.; Pan, G.; Yin, S.; Chen, Y.; Wei, J.2009,, 894. doi: 10.1002/adfm.200800954
(30) Zhai, C.; Zhu, M.; Lu, Y.; Ren, F.; Wang, C.; Du, Y.; Yang, P.2014,, 14800. doi: 10.1039/C4CP01401D
(31) Chang, H.; Lv, X.; Zhang, H.; Li, J.2010,, 483. doi: 10.1016/j.elecom.2010.01.025
(32) Xiang, Q.; Cheng, B.; Yu, J.2015,, 11350. doi: 10.1002/anie.201411096
(33) Tran, P. D.; Wong, L. H.; Barber, J.; Loo, J. S. C.2012,, 5902. doi: 10.1039/C2EE02849B
(34) Habisreutinger, S. N.; Schmidt-Mende, L.; Stolarczyk, J. K.2013,, 7372. doi: 10.1002/anie.201207199
(35) Li, H.; Gan, S.; Wang, H.; Han, D.; Niu, L.2015,, 6906. doi: 10.1002/adma.201502755
(36) Sato, S.; Arai, T.; Morikawa, T.; Uemura, K.; Suzuki, T. M.; Tanaka, H.; Kajino, T.2011,, 15240. doi: 10.1021/ja204881d
(37) Magesh, G.; Kim, E. S.; Kang, H. J.; Banu, M.; Kim, J. Y.; Kim, J. H.; Lee, J. S.2014,, 2044. doi: 10.1039/C3TA14408A
(38) Chen, W. Y.; Mattern, D. L.; Okinedo, E.; Senter, J. C.; Mattei, A. A.; Redwine, C. W.2014,, 1054. doi: 10.1002/aic.14347
(39) Xie, S.; Zhang, Q.; Liu, G.; Wang, Y.2016,, 35. doi: 10.1039/C5CC07613G
(40) Tinnemans, A.; Koster, T.; Thewissen, D.; Mackor, A.1984,, 288. doi: 10.1002/recl.19841031004
(41) Zhao, W. W.; Xiong, M.; Li, X. R.; Xu, J. J.; Chen, H. Y.2014,, 40. doi: 10.1016/j.elecom.2013.10.035
(42) Li, X.; Wen, J.; Low, J.; Fang, Y.; Yu, J.2014,, 70. doi: 10.1007/s40843-014-0003-1
(43) Schouten, K. J. P.; Kwon, Y.; van der Ham, C. J. M.; Qin, Z.; Koper, M. T. M.2011,, 1902. doi: 10.1039/C1SC00277E
(44) Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K.1979,, 637. doi: 10.1038/277637a0
(45) Anpo, M.; Yamashita, H.; Ichihashi, Y.; Ehara, S.1995,, 21. doi: 10.1016/0022-0728(95)04141-A
(46) Yang, C. C.; Vernimmen, J.; Meynen, V.; Cool, P.; Mul, G.2011,, 1. doi: 10.1016/j.jcat.2011.08.005
(47) Ulagappan, N.; Frei, H.2000,, 7834. doi: 10.1021/jp001470i
(48) Amatore, C.; Saveant, J. M.1981,, 5021. doi: 10.1021/ja00407a008
(49) Chang, X.; Wang, T.; Gong, J.2016,, 2177. doi: 10.1039/C6EE00383D
(50) Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O.1994,, 1833. doi: 10.1016/0013-4686(94)85172-7
(51) Koppenol, W. H.; Rush, J. D.1987,, 4429. doi: 10.1021/j100300a045
(52) Centi, G.; Perathoner, S.; Wine, G.; Gangeri, M.2007,, 671. doi: 10.1039/B615275A
(53) Wu, T.; Zou, L.; Han, D.; Li, F.; Zhang, Q.; Niu, L.2014,, 2142. doi: 10.1039/C3GC42454E
(54) Ong, W. J.; Tan, L. L.; Chai, S. P.; Yong, S. T.2015,, 858. doi: 10.1039/C4CC08996K
(55) Yu, J.; Jin, J.; Cheng, B.; Jaroniec, M.2014,, 3407. doi: 10.1039/C3TA14493C
(56) Stoller, M. D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R. S.2008,, 3498. doi: 10.1021/nl802558y
(57) Gattrell, M.; Gupta, N.; Co, A.2006,, 1. doi: 10.1016/j.jelechem.2006.05.013
(58) Yoneyama, H.; Sugimura, K.; Kuwabata, S.1988,, 143. doi: 10.1016/0022-0728(88)80355-3
(59) Chang, X.; Wang, T.; Zhang, P.; Wei, Y.; Zhao, J.; Gong, J.2016,, 8986. doi: 10.1002/anie.201602973
(60) Allam, N. K.; Shankar, K.; Grimes, C. A.2008,, 2341. doi: 10.1039/B718580D
(61) Luo, J.; Im, J. H.; Mayer, M. T.; Schreier, M.; Nazeeruddin, M. K.; Park, N. G.; Tilley, S. D.; Fan, H. J.; Grätzel, M.2014,, 1593. doi: 10.1126/science.1258307
(62) Minguez-Bacho, I.; Courte, M.; Fan, H. J.; Fichou, D.2015,, 185401. doi: 10.1088/0957-4484/26/18/185401
(63) Chua, L. L.; Zaumseil, J.; Chang, J. F.; Ou, E. C. W.; Ho, P. K. H.; Sirringhaus, H.; Friend, R. H.2005,, 194. doi: 10.1038/nature03376
(64) Koval, C. A.; Howard, J. N.1992,, 411. doi: 10.1021/cr00011a004
(65) Gao, Y. Q.; Georgievskii, Y.; Marcus, R. A.2000,, 3358. doi: 10.1063/1.480918
(66) White, J. L.; Baruch, M. F.; Pander Iii, J. E.; Hu, Y.; Fortmeyer, I. C.; Park, J. E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; Shaw, T. W.; Abelev, E.; Bocarsly, A. B.2015,, 12888. doi: 10.1021/acs.chemrev.5b00370
(67) Peng, Y. P.; Yeh, Y. T.; Shah, S. I.; Huang, C. P.2012,, 414. doi: 10.1016/j.apcatb.2012.04.037
(68) Chen, D.; Zhang, H.; Liu, Y.; Li, J.2013,, 1362. doi: 10.1039/c3ee23586f
(69) Lightcap, I. V.; Kamat, P. V.2013,, 2235. doi: 10.1021/ar300248f
(70) Low, J.; Yu, J.; Ho, W.2015,, 4244. doi: 10.1021/acs.jpclett.5b01610
(71) Lightcap, I. V.; Murphy, S.; Schumer, T.; Kamat, P. V.2012,, 1453. doi: 10.1021/jz3004206
(72) Zhang, N.; Yang, M. Q.; Liu, S.; Sun, Y.; Xu, Y. J.2015,, 10307. doi: 10.1021/acs.chemrev.5b00267
(73) Tu, W.; Zhou, Y.; Liu, Q.; Tian, Z.; Gao, J.; Chen, X.; Zhang, H.; Liu, J.; Zou, Z.2012,, 1215. doi: 10.1002/adfm.201102566
(74) Han, C.; Chen, Z.; Zhang, N.; Colmenares, J. C.; Xu, Y. J.2015,, 221. doi: 10.1002/adfm.201402443
(75) Gao, E.; Wang, W.; Shang, M.; Xu, J.2011,, 2887. doi: 10.1039/C0CP01749C
(76) Wang, P. Q.; Bai, Y.; Luo, P. Y.; Liu, J. Y.2013,, 82. doi: 10.1016/j.catcom.2013.04.020
(77) Cheng, J.; Zhang, M.; Wu, G.; Wang, X.; Zhou, J.; Cen, K.2014,, 7076. doi: 10.1021/es500364g
(78) Yang, K. D.; Ha, Y.; Sim, U.; An, J.; Lee, C. W.; Jin, K.; Kim, Y.; Park, J.; Hong, J. S.; Lee, J. H.; Lee, H. E.; Jeong, H. Y.; Kim, H.; Nam, K. T.2016,, 233. doi: 10.1002/adfm.201502751
(79) 79) Xiao, F. X.; Pagliaro, M.; Xu, Y. J.; Liu, B.2016, (, 3088. doi: 10.1039/C5CS00781J
(80) Li, Z.; Luo, W.; Zhang, M.; Feng, J.; Zou, Z.2013,, 347. doi: 10.1039/C2EE22618A
(81) Kecenovic, E.; Endrődi, B.; Pápa, Z.; Hernadi, K.; Rajeshwar, K.; Janaky, C.2016,, 3139. doi: 10.1039/C5TA10457B
(82) Shen, Q.; Chen, Z.; Huang, X.; Liu, M.; Zhao, G.2015,, 5828. doi: 10.1021/acs.est.5b00066
(83) Kim, K. S.; Zhao, Y.; Jang, H.; Lee, S. Y.; Kim, J. M.; Kim, K. S.; Ahn, J. H.; Kim, P.; Choi, J. Y.; Hong, B. H.2009,, 706. doi: 10.1038/nature07719
(84) Juang, Z. Y.; Wu, C. Y.; Lu, A. Y.; Su, C. Y.; Leou, K. C.; Chen, F. R.; Tsai, C. H.2010,, 3169. doi: 10.1016/j.carbon.2010.05.001
(85) Huang, X.; Qi, X.; Boey, F.; Zhang, H.2012,, 666. doi: 10.1039/C1CS15078B
(86) Xu, C.; Xu, B.; Gu, Y.; Xiong, Z.; Sun, J.; Zhao, X. S.2013,, 1388. doi: 10.1039/C3EE23870A
(87) Chen, J.; Shi, J.; Wang, X.; Cui, H.; Fu, M.2013,, 621. doi: 10.1016/S1872-2067(12)60530-0
(88) Xiang, Q.; Yu, J.; Jaroniec, M.2012,, 782. doi: 10.1039/C1CS15172J
(89) Ng, Y. H.; Iwase, A.; Kudo, A.; Amal, R.2010,, 2607. doi: 10.1021/jz100978u
(90) Sun, L.; Bai, Y.; Zhang, N.; Sun, K.2015,, 1846. doi: 10.1039/C4CC08288E
(91) Li, H.; Pang, S.; Wu, S.; Feng, X.; Müllen, K.; Bubeck, C.2011,, 9423. doi: 10.1021/ja201594k
(92) Eda, G.; Emrah Unalan, H.; Rupesinghe, N.; Amaratunga, G. A. J.; Chhowalla, M.2008,, 233502. doi: 10.1063/1.3028339
(93) Annamalai, A.; Kannan, A. G.; Lee, S. Y.; Kim, D. W.; Choi, S. H.; Jang, J. S.2015,, 19996. doi: 10.1021/acs.jpcc.5b06450
(94) Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H. M.2011,, 424. doi: 10.1038/nmat3001
(95) Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.;
Velamakanni, A.; Jung, I.; Tutuc, E.; Banerjee, S. K.; Colombo,
L.; Ruoff, R. S.2009,, 1312. doi: 10.1126/science.1171245
(96) Yu, C.; Meng, X.; Song, X.; Liang, S.; Dong, Q.; Wang, G.; Hao, C.; Yang, X.; Ma, T.; Ajayan, P. M.; Qiu, J.2016,, 474. doi: 10.1016/j.carbon.2016.01.042
(97) Shin, S.; Kim, S.; Kim, T.; Du, H.; Kim, K. S.; Cho, S.; Seo, S.2017,, 215. doi: 10.1016/j.carbon.2016.09.077
(98) Gao, L.; Ren, W.; Xu, H.; Jin, L.; Wang, Z.; Ma, T.; Ma, L. P.; Zhang, Z.; Fu, Q.; Peng, L. M.; Bao, X.; Cheng, H. M.2012,, 699. doi: 10.1038/ncomms1702
(99) Wang, H.; Yu, G.2016,, 4956. doi: 10.1002/adma.201505123
(100) Teng, P. Y.; Lu, C. C.; Akiyama-Hasegawa, K.; Lin, Y. C.; Yeh, C. H.; Suenaga, K.; Chiu, P. W.2012,, 1379. doi: 10.1021/nl204024k
(101) Wei, D.; Lu, Y.; Han, C.; Niu, T.; Chen, W.; Wee, A. T.2013,, 14121. doi: 10.1002/anie.201306086
(102) Yang, W.; Chen, G.; Shi, Z.; Liu, C. C.; Zhang, L.; Xie, G.; Cheng, M.; Wang, D.; Yang, R.; Shi, D.; Watanabe, K.; Taniguchi, T.; Yao, Y.; Zhang, Y.; Zhang, G.2013,, 792. doi: 10.1038/nmat3695
(103) Yuan, Y. P.; Ruan, L. W.; Barber, J.; Joachim Loo, S. C.; Xue, C.2014,, 3934. doi: 10.1039/C4EE02914C
(104) An, S. J.; Zhu, Y.; Lee, S. H.; Stoller, M. D.; Emilsson, T.; Park, S.; Velamakanni, A.; An, J.; Ruoff, R. S.2010,, 1259. doi: 10.1021/jz100080c
(105) Yang, Y.; Li, J.; Chen, D.; Zhao, J.2016,, 26730. doi: 10.1021/acsami.6b07990
(106) Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S.2011,, 133. doi: 10.1016/j.elecom.2010.11.033
(107) Li, F.; Zhang, L.; Tong, J.; Liu, Y.; Xu, S.; Cao, Y.; Cao, S., 320. doi: 10.1016/j.nanoen.2016.06.056
(108) Liu, C.; Teng, Y.; Liu, R.; Luo, S.; Tang, Y.; Chen, L.; Cai, Q.2011,, 5312. doi: 10.1016/j.carbon.2011.07.051
(109) Liu, C.; Wang, K.; Luo, S.; Tang, Y.; Chen, L.2011,, 1203. doi: 10.1002/smll.201002340
(110) Tang, J.; Zhang, Y.; Kong, B.; Wang, Y.; Da, P.; Li, J.; Elzatahry, A. A.; Zhao, D.; Gong, X.; Zheng, G.2014,, 2702. doi: 10.1021/nl500608w
(111) Bessegato, G. G.; Guaraldo, T. T.; Brito, J. F.; Brugnera, M. F.; Zanoni, M. V. B.2015,, 415. doi: 10.1007/s12678-015-0259-9
(112) Zhang, M.; Cheng, J.; Xuan, X.; Zhou, J.; Cen, K.2016,, 6344. doi: 10.1021/acssuschemeng.6b00909
(113) Cheng, J.; Zhang, M.; Wu, G.; Wang, X.; Zhou, J.; Cen, K.2015,, 606. doi: 10.1016/j.solmat.2014.10.015
(114) Pathak, P.; Gupta, S.; Grosulak, K.; Imahori, H.; Subramanian, V.2015,, 7543. doi: 10.1021/jp512160h
(115) Ng, Y. H.; Lightcap, I. V.; Goodwin, K.; Matsumura, M.; Kamat, P. V.2010,, 2222. doi: 10.1021/jz100728z
(116) Lightcap, I. V.; Kamat, P. V.2012,, 7109. doi: 10.1021/ja3012929
(117) Hasan, M. R.; Abd Hamid, S. B.; Basirun, W. J.; Meriam Suhaimy, S. H.; Che Mat, A. N.2015,, 77803. doi: 10.1039/C5RA12525A
(118) Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H.2011,, 780. doi: 10.1038/nmat3087
(119) Wu, Z. S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K.2012,, 9082. doi: 10.1021/ja3030565
(120) Huang, X.; Cao, T.; Liu, M.; Zhao, G.2013,, 26432. doi: 10.1021/jp408630s
(121) Sekizawa, K.; Maeda, K.; Domen, K.; Koike, K.; Ishitani, O.2013,, 4596. doi: 10.1021/ja311541a
(122) Jin, J.; Yu, J.; Guo, D.; Cui, C.; Ho, W.2015,, 5262. doi: 10.1002/smll.201500926
(123) Iwashina, K.; Iwase, A.; Ng, Y. H.; Amal, R.; Kudo, A.2015,, 604. doi: 10.1021/ja511615s
(124) Xian, J.; Li, D.; Chen, J.; Li, X.; He, M.; Shao, Y.; Yu, L.; Fang, J.2014,, 13157. doi: 10.1021/am5029999
(125) Li, P.; Zhou, Y.; Li, H.; Xu, Q.; Meng, X.; Wang, X.; Xiao, M.; Zou, Z.2015,, 800. doi: 10.1039/C4CC08744E
(126) Maeda, K.2013,, 1486. doi: 10.1021/cs4002089
(127) Zhou, P.; Yu, J.; Jaroniec, M.2014,, 4920. doi: 10.1002/adma.201400288
(128) Arai, T.; Sato, S.; Kajino, T.; Morikawa, T.2013,, 1274. doi: 10.1039/C3EE24317F
(129) Arai, T.; Sato, S.; Uemura, K.; Morikawa, T.; Kajino, T.; Motohiro, T.2010,, 6944. doi: 10.1039/C0CC02061C
(130) Iwase, A.; Yoshino, S.; Takayama, T.; Ng, Y. H.; Amal, R.; Kudo, A.2016,, 10260. doi: 10.1021/jacs.6b05304
(131) Kondratenko, E. V.; Mul, G.; Baltrusaitis, J.; Larrazabal, G. O.; Perez-Ramirez, J.2013,, 3112. doi: 10.1039/C3EE41272E
(132) Christensen, P. A.; Curtis, T. P.; Egerton, T. A.; Kosa, S. A. M.; Tinlin, J. R.2003,, 371. doi: 10.1016/s0926-3373(02)00172-8
(133) Gangeri, M.; Perathoner, S.; Caudo, S.; Centi, G.; Amadou, J.; Bégin, D.; Pham-Huu, C.; Ledoux, M. J.; Tessonnier, J. P.; Su, D. S.; Schlögl, R.2009,, 57. doi: 10.1016/j.cattod.2008.11.00
石墨烯基复合材料应用于光电二氧化碳还原的基本原理,研究进展和发展前景
全 泉1谢顺吉2王 野2,*徐艺军1,*
(1福州大学化学学院,能源与环境光催化国家重点实验室,福州 350116;2厦门大学化学化工学院,固体表面物理化学国家重点实验室,能源材料化学协同创新中心,福建 厦门 361005)
面对日益严重的化石能源消耗和温室效应问题,二氧化碳还原正成为一个重要的全球性研究课题,其通过消耗二氧化碳来生成可用于能源供应的产物。光电催化技术同时利用光能和外部电压,是一种用于二氧化碳还原的可行且有效的途径。因为石墨烯具有增强二氧化碳吸附和促进光生电子转移的特性能够提升石墨烯基复合电极的性能,所以引入石墨烯用于调优光电催化二氧化碳还原体系已经引起了广泛关注。本篇综述详细陈述了石墨烯基复合材料应用于光电二氧化碳还原的基本原理,电极制备方法以及目前的研究进展。我们也对这个蓬勃发展的领域未来可能会遇到的机遇和挑战进行了展望,同时提出了潜在可行的革新策略用于提升光电二氧化碳还原方面的研究。
光电催化;二氧化碳还原;石墨烯基复合材料
O649
10.3866/PKU.WHXB201706263
June 5, 2017;
June 19, 2017;
June 26, 2017.
Corresponding authors.WANG Ye, Email: wangye@xmu.edu.cn. XU Yi-Jun, Email: yjxu@fzu.edu.cn.
The project was supported by the National Natural Science Foundation of China (U1463204, 20903023 and 21173045), the Award Program for Minjiang Scholar Professorship, the Natural Science Foundation of Fujian Province for Distinguished Young Investigator Grant (2012J06003), the Independent Research Project of State Key Laboratory of Photocatalysis on Energy and Environment (2014A05), the first Program of Fujian Province for Top Creative Young Talents, the Open Research Project of State Key Laboratory of Physical Chemistry of Solid Surfaces of Xiamen University (201519), the Program for Returned High-Level Overseas Chinese Scholars of Fujian province, and the Natural Science Foundation of Fujian Province for Distinguished Young Investigator Rolling Grant (2017J07002).
国家自然科学基金(U1463204, 20903023和21173045),闽江学者特聘教授科研启动基金,福建省杰出青年自然科学基金(2012J06003),能源与环境光催化国家重点实验室自主课题(2014A05),福建省首批特支人才“双百计划”青年拔尖创新人才,厦门大学固体表面物理化学国家重点实验室开放课题(201519),教育部留学回国人员科研启动基金项目,福建省杰出青年自然科学基金滚动资助项目(2017J07002)
猜你喜欢
杂志排行
物理化学学报的其它文章
- AlN-Fe纳米复合薄膜:一种新型锂离子电池负极材料
- Photocatalytic Production of Hydrogen Peroxide Using g-C3N4 Coated MgO-Al2O3-Fe2O3 Heterojunction Catalysts Prepared by a Novel Molten Salt-Assisted Microwave Process
- Theoretical and Experimental Studies on the Crystal Morphologyof Transition-Metal Carbohydrazide Perchlorate Complexes
- 无定型钼硫化物/还原氧化石墨烯的辐射合成及其电催化析氢性能
- Synthesis of Poly(bis-3,4-ethylenedioxythiophene methine)s with Side-Chain Comprising Electro-Optical Moieties and Alkyl Chain Effect in Solid State Polymerization
- 介孔TiO2薄膜光波导共振传感器对苯并(a)芘的探测灵敏度
