Efficient Synthesis of Sulfur and Nitrogen Co-Doped Porous Carbon by Microwave-Assisted Pyrolysis of Ionic Liquid
2018-01-10MENGYanShuangWANGChenWANGLeiWANGGongRuiXIAJunZHUFuLiangZHANGYue
MENG Yan-Shuang WANG Chen WANG Lei WANG Gong-Rui XIA Jun ZHU Fu-Liang,* ZHANG Yue
Efficient Synthesis of Sulfur and Nitrogen Co-Doped Porous Carbon by Microwave-Assisted Pyrolysis of Ionic Liquid
MENG Yan-Shuang1,3WANG Chen1WANG Lei1WANG Gong-Rui1XIA Jun1ZHU Fu-Liang1,3,*ZHANG Yue2,*
(1;2;3)
Sulfur and nitrogen co-doped porous carbon (SNDPC) was successfully synthesized using one-step microwave-assisted pyrolysis of ionic liquid. The structure and morphology of the pyrolysis products were characterized by Fourier-transform infrared (FT-IR) spectroscopy, Raman spectroscopy, X-ray diffraction (XRD), electron microscopy, and X-ray photoelectron spectroscopy (XPS). The pyrolysis mechanism of the ionic liquid 1-ethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide (EMImNTf2) under microwave irradiation was discussed. Microwave irradiation was found to accelerate the pyrolysis of EMImNTf2. The cation of EMImNTf2works as the precursor of the carbon backbone of the porous carbon, while the anion acts as sulfur source and porosity-directing regulator. The SNDPC was obtained at 320 °C and exhibited graphitic structure with numerous surface defects. The atomic percentages of N and S in SNDPC were 12.84% and 1.07%, respectively. The N atoms mainly substitute the C sites in the graphitic carbon matrix, whereas the S atoms mainly bond to the ledges and defects of the carbon matrix.
Sulfur and nitrogen co-doped; Porous carbon; Microwave; Pyrolysis; Ionic liquid
1 Introduction
Recently, the heteroatoms (e.g. N, S, P, B and O) doped and their dual or multiple doped porous carbon materials have attracted tremendous attentions owing to their various applications as oxygen reduction reaction (ORR) electrocatalysts1–3, adsorbents4, supercapacitors materials5–9, hydrogen storage materials10, vanadium redox flow batteries (VRFBs)11, lithium-sulfur batteries12,13, and lithium-ion batteries electrode materials14,15. The sulfur and nitrogen co-doped porous carbon (SNDPC) had been studied in the past decades and experienced great progress16–19. The presence of heteroatoms, such as nitrogen, in the carbon framework can enhance the electrode/electrolyte wettability, the reactivity, the electronical conductivity and, therefore, the electrochemical performance2,7–9.
Sulfur and nitrogen co-doped carbon materials SNDPC materials were generally synthesized by thermal pyrolysis of biomass or polymers in anor a post-treatment manner. In themethod, carbon precursors were pretreated with N and S containing components (e.g. polydopamine, melamine, benzyl disulfide) and then pyrolyzed at high temperature20,21. In the post-treatment method, the as-prepared carbon materials were heated in the gas atmosphere containing N and S (e.g. NH3, pyridine, H2S, SO2, and thiophene)22,23. However, these pyrolysis processes are limited by the harsh processing conditions and low carbon yields24.
Recently, the ionic liquids (ILs) have attracted an increasing number of attentions since ILs possess excellent properties as the as the precursors of doped porous carbon, such as negligible vapor pressure, structure designability, and simple processing17,25–29. Due to the negligible vapor of ILs, the materials do not evaporate during the carbonizations process, therefore, makes the processing and shaping process simple. In addition, as the chemical structure of ILs is desirable, it is easy to tune the doping content of heteroatoms by adjusting the ratio of cation and anion components in ILs. The unique properties of ILs lead to a higher doping contents of IL-derived carbons when compare with other carbon precursors30. Recently, Lee.31employed ILs, such as [EMIm][C(CN)3], [BMIm][C(CN)3] and [BCNIm][C(CN)3], as carbon precursor. Their work indicated that the doping content was closely related to the type and the pyrolysis temperature of the ILs. Fulvio and co-workers obtained the carbon materials with different structures and morphology by adjusting the ratio of two different ILs ([BMIm][C(CN)3] and [EMIm][B(CN)4]) and pyrolysis temperature. Additionally, the porosity of the resulting carbon can be induced by the anion or cation without employing any other porogens17,24.
In this work, we present a method for synthesis of sulfur and nitrogen co-doped porous carbon (SNDPC) by a microwave-assisted pyrolysis of ionic liquid. The microwave-assisted pyrolysis mechanism of the ionic liquid EMImNTf2was discussed for the first time ever. The morphology and structure of the SNDPC were investigated. The results showed that microwave-assisted pyrolysis of ionic liquid was a facile and time-saving method to prepare SNDPC.
2 Experimental and computational section
2.1 Preparation of SNDPC
The ionic liquid 1-ethyl-3-methylimidazolium bis(( trifluoromethyl)sulfonyl)imide EMImNTf2(analytical reagent) was purchased from Lanzhou Institute of Chemical Physics and used without further purification. EMImNTf2was loaded into a quartz boat, which was put into HAMiLab-V1500 microwave sintering furnace (Synotherm Corporation, China) and heated to different temperatures in a nitrogen atmosphere with 800 W. The pyrolysis products were denoted as SNDPC-, whereindicated the pyrolysis temperatures (°C). Pyrolysis products from different temperatures were weighted.
2.2 Structural and Morphology Characterization
Thermal properties were studied by NETZSCH STA 449F3 TG-DSC (thermogravimetry-differential scanning calorimetry) thermal analyzer (Netzsch, Germany) with different ramp rates in nitrogen atmosphere. Fourier transform infrared (FT-IR) spectra were recorded on a Nexus 670 FT-IR spectrophotometer (Thermo Nicolet Corporation, USA). The spectra of solids were carried out using KBr pellets. X-ray diffraction (XRD) characterization of the samples was performed with a Rigaku D/MAX 2500V X-ray diffractometer (Rigaku Corporation, Japan) and filtered Curadiation. Raman spectra were recorded on a LabRAM HR UV/Vis/NIR (Horiba Jobin Yvon, France) using a CW Ar-ion laser (514.5 nm) as an excitation source. Field- emission scanning electron microscopy (FT-SEM) was carried out with a JEOL-7500F microscope (JEOL, Japan) equipped with an energy-dispersive spectroscopy (EDS) instrument. Transmission electron microscopy (TEM) characterizations were carried out with a JEOL JEM-2010F equipment (JEOL, Japan). X-ray photoelectron spectroscopy (XPS) was recorded using a Kratos XSAM 800 spectrometer (Manchester, UK). The N2adsorption-desorption isotherm was obtained using an N2adsorption analyzer (Autosorb- IQ2-MP-C system, Quantachrome, USA). The specific surface area of SNDPC was calculated by BET method.
3 Results and discussion
The TG and DTG (derivative thermogravimetry) curves of EMImNTf2recorded at different ramp rates were shown in Fig.1(a, b), respectively. The abrupt weight loss arose at the range of 400 to 520 °C, owing to the pyrolysis and carbonization of EMImNTf2. The pyrolysis kinetic parameters of EMImNTf2were calculated on the Kissinger Eq.(1).

where,is the heating rate,pis the peak temperature of the pyrolysis in K,ais the activation energy,is the nucleating rate,is the gas constant.
Fig.1(b) showed that the pyrolysis peak temperatures,p, increased with the heating rates. Plots of the pyrolysis data for EMImNTf2, fit to Eq.(1), was shown in Fig.2. It indicated that a linear line was obtained. The activation energyaand nucleating ratewere calculated from the slope and the intercept of the line, respectively. The activation energy of the pyrolysis of EMImNTf2was 3.10 kJ∙mol−1, as shown in Table 1.
The ionic liquid EMImNTf2has high dipole moment, so itabsorbed the microwave energy and can be heated under the microwave irradiation. The mass loss of EMImNTf2during the microwave irradiation was plotted in Fig.3. Fig.3 showed that an abrupt mass loss arose in a large range of 130 to 320 °C under the microwave irradiation, indicating that the pyrolysis of EMImNTf2was accelerated by the microwave irradiation. EMImNTf2was pyrolyzed and carbonized gradually under the microwave irradiation. The residue product was sulfur and nitrogen co-doped porous carbon with a mass of about 9.2%. The carbon was mainly derived from the cation, with the anion acting as sulfur sources and porosity-directing regulator.
To investigate the chemical evolution of the EMImNTf2during the pyrolysis by microwave irradiation, FT-IR test was performed on the pristine EMImNTf2andthe products produced at different pyrolysis temperatures (Fig.4). The typical peaks at 3160 and 3117 cm−1were attributed to the stretching vibrations of aromatic C―H. The peak at 2989 cm−1stemmed from the stretching vibrations of the aliphatic C―H bond32. The peaks at 1578 and 1458 cm−1arose from the deformation of aliphatic C―H bonds. The broad peak at 1197 cm−1was attributed to the stretching vibrations of the imidazole ring. The typical peaks at 1147 and 1056 cm−1were ascribed to the stretching vibrations of C―F. The broad peak at 1345 cm−1arose from the S=O. When the temperature reached 220 °C, the peaks at 3160, 3117, 2989, 1578 and 1458 cm−1disappeared, indicating the decomposition of the alkyl substituent groups and the imidazolium ring. With the temperature increasing from 220 to 320 °C, the other characteristic peaks at 1345, 1197, 1147 and 1056 cm−1gradually decreased. This indicated that EMImNTf2carbonized at 320 °C under the microwave irradiation.
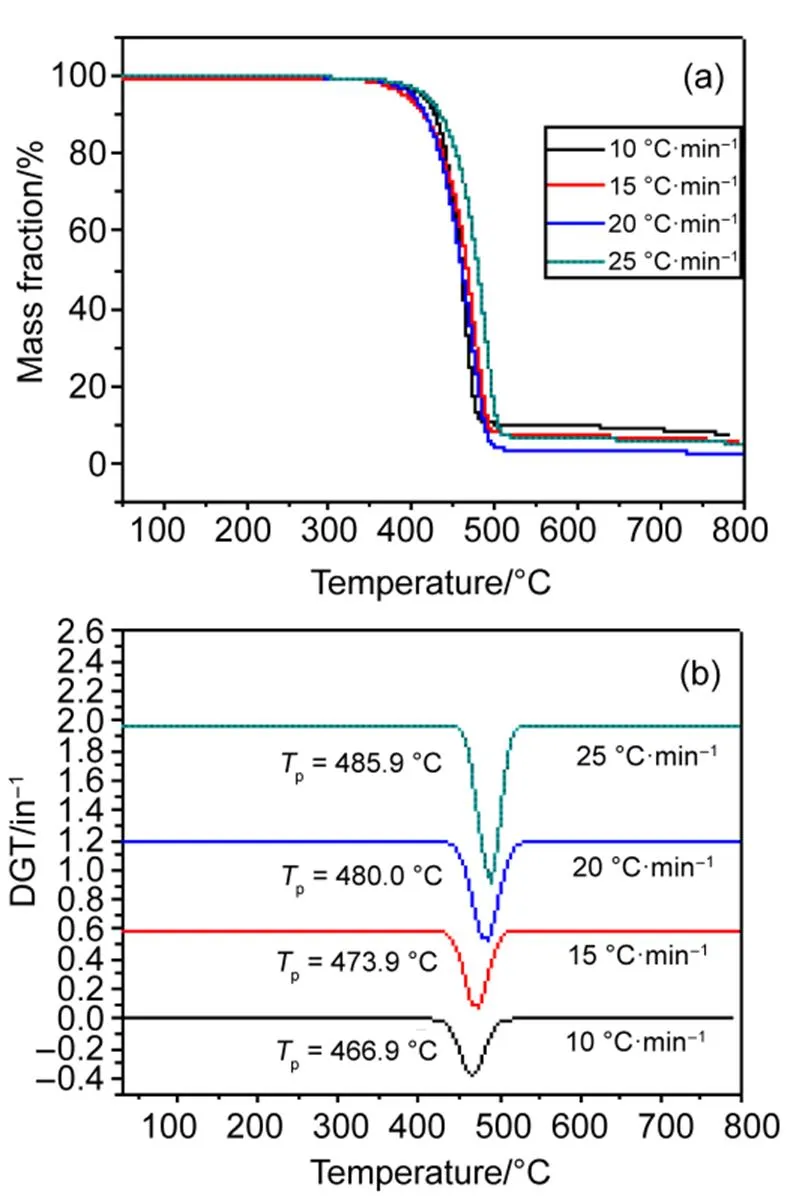
Fig.1 TG (a) and DTG (b) curves of EMImNTf2 at different ramp rates.
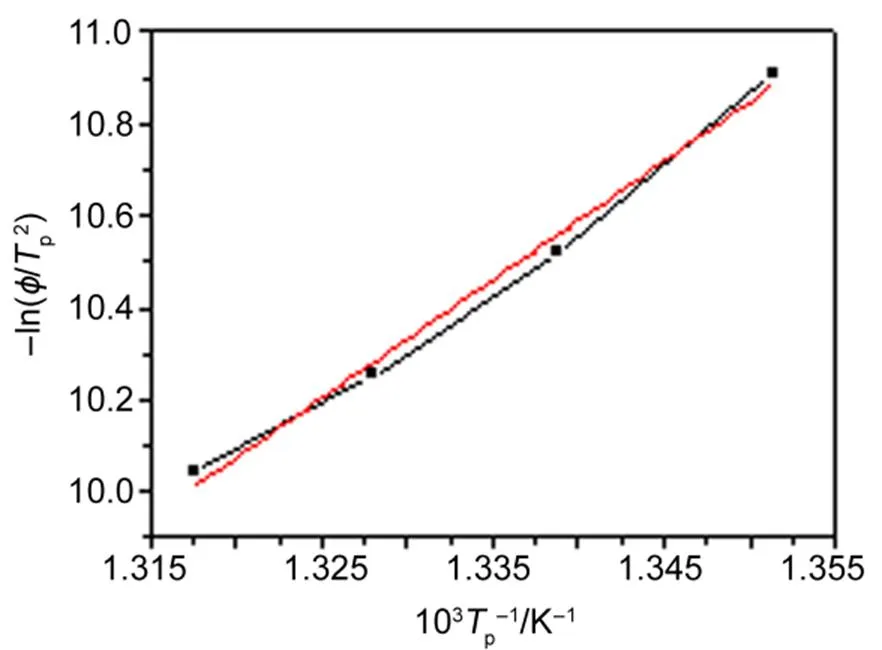
Fig.2 Curves of EMImNTf2 of −ln(ϕ/Tp2) vs 1/Tp.
The XRD patterns of pyrolysis products from different temperatures were shown in Fig.5. When the temperature was below 320 °C, there was no obvious diffraction peak in the diffraction patterns. When the temperature was above 320 °C, the pyrolysis products possessed peaks at 2of 23.6°, corresponding to the (002) reflection of carbon15,33. This was agreed well with that of the FTIR result. The broad characteristic peaks at 23.6° indicated a large amount of edges and defects in the carbon matrix.

Fig.3 Temperature rising and mass loss curves of EMImNTf2 during microwave irradiation.

Table 1 Kinetic parameters of the pyrolysis of EMImNTf2.
The Raman spectra of the pyrolysis products from different temperatures were shown in Fig.6. Two broaden peaks at 1350 and 1570 cm−1were corresponded to the D and G bands, respectively34. The G band was attributed to the2gvibration mode of2bonded graphitic carbons35,36. The relative intensity of D band between 1200 and 1450 cm−1stands for the disorder of graphite crystal structure of carbon materials. The disorder degree of carbon materials was generally evaluated by the intensity ratio of D band to G band37–39. Theband andband of the pyrolysis products were fitted and the values ofI/Iwere calculated (showed in Fig.6). It was observed that the ratioI/Ifor all spectra was above 1, indicating a large number of defects in the pyrolysis products. TheI/Iratios were about 1.25 when the pyrolysis temperature increased from 320 to 500 °C. This indicated that the SNDPC was formed at 320 °C and exhibited a stable graphitic structure with numerous surface defects.
The morphology of the SNDPC from 500 °C was characterized by the electron microscopy. As shown in Fig.7(a, b), the porous structure of the obtained SNDPC was in nano-scale. Fig.7(c) further revealed many pores and defects in the SNDPC, which was due to the heteroatoms doping in the carbon matrix. Fig.7(d–f) indicated that the carbon matrix was uniformly doped with nitrogen and sulfur in a high level. The nitrogen adsorption-desorption isotherm at 77 K of the SNDPC-500 was shown in Fig.8. It indicated that the pores of adsorbents were grouped into micropore (< 2 nm) and mesopore (= 2–50 nm). The pore-size distribution of SNDPC-500 was calculated from nitrogen desorption using the BJH model. The two sharpest peaks occurred at pore diameters of 1.72 and 2.15 nm, respectively, indicating that a vast majority of the pores fell into the micropore range. A large surface area of 385.677 m2∙g−1was calculatedBET analysis. The nitrogen adsorption- desorption isotherm results indicated that the obtained SNDPC had a porous structure.

Fig.4 FT-IR spectra of EMImNTf2 and pyrolysis products at different temperatures.

Fig.5 XRD patterns of pyrolysis products at different temperatures.

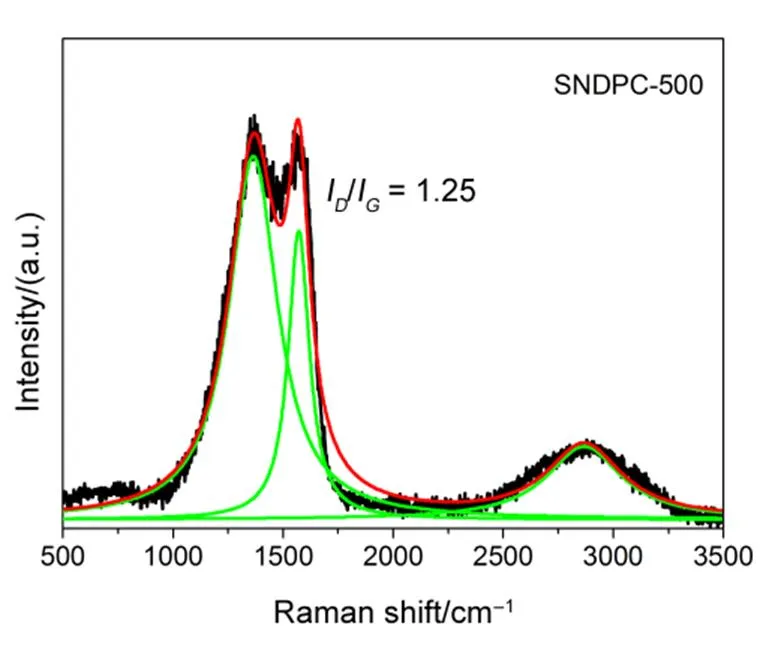
Fig.6 Raman spectra of pyrolysis products at different temperatures.

Fig.7 SEM image (a), TEM images (b, c), EDS map for carbon (d), EDS map for nitrogen (e) and EDS map for sulfur (f) of SNDPC-500.
To study the surface chemical compositions of as-prepared SNDPC, XPS spectra were performed and analyzed and the results were shown in Fig.9. The XPS results showed that the surface atomic percentages of C, N and S for the SNDPC-500 were 86.09%, 12.84% and 1.07% (atomic fraction ()), respectively (Fig.9(a)). A similar ionic liquid BMIMNTf2was used by Leung and collaborators40to prepare SNDPC material. The authors performed pyrolysis without microwave at 900 °C and the nitrogen and sulfur contents were estimated to be 9.88% and 0.38% () respectively. Tang and coworkers41used the ion liquid 1-methyl-3-propargyl-imidzolium NTf2to prepare SNDPC using silica as hard template. In this case, the nitrogen and sulfur contents were determined to be 2.6% and 0.91% () at 900 °C. In the present paper, the nitrogen and sulfur contents are higher, 12.84% and 1.07% () for N and S respectively, as expected from the lower temperature by microwave- assisted pyrolysis
As showed in Fig.9(b), the complex C 1spectrum of SNDPC-500 was deconvoluted into four different peaks, corresponding to C=C (284.7 eV), C―S (285.2 eV), C―C (285.5 eV), and C―N (287.3 eV), respectively42,43. The high resolution N 1spectrum of the SNDPC-500 was fitted into two N species (Fig.9(c)), corresponding to graphitic-N (400.9 eV) and pyridinic-N (398.5 eV), respectively44,45. The graphitic N―(C)3and pyridinic C―N―C indicated that the N atoms mainly substituted for the C sites in the graphitic carbon matrix. Zhu and collaborators46found that nitrogen-doped porous carbon had high capacitive performance. It was attributed to the hierarchical porosity combined with high effective surface area and heteroatom doping effects, which resulted in both electrochemical double layer and Faradaic capacitance contributions. The nitrogen dopant also modified the surface basicity of carbon material and thus improved the SO2adsorption of the carbon material47. In addition, doping with nitrogen can also improve the electrocatalytic activities of carbon materials, leading to higher catalytic performance of ORR48.
The S 2spectrum (Fig.9(d)) can be deconvoluted into three different peaks at the binding energies about 163.9, 165.0 and 168.2 eV, corresponding to the thiophene-S (―C―S―C―), ―C=S― and oxidized S (C―SOX―C), respectively49−51. The intense oxidized S (C―SOX―C) indicated that the S atoms mainly bonded to the edges and defects of the carbon matrix. The performance of carbon matrix can be significantly improved by the sulfur dopant. It was found that sulfur doped carbon exhibited high hydrogen uptake properties because the sulfur dopant induced a stronger interaction between the carbon host and the hydrogen molecules52. The sulfur dopant also increased the amount of nanopore moieties in the carbon material and provided ideal preconditions for its application in lithium ion batteries53.
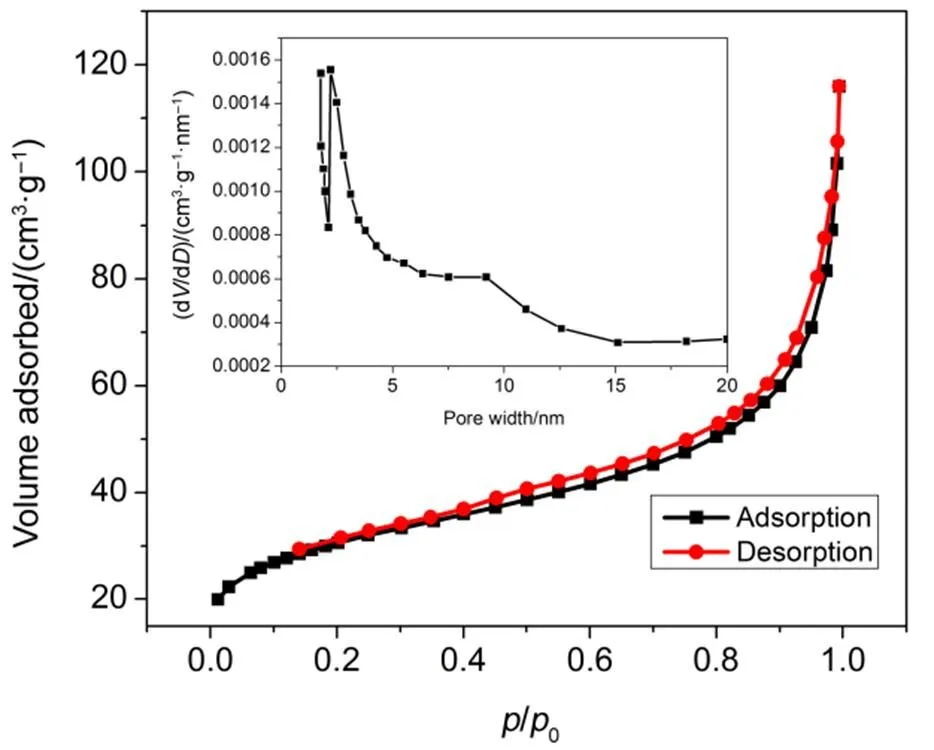
Fig.8 Nitrogen adsorption-desorption isotherms at 77 K and pore size distribution (inset) for the SNDPC-500.

Fig.9 XPS survey (a), the high-resolution C 1s XPS spectrum (b), the high-resolution N 1s XPS spectrum (c), and the high-resolution S 2p XPS spectrum (d) of the SNDPC-500.
Co-doping carbons with both sulfur and nitrogen can probably create synergistic effects54. Liang.21employed density functional theory calculations and confirmed that the charge and spin density at the different sites of the dually doped materials were enhanced by the different behaviors of nitrogen and sulfur. Accordingly, the sulfur and nitrogen co-doped carbon material had superior properties than the merely sulfur or nitrogen doped carbon materials. Co-doped with sulfur and nitrogen, the carbon anode materials exhibited excellent electrochemical properties. It was attributed to the increased number of active sites, the large specific surface area and the high level of heteroatoms14,55. In addition, the larger atoms like N, S and P are favorable for the polarizablity of the carbon materials and thus they have an important influence on electrochemical applications56.
4 Conclusions
Due to the high dipole moment and dielectric constant of ionic liquid EMImNTf2, SNDPC was produced by heating and carbonizing EMImNTf2under the microwave irradiation. The obtained SNDPC had graphitic structure with a large amount of edges and defects. From the results, it was found that the high content doping of nitrogen (12.84%) was mainly originated from the cation of EMImNTf2. The nitrogen dopant existed in two groups of graphitic-N and pyridinic-N. The sulfur dopant was originated from the anion of EMImNTf2and deconvoluted into three groups of thiophene-S, ―C=S― and oxidized S. The SNDPC was successfully prepared by pyrolysis of EMImNTf2without any other porogens or template species. This approach may provide an efficient platform for the synthesis of a series of heteroatom-doped carbon materials for different applications.
(1) He, Y.; Han, X.; Du, Y.; Song, B.; Xu, P.; Zhang, B.2016,, 3601. doi: 10.1021/acsami.5b07865
(2) Pašti, I. A.; Gavrilov, N. M.; Dobrota, A. S.; Momčilović, M.; Stojmenović, M.; Topalov, A.; Stanković, D. M.; Babić, B.; Ćirić-Marjanović, G.; Mentus, S. V.2015,, 498. doi: 10.1007/s12678-015-0271-0
(3) Zhao, S.; Liu, J.; Li, C.; Ji, W.; Yang, M.; Huang, H.; Liu, Y.; Kang, Z.2014,, 22297. doi: 10.1021/am506284k
(4) Sun, F.; Gao, J.; Liu, X.; Yang, Y.; Wu, S.2016,, 116. doi: 10.1016/j.cej.2015.12.044
(5) Chen, K.; Huang, X.; Wan, C.; Liu, H.2015,, 85. doi: 10.1016/j.matchemphys.2015.08.027
(6) Zhu, T.; Zhou, J.; Li, Z.; Li, S.; Si, W.; Zhuo, S.2014,, 12545. doi: 10.1039/c4ta01465k
(7) Wang, C.; Sun, L.; Zhou, Y.; Wan, P.; Zhang, X.; Qiu, J.2013,, 537. doi: 10.1016/j.carbon.2013.03.052
(8) Nasini, U. B.; Bairi, V. G.; Ramasahayam, S. K.; Bourdo, S. E.; Viswanathan, T.; Shaikh, A. U.2014,, 257. doi: 10.1016/j.jpowsour.2013.11.014
(9) Si, W.; Zhou, J.; Zhang, S.; Li, S.; Xing, W.; Zhuo, S.2013,, 397. doi: 10.1016/j.electacta.2013.06.065
(10) Kim, H. S.; Kang, M. S.; Yoo, W. C.2015,, 28512. doi: 10.1021/acs.jpcc.5b10552
(11) Ryu, J.; Park, M.; Cho, J.2015,, A5144. doi: 10.1149/2.0191601jes
(12) Chen, F.; Yang, J.; Bai, T.; Long, B.; Zhou, X.2016,, 99. doi: 10.1016/j.electacta.2016.01.192
(13) Zhou, X.; Liao, Q.; Tang, J.; Bai, T.; Chen, F.; Yang, J.2016,, 55. doi: 10.1016/j.jelechem.2016.02.037
(14) Yun, Y. S.; Jin, H.-J.2013,, 311. doi: 10.1016/j.matlet.2013.07.026
(15) Ou, J.; Zhang, Y.; Chen, L.; Yuan, H.; Xiao, D.2014,, 63784. doi: 10.1039/c4ra12121j
(16) Paraknowitsch, J. P.; Thomas, A.2013,, 2839. doi: 10.1039/c3ee41444b
(17) Paraknowitsch, J. P.; Thomas, A.; Schmidt, J.2011,, 8283. doi: 10.1039/c1cc12272j
(18) Schmidt, J.; Weber, J.; Epping, J. D.; Antonietti, M.; Thomas, A.2009,, 702. doi: 10.1002/adma.200802692
(19) Thomas, A.2010,, 8328. doi: 10.1002/anie.201000167
(20) Qu, K.; Zheng, Y.; Dai, S.; Qiao, S. Z.2016,, 373. doi: 10.1016/j.nanoen.2015.11.027
(21) Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S. Z.2012,, 11496. doi: 10.1002/anie.201206720
(22) Yang, S.; Zhi, L.; Tang, K.; Feng, X.; Maier, J.; Müllen, K.2012,, 3634. doi: 10.1002/adfm.201200186
(23) Ito, Y.; Cong, W. T.; Fujita, T.; Tang, Z.; Chen, M. W.2015,, 2131. doi: 10.1002/anie.201410050
(24) Xie, Z. L.; Su, D. S.2015, 1137. doi: 10.1002/ejic.201402607
(25) Wang, X. Q.; Dai, S.2010,, 6664. doi: 10.1002/anie.201003163
(26) Lee, J. S.; Wang, X. Q.; Luo, H. M.; Baker, G. A.; Dai, S.2009,, 4596. doi: 10.1021/ja900686d
(27) Zhi, L.; Hu, Y. S.; El, H. B.; Wang, X.; Ingo, L.; Ute, K.; Joachim, M.; Klaus, M.2008,, 1727. doi: 10.1002/adma.200702654
(28) Fulvio, P. F.; Lee, J. S.; Mayes, R. T.; Wang, X. Q.; Mahurin, S. M.; Dai, S.2011,, 13486. doi: 10.1039/c1cp20631a
(29) Wan, M. M.; Sun, X. D.; Li, Y. Y.; Zhou, J.; Wang, Y.; Zhu, J. H.2016,, 1252. doi: 10.1021/acsami.5b09759
(30) Paraknowitsch, J. P.; Thomas, A.2012,, 1132. doi: 10.1002/macp.201100573
(31) Lee, J. S.; Wang, X.; Luo, H.; Dai, S.2010,, 1004. doi: 10.1002/adma.200903403
(32) Xiao, D. L.; Yuan, D. H.; He, H.; Gao, M. M.2013,, 120. doi: 10.1016/j.jlumin.2013.02.032
(33) Tang, Z. H.; Song, Y.; He, X.; Yang, J. H.2012,, 330. doi: 10.1016/j.matlet.2012.08.105
(34) Ferrari, A.; Meyer, J.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.; Roth, S.2006,, 187401. doi: 10.1103/PhysRevLett.97.187401
(35) Dresselhaus, M. S.; Dresselhaus, G.; Saito, R.; Jorio, A.2005,, 47. doi: 10.1016/j.physrep.2004.10.006
(36) Han, P. X.; Yue, Y. H.; Zhang, L. X.; Xu, H. X.; Liu, Z. H.; Zhang, K. J.; Zhang, C. J.; Dong, S. M.; Ma, W.; Cui, G. L.2012,, 1355. doi: 10.1016/j.carbon.2011.11.007
(37) Nakamizo, M.; Kammereck, R.; Walker, P. L.1974,, 259. doi: 10.1016/0008-6223(73)90397-7
(38) Yen, B.; Tadashi, i.1996,, 489. doi: 10.1016/0008-6223(95)00205-7
(39) Zou, L.; Huang, B.; Huang, Y.; Huang, Q.; Wang, C. A.2003,, 654. doi: 10.1016/S0254-0584(03)00332-8
(40) (40) She, Y.; Lu, Z.; Ni, M.; Li, L.; Leung, M. K.2015,, 7214. doi: 10.1021/acsami.5b00222
(41) (41) Wu, H.; Shi, L.; Lei, J.; Liu, D.; Qu, D.; Xie, Z.; Du, X.; Yang, P.; Hu, X.; Li, J.2016,, 90. doi: 10.1016/j.jpowsour.2016.05.044
(42) Cheng, X. B.; Zhang, Q.; Wang, H. F.; Tian, G. L.; Huang, J. Q.; Peng, H. J.; Zhao, M. Q.; Wei, F.2015,, 244. doi: 10.1016/j.cattod.2014.10.047
(43) Liu, X. W.; Wu, Y.; Yang, Z. Z.; Pan, F. S.; Zhong, X. W.; Wang, J. Q.; Gu, L.; Yu, Y.2015,, 799. doi: 10.1016/j.jpowsour.2015.05.074
(44) Qian, Z.; Ma, J.; Shan, X.; Feng, H.; Shao, L.; Chen, J.2014,, 2983. doi: 10.1002/chem.201400424
(45) Ding, H.; Wei, J. S.; Xiang, H. M.2014,, 13817. doi: 10.1039/c4nr04267k
(46) Zhu, T. T.; Zhou, J.; Li, Z. H.; Li, S. J.; Si, W. J.; Zhuo, S. P.2014,, 12545. doi: 10.1039/c4ta01465k
(47) Sun, F.; Gao, J. H.; Liu, X.; Yang, Y. Q.; Wu, S. H.2016,, 116. doi: 10.1016/j.cej.2015.12.044
(48) He, Y. Z.; Han, X. J.; Du, Y. C.; Song, B.; Xu, P.; Zhang, B.2016,, 3601. doi: 10.1021/acsami.5b07865
(49) Jiang, T. T.; Wang, Y.; Wang, K.; Liang, Y. R.; Wu, D. C.; Tsiakaras, P.; Song, S. Q.2016,, 1. doi: 10.1016/j.apcatb.2016.02.009
(50) Herrmann, I.; Kramm, U. I.; Radnik, J.; Fiechter, S.; Bogdanoff, P.2009,, B1283. doi: 10.1149/1.3185852
(51) Yang, S. B.; Zhi, L. J.; Tang, K.; Feng, X. L.; Maier, J.; Mullen, K.2012,, 3634. doi: 10.1002/adfm.201200186
(52) Xia, Y. D.; Zhu, Y. Q.; Tang, Y.2012,, 5543. doi: 10.1016/j.carbon.2012.07.044
(53) Yan, Y.; Yin, Y. X.; Xin, S.; Guo, Y. G.; Wan, L. J.2012,, 10663. doi: 10.1039/c2cc36234a
(54) Jiang, T.; Wang, Y.; Wang, K.; Liang, Y.; Wu, D.; Tsiakaras, P.; Song, S.2016,, 1. doi: 10.1016/j.apcatb.2016.02.009
(55) Song, H.; Yang, G.; Wang, C.2014,, 21661. doi: 10.1021/am506747z
(56) Paraknowitsch, J. P.; Thomas, A.2013,, 2839. doi: 10.1039/c3ee41444b
微波辅助裂解离子液体制备硫氮共掺杂多孔碳材料
蒙延双1,3王 琛1王 磊1王功瑞1夏 军1朱福良1,3,*ZHANG Yue2,*
(1兰州理工大学材料科学与工程学院,兰州 730050;2Department of Mechanical and Industrial Engineering, Texas A&M University-Kingsville, Kingsville, TX 78363, USA;3省部共建有色金属先进加工与再利用国家重点实验室,兰州 730050)
采用一步微波辅助裂解离子液体法制备了硫氮共掺杂多孔碳材料。用傅里叶变换红外(FT-IR)光谱,拉曼光谱,X射线衍射(XRD),电子显微镜和X射线光电子能谱(XPS)等检测手段对裂解产物的结构和形貌进行了表征。对微波辐照下离子液体1-乙基-3-甲基咪唑双三氟甲磺酰亚胺盐(EMImNTf2)的裂解机理进行了分析。结果表明,微波辐照能够促进EMImNTf2的裂解,使其在320 °C下裂解得到硫氮共掺杂的多孔碳材料。离子液体EMImNTf2的阳离子作为多孔碳材料骨架的前驱体,而阴离子作为硫源和造孔剂。制备的硫氮共掺杂多孔碳材料具有含缺陷的石墨结构。石墨碳基体中氮和硫的原子百分含量分别为12.84%和1.07%,其中N原子主要取代C成为活性点,而S原子主要存在于边界和缺陷处。
硫氮共掺杂;多孔碳;微波;裂解;离子液体
O646
10.3866/PKU.WHXB201705083
March 22, 2017;
April 28, 2017;
May 8, 2017.
. ZHU Fu-Liang, Email: chzfl@126.com; Tel: +86-139-19491509. ZHANG Yue, Email: yue.zhang@tamuk.edu.
The project was supported by the National Natural Science Foundation of China (51364024, 51404124), Natural Science Foundation of Gansu Province, China (1506RJZA100), and the Foundation for Innovation Groups of Basic Research in Gansu Province, China (1606RJIA322).
国家自然科学基金(51364024, 51404124),甘肃省自然科学基金(1506RJZA100),甘肃省基础研究创新群体计划项目(1606RJIA322)资助
猜你喜欢
杂志排行
物理化学学报的其它文章
- Development and Validation of a Reduced Chemical Kinetic Mechanism for HCCI Engine of Biodiesel Surrogate
- Cobalt@cobalt Carbide Supported on Nitrogen and Sulfur Co-Doped Carbon: an Efficient Non-Precious Metal Electrocatalyst for Oxygen Reduction Reaction
- OL负载Cu催化剂及其催化氧化CO及乙酸乙酯性能
- Combined Effects of the Hole and Twin Boundary on the Deformation of Ag Nanowires: a Molecular Dynamics Simulation Study
- First-Principles Study: the Structural Stability and Sulfur Anion Redox of Li1−xNiO2−ySy
- S掺杂促进Fe/N/C催化剂氧还原活性的实验与理论研究
