Cobalt@cobalt Carbide Supported on Nitrogen and Sulfur Co-Doped Carbon: an Efficient Non-Precious Metal Electrocatalyst for Oxygen Reduction Reaction
2018-01-10SHENHaiBoJIANGHaoLIUYiSiHAOJiaYuLIWenZhangLIJie
SHEN Hai-Bo JIANG Hao LIU Yi-Si,3 HAO Jia-Yu LI Wen-Zhang,2,4,* LI Jie,*
Cobalt@cobalt Carbide Supported on Nitrogen and Sulfur Co-Doped Carbon: an Efficient Non-Precious Metal Electrocatalyst for Oxygen Reduction Reaction
SHEN Hai-Bo1JIANG Hao1LIU Yi-Si1,3HAO Jia-Yu1LI Wen-Zhang1,2,4,*LI Jie1,*
(1;2;3;4)
Development of high-efficiency and low-cost electrocatalysts for large-scale oxygen reduction reaction (ORR) remains a challenge. In this study, we employed melamine, trithiocyanuric acid, and cobaltous nitrate to fabricate a novel ORR electrocatalyst with cobalt and cobalt carbide supported on carbon co-doped with nitrogen and sulfur (hereafter referred to as MTC-0.1-900) by two-step pyrolysis. The MTC-0.1-900 was characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), Raman spectroscopy, Brunauer-Emmett-Teller (BET) specific surface area analysis, and X-ray photoelectron spectroscopy (XPS). The electrochemical performance for ORR was investigated by cyclic voltammetry and linear sweep voltammetry in 0.1 mol∙L−1KOH solutions. The results showed that the onset potential and half-wave potential of MTC-0.1-900 were 29 and 5 mV superior to the commercial Pt/C catalyst, respectively. After 12000 s operation at the potential of −0.3 V (Ag/AgCl), the current retention capacities of MTC-0.1-900 and Pt/C were 97.1% and 76.7%, respectively. MTC-0.1-900 also showed better methanol tolerance than Pt/C. These unique properties of MTC-0.1-900 provide us with an alternative for replacing or reducing the use of Pt catalyst in metal-air battery cathode materials.
Nitrogen and sulfur co-doped carbon; Cobalt@cobalt carbide; Graphitic carbon; Core-shell; Oxygen reduction reaction
1 Introduction
Oxygen reduction reaction (ORR) has attracted a lot of attention in the new energy devices. Traditionally, platinum-based catalysts are widely accepted for the high ORR catalytic performance. But the low content, high cost and instability of Pt largely limit its wide commercialization1–6. Thus, it is urgent to find novel catalysts with low cost and high ORR activity to satisfy the demand of new energy7–10. Carbon based materials have been regarded as promising candidates for ORR, which show excellent resistance to CO poisoning and better durability than Pt/C catalyst. Especially, the ORR performance would be largely enhanced with heteroatom doping in carbon11–14. Gong.15reported that the nitrogen doped carbon nanotube arrays showed a four-electron pathway and high electrocatalytic performance for ORR. Wu16synthesized a nitrogen and sulfur co-doped three-dimensional carbon, which showed excellent durability and ORR property. Dou17demonstrated a nitrogen, phosphorus and sulfur tri-doped graphene for ORR, and the catalyst showed long-term stability in KOH solutions with negligible fuel crossover effect. Although many efforts have been taken to promote the ORR property of carbon materials, it is still hard to obtain satisfactory ORR catalysts for the application of the new energy devices.
It has been proved that carbon supported metal materials with core-shell structures showed excellent ORR activity in both alkaline and acidic solutions. Zhang18fabricated a nitrogen doped graphene with cobalt nano-particles encapsulated in N-doped graphitic carbon shells, which showed a four-electron pathway in alkaline solutions. Wen19reported a facile method to synthesize core-shell Fe/Fe3C-C nanorods as an efficient ORR catalyst. In addition, the catalyst can be an alternative to Pt/C on ORR in neutral phosphate buffer solutions. Xia20rationally synthesized a Co@Co3O4@C-CM bishell structure based on Metal-Organic Frameworks (MOFs) materials, they introduced the Co@Co3O4@C into carbon matrix and the hybrid showed even better ORR activity than 20% Pt/C catalyst. With metal nano-particles encased in graphitic carbon layers, the core and shell structure plays an important role in ORR process. It is generally accepted that the metal nano-particles may activate the graphitic carbon layers and the graphitic carbon layers may prevent the metal nano-particles away from the oxygen and electrolyte21,22. Thus, the core-shell structure of non-precious metals nano-particles encased in carbon layers is a promising candidate for ORR. Even efforts have been devoted to enhancing the ORR performance, those materials still suffered from some weaknesses, such as the complex process of synthesis, extra nitrogen dopant or carbon materials. It is urgent to develop a simple method and low cost to fabricate efficient catalysts with durability for commercialization.
To the best of our knowledge, the cobalt@cobalt carbide structure has not been reported and applied for ORR. In this article, we first reported a nitrogen and sulfur co-doped carbon supported cobalt@cobalt carbide (MTC-0.1-900) by facile pyrolysis under high temperature. Melamine and trithiocyanuric acid were employed to fabricate supramolecular structure by hydrogen-bonding with cobalt nitrate embedded in. The supramolecular transformed into nitrogen and sulfur co-doped carbon with cobalt@cobalt carbide forming during the pyrolysis. The electrocatalyst shows better ORR activity than nitrogen doped carbon supported cobalt@cobalt carbide (named as MC-0.1-900) and Pt/C catalyst. More importantly, the MTC-0.1-900 shows comparable onset potential and similar half-wave potentials with Pt/C catalyst. The obtained catalysts exhibit higher methanol tolerance and durability in alkaline solutions. It should be noticed that the MTC-0.1-900 is much economical and available when compared with traditional noble metals materials.
2 Experimental section
2.1 Synthesis of nitrogen and sulfur co-doped carbon supported cobalt@cobalt carbide
In a typical synthesis, 1.000 g melamine (99%, 7.94 mmol) was firstly dispersed in 40 mL ethanol, 0.100 g trithiocyanuric acid (95%, 0.56 mmol, dispersed in ethanol) was added under magnetic stirring to form supermolecule structure. Then, 0.100 g Co(NO3)2·6H2O (99%, 0.34 mmol, dispersed in ethanol) was added drop by drop. The ethanol was removed under the temperature of 70 °C for 48 h. Next, the dried mixture was heated up to 550 °C at a rate of 5 °C∙min−1and remained at this temperature for 3 h, then the temperature was continuously heated up to 900 °C for 0.5 h at a rate of 10 °C∙min−1. Black composite was obtained and marked as MTC-0.1-900, where M, T and C represented as melamine, trithiocyanuric acid and cobalt nitrate respectively. Similarly, the electrocatalyst donated as MTC-0.05-900 and MTC-0.15-900 represented the mass of cobalt nitride was 0.05 and 0.15 g. MC-0.1-900 was synthesized under the same condition without trithiocyanuric acid. Melamine and cobalt nitrate are purchased from Sinopharm Chemical Reagent Co., Ltd. Trithiocyanuric acid is purchased from adamas reagent Co., Ltd.
It should be noticed that melamine and trithiocyanuric acid composite will totally decompose when the temperature is higher than 750 °C23. While, with the metal (Fe and Co etc) introduction, carbon based metal materials would fabricate under high temperature pyrolysis19,24.
2.2 Material characterization
X-ray diffraction (XRD, D/Max2250, Rigaku Corporation, Japan) with Cu Kradiation (= 0.15406 nm) is employed to analyze the crystal structure of the catalysts. The diffraction angle is ranging from 10° to 80° with a scan rate of 10 (°)∙min−1. Raman Spectrometer (Renishaw PLC, UK) measurement was performed with an excitation wavelength of 532 nm. X-ray photoelectron spectroscopy (XPS, K-Alpha 1063, Thermo Fisher Scientific) was implied to analysis the valence state of the element in MTC-0.1-900. Brunauer-Emmett-Teller (BET, JW-BK132F, China) method was used to analyze the specific surface area and pore-size distribution. The morphology of the catalyst was investigated by field emission scanning electron microscope (SEM, Nova NanoSEM 230) and transmission electron microscope (TEM) and high resolution transmission electron microscope (HRTEM, FEI TECNAI G2 F20).
2.3 Electrochemical measurements
The electrochemical performance was evaluated on an electrochemical workstation (Zennnium, Zahner, Germany). The three-electrode system includes a platinum sheet (counter electrode), an Ag/AgCl in KCl saturated solution (reference electrode) and a glassy carbon electrode (working electrode with a diameter of 5 mm). The catalyst was prepared as follows: 6 mg as-prepared sample was sonicated in 1 ml isopropanol to a homogeneous solution. Then 20 μL 5% (, mass fraction) nafion solution was added with a following 30 min ultrasonic treatment. The Pt/C for comparison was prepared using the same process.
Cyclic voltammetrys were measured with potentials ranging from −1.2 to 0.2 V. In the roating disk electrode (RDE) tests, linear sweep voltammetry was performed from −1.0 to 0.2 V with a scan rate of 5 mV∙s−1. The chronoamperometric current test was evaluated at a constant potential (−0.3 V) at a rotating speed of 1600 r∙min−1. All of the electrochemical measurements were performed in 0.1 mol∙L−1KOH solutions with O2/N2saturated.
The electron transferred number () during oxygen reduction is calculated from Koutechy-Levich (K-L) equation as follows25,26:


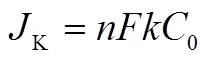
whereis the measured current density,Kis the kinetic current density.is the angular velocity (r∙min−1),is the electron transferred number of a single O2molecule, andis the Faraday constant (= 96485 C∙mol−1).0is the bulk concentration of O2in the 0.1 mol∙L−1KOH electrolyte (1.2 × 10−6mol∙cm3),0is the diffusion coefficient of O2in the 0.1 mol∙L−1KOH electrolytes (1.9 × 10−5cm2∙s−1),is the viscosity of 0.1 mol∙L−1KOH (0.01 m2∙s−1) andis the electron transfer rate constant.
3 Result and discussion
The precursors of all catalysts were fabricated at room temperature. Melamine and trithiocyanuric acid formed supramolecular structure by N―H…S and N…H hydrogen bond, and the cobalt nitrate was embedded in the supramolecular structure27. The morphology of MTC-0.1-900 was investigated by SEM and TEM. Fig.1(a) shows the SEM image of MTC-0.1-900. It can be observed that the nano-particles are distributed in the composite. TEM images (Fig.1(b, c)) are provided to better understand the morphology of the catalyst, where the graphite structure is clearly observed. Moreover, the Co@Co2C particles are encapsulated in graphitic carbon layer, which may due to that the melamine, serving as nitrogen and carbon source, converts into carbon during the pyrolysis progress21. The graphitic carbon layer is clearly observed which was formed during high temperature pyrolysis with the existence of cobalt (Fig.1(d)). Fig.1(e) shows the high-resolution TEM image of MTC-0.1-900 with an interlayer spacing of 0.34 nm18,28, which is consistent with the (002) diffraction peak of graphite. The lattice fringes of cobalt and cobalt carbide are also observed (Fig.1(f)), which correspond to the diffraction peaks of (200) and (210), respectively. The TEM images of MTC-0.1-850, MTC-0.15-900, MTC-0.05-900 and MTC-0.1-950 are shown in Fig.S1 (in Supporting Information). At the temperature of 850 °C, the graphite and irregular particles are found in the composite. When the temperature is up to 950 °C, the particles with minor diameters can be observed (Fig.S2(d), in Supporting Information). The aggregated nano-particles (Fig.S1(d), in Supporting Information) can be attributed to the high temperature of 950 °C during the pyrolysis. As for MTC-0.15-900, the particles with various diameters are also observed in Fig.S1(b) (in Supporting Information). However, almost no obvious particles can be observed in MTC-0.05-900 (Fig.S1(c), in Supporting Information) compared to MTC-0.15-900 (Fig.S1(b) in Supporting Information), which is attributed to the low content of cobalt nitrate in the precursor. Notably, it is noticed that the carbon flake in MTC-0.05-900 varies from the carbon ribbon in MTC-0.15-900. The different carbon structure is attributed to the different content of cobalt nitrate in the precursors. To better understand the catalysts structure, the samples were further investigated by HRTEM measurements. The HRTEM images of Fig.S2 (in Supporting Information) show that all the samples have the graphitic carbon shell structure. The thickness of the graphitic carbon shell is different, which could be related to the pyrolysis temperature and the content of cobalt nitrate in the precursor.
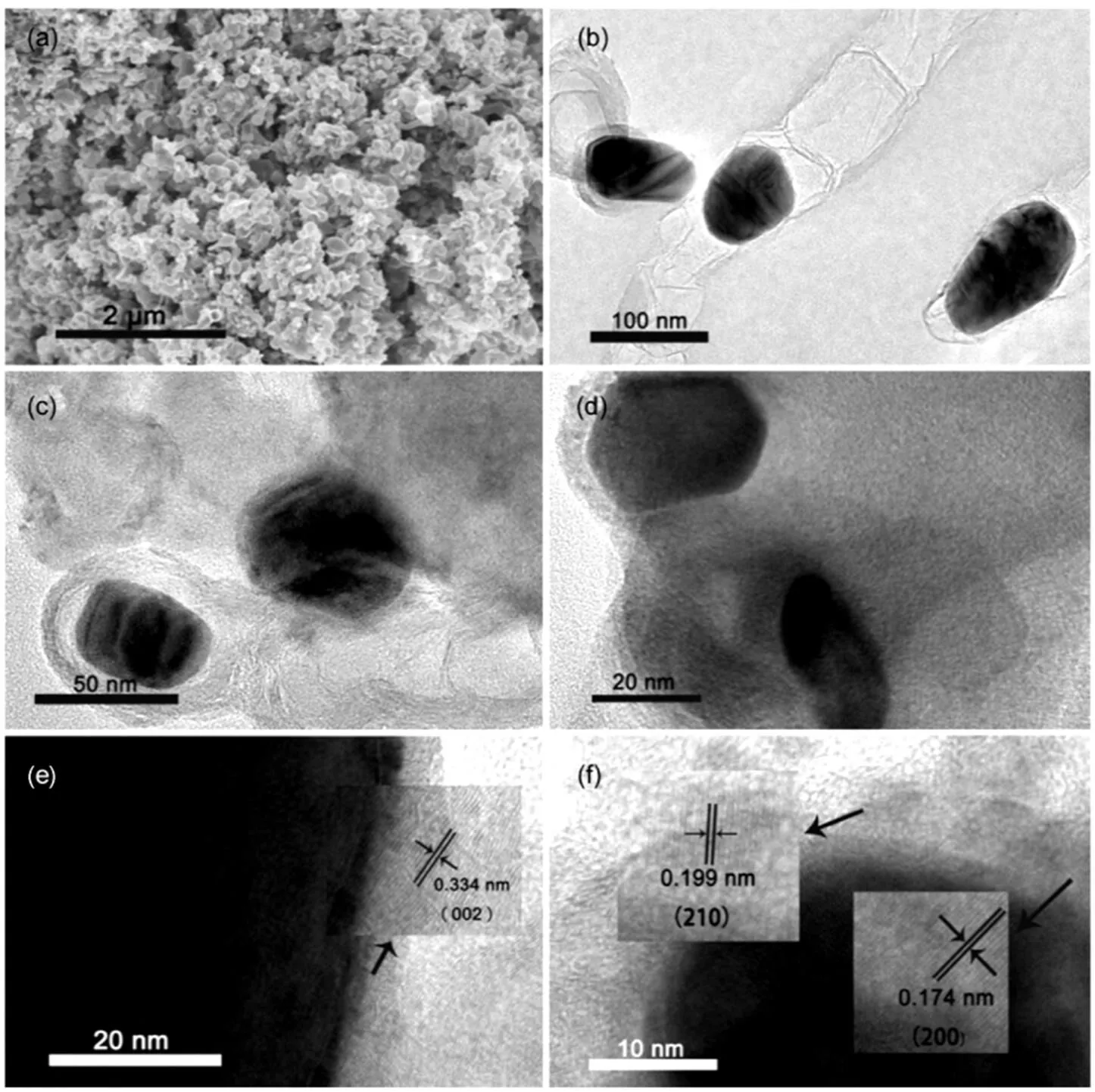
Fig.1 (a) SEM image of the MTC-0.1-900, (b, c) TEM image of the MTC-0.1-900, (d) TEM image of the MTC-0.1-900 with graphitic shell, (e, f) HRTEM image of MTC-0.1-900 (inset is the zoom-in of the HRTEM images).
The XRD patterns of MTC-0.1-900 and MC-0.1-900 are shown in Fig.2(a). A sharp peak corresponded to 26.5° is attributed to (002) peak of graphite, indicating the formation of carbon-based materials under 900 °C. The characteristic diffraction peaks of metallic Co (JCPDs No. 15-0806) are observed at 44.2°, 51.5° and 75.8°29, which are corresponded to (111), (200) and (220) crystal facets, respectively. The metallic Co can be obtained from the decomposed Co(NO3)2by the reductive carbon at 900 °C18,30,31. The rest peaks belong to the crystalline facets of Co2C (JCPDs No. 05-0704). The 2degrees appeare at 37.2°, 41.5°, 42.7°, 45.7°, 56.6°, 71.6°, 76.3° and 78.4° belong to the facets of (011), (020), (111), (210), (121), (301), (131) and (230), respectively. The XRD results also confirm the existence of graphite and Co and Co2C in the composite of MTC-0.1-900. The XRD patterns of other samples are also shown in Fig.S3(a) (in Supporting Information). The peaks of graphite and metallic cobalt are observed for the MTC-0.1-850 sample. There are no obvious peaks for Co2C in the composite of MTC-0.1-850. When the temperature is risen up to 900 or 950 °C, the peaks of Co2C are observed obviously. Thus, it can be concluded that the temperature plays an important role for the formation of Co2C. The influence of cobalt nitrate content in the precursor was also investigated for the formation of Co@Co2C. In MTC-0.05-900, there are just two peaks corresponded to (020) and (111) facets. Also, only three peaks of Co2C are observed in MTC-0.15-900. The few peaks of Co2C in MTC-0.05-900 and MTC-0.15-900 depend on the material content in the precursor. Thus, it can be concluded that the formation of Co2C is related to the temperature and cobalt nitrate content before pyrolysis.
Raman spectra were employed to investigate the structure and defects in MTC-0.1-900 and MC-0.1-900 as shown in Fig.2(b). Theband (1348 cm−1) corresponds to the defects of disordered carbons and theband (1582 cm−1) originates from the graphitic structure at high temperature20. The intensity ratio ofband andband is also implied to verify the defects in carbon-based materials. The intensity ratio value of MTC-0.1-900 (I/I= 0.96) is slightly higher than MC-0.1-900 (I/I= 0.95), indicating more defects are introduced with the sulfur doping. And the appearance of 2band is also attributed to the introduction of sulfur atoms, which cause more defects32. The MTC-0.1-900 shows nitrogen adsorption-desorption isotherms of type IV in Fig.2(c). The BET surface area of MTC-0.1-900 is 103.5 m2·g−1, which is larger than MC-0.1-900 (91.3 m2·g−1). With relative higher specific surface area, more active sites will be exposed for ORR33. Obviously, it can be observed that the pore-size distributions of MC-0.1-900 mainly focus on 3.96 nm and the pores of MTC-0.1-900 distribute at 3.8 and 5.6 nm (Fig.2(d)). The higher BET surface area of MTC-0.1-900 can provide more active sites for the electrolyte and oxygen. Moreover, the high surface area also accelerates transportation of ORR-relevant species of the catalyst34,35.
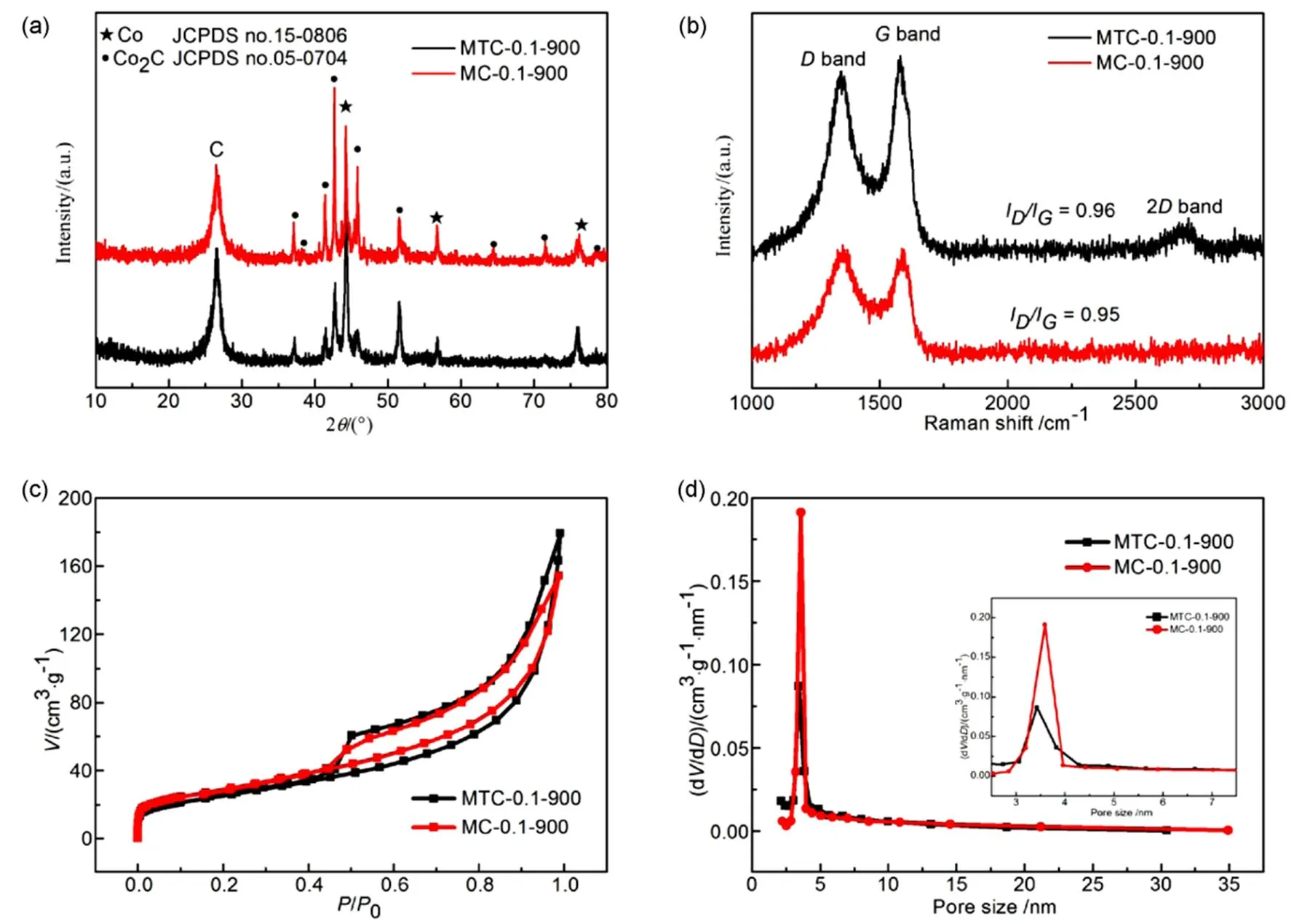
Fig.2 (a) XRD pattern of MTC-0.1-900 and MC-0.1-900, (b) Raman spectra of MTC-0.1-900 and MC-0.1-900, (c) N2 adsorption-desorption isotherms of MTC-0.1-900 and MC-0.1-900, (d) pore size distribution of MTC-0.1-900 and MC-0.1-900 (inset is the zoom-in of the pore size distribution).
The XPS measurement was employed to reveal the presence of sulfur, carbon, nitrogen and cobalt, as shown in Figs.3, 4(a) and Table S1. It should be noticed that the mere presence of S 2in Fig.3(a) can be divided in two peaks corresponding to 163.1 and 168.9 eV. The peak at 168.9 eV is assigned to the carbon bonded sulfur oxide groups of C-SO-C and the peak at 163.1 eV is assigned to the elemental sulfur bonded carbon atoms of MTC-0.1-90036. The presence of carbon, sulfur and cobalt is further proved by energy dispersive spectrometer (EDS) and elemental mapping in Fig.4(b–f). The corresponding elemental mapping of S in Fig.4(f) with a content of 0.1% (atomic fraction), matching the result of XPS (0.07%, atomic fraction). Fig.3(b) displays the C 1spectrum, which is fitted up with three peaks of C=C (284.7 eV), C―N (285.6 eV) and C―O (287.0 eV)37,38. The N 1spectrum is divided into three peaks of 398.7, 401.1 and 403.9 eV, which correspond to the N species of pyridinic N, graphitic N and pyridinic N oxide, respectively39–43. It can be concluded that the successful doping of S and N in graphite. The Co 2spectrum is also shown in Fig.3(d). Five peaks are obviously observed at 803.3, 796.6, 786.9, 781.3 and 778.4 eV. The peaks at 781.3 and 796.6 eV can be attributed to the Co 23/2and Co 21/244,45. And the other two peaks of 803.3 and 786.9 eV are two shake-up peaks of the Co 23/2and Co 21/246,47. The four peaks indicate the existence of Co (II). An extra peak corresponds at 778.4 eV is attributed to the metallic cobalt48. The XPS spectra of MTC-0.1-850, MTC-0.1-950, MTC-0.05-900 and MTC-0.15-900 are also shown in Fig.S3(b) (in Supporting Information). It can be observed that the presence of sulfur, carbon, nitrogen and cobalt, which are similar to the elemental composition of MTC-0.1-900. The high resolution of Co 2from different samples is shown in Fig.S4 (in Supporting Information). The presence of Co(II) and metallic cobalt is also proved with different content as shown in Fig.S4 and Table S2 (in Supporting Information).
Cyclic voltammetrys (CVs) are firstly used to investigate the ORR performance of Pt/C, MTC-0.1-900, MTC-0.1-850, MTC-0.1-950, MTC-0.05-900, MTC-0.15-900 and MC-0.1-900 in O2saturated 0.1 mol∙L−1KOH with a scan rate of 50 mV·s−1. In Fig.5(a), the ORR peak of MTC-0.1-900 situates at −0.153 V, which is positive than those of MTC-0.1-850 (−0.155 V), MTC-0.1-950 (−0.183 V), MTC-0.05-900 (−0.169 V), MTC-0.15-900 (−0.169 V), MC-0.1-900 (−0.181 V) and Pt/C (−0.160 V). Without obvious oxygen reduction peak can be found when the alkaline solution is saturated with N2(Fig.7(a)), which is perceptible different from CVs with O2saturated.

Fig.3 XPS spectra of MTC-0.1-900, (a) high resolution of S 2p region, (b) C 1s region, (c) N 1s region and (d) Co 2p region.
RDE measurements are carried out to further assess the ORR property of all catalysts with a scan rate of 5 mV·s−1. The LSV curves of MTC-0.1-900 at the rotation speeds (400, 625, 900, 1225 and 1600 r·min−1) are shown in Fig.5(c). The diffusion current densities increase as the rotation speed increase. The LSVs of Pt/C, MTC-0.1-900, MTC-0.1-850, MTC-0.1-950, MTC-0.05-900, MTC-0.15-900 and MC-0.1-900 at 1600 r·min−1are also shown in Fig.5(b). The MTC-0.1-900 displays more positive half-wave potential (1/2, −0.132 V) in comparison with Pt/C (−0.137 V), MTC-0.1-850 (−0.138 V), MTC-0.1-950 (−0.147 V), MTC-0.05-900 (−0.138 V), MTC-0.15-900 (−0.138 V) and MC-0.1-900 (−0.168 V), which reveal that the MTC-0.1-900 exhibits the highest ORR performance. The different ORR performances of all catalysts are attributed to the components in the composites. The MTC-0.1-900 with Co@Co2C structure shows better ORR performance than other catalysts. Besides, the onset potential of MTC-0. 1-900 (0.12 V) is also more positive than those of MC-0.1-900 (0.069 V) and Pt/C (0.091 V). The Kouteckye-Levich plots (Fig.6(c)) are collected and calculated according to Fig.6(a). After subtracting the background current (Fig.6(b)), the retained Kouteckye-Levich plots are collected and shown in Fig.6(d) with linear plots. Electron transfer number () is another factor to evaluate the ORR performance. The valueofis calculated after subducting the background current, which varies from 3.74 to 3.87, indicating a close 4 e−transfer process. The Tafel slopes of different samples are also shown in Fig.5(d). It can be calculated that the Tafel slope of MTC-0.1-900 (−82.9 mV·dec−1) is lower than that of Pt/C (−112.9 mV·dec−1). The smaller Tafel slope indicates a faster dynamic reaction with higher ORR activity at low overpotential18,49.
The excellent ORR performance of MTC-0.15-900 is attributed to the aspects as follows. Firstly, the nitrogen and sulfur atoms result in the redistribution of spin and charge densities50, leading to more carbon atoms active sites for ORR in the catalysts. Another reason is the existence of Co@Co2C, which activate the graphitic carbon layers for enhanced ORR performance. Meanwhile, the synergistic effect in graphite, cobalt and cobalt carbide is another reason for the enhancement of ORR activity. The third reason is that the structure with a carbon shell exhibits electronic interactions in the graphite and graphitic carbon encapsulated structure51,52. And the graphitic carbon layers prevent Co@Co2C away from the oxygen and electrolyte. Therefore, the MTC-0.1-900 shows excellent ORR activity.
In order to further explore the main role for the higher ORR activity. The MTC-0.1-900 was etched in 3 mol∙L−1HCl for 3 days to reduce the content of the Co@Co2C in MTC-0.1-900.There is almost no change about current density before and after etching in acid. But the half-wave potential of MTC-0.1-900 shifts to −0.144 V (Ag/AgCl) after etching, which is negative than that of MTC-0.1-900 (−0.132 V) (Fig.S5) (in Supporting Information). The negative shift of half-wave potential indicates that the part removal of Co@Co2C lowers the ORR activity. Since the Co@Co2C can not be absolutely removed and the nitrogen, sulfur co-doped carbon can not be directly synthesized, it is difficult to realize the dominant contribution of nitrogen and sulfur co-doped carbon and Co@Co2C in the MTC-0.1-900 for ORR. It is concluded that the synergistic effect in nitrogen, sulfur co-doped carbon and Co@Co2C leads a higher ORR performance than Pt/C in 0.1 mol·L−1KOH solutions.
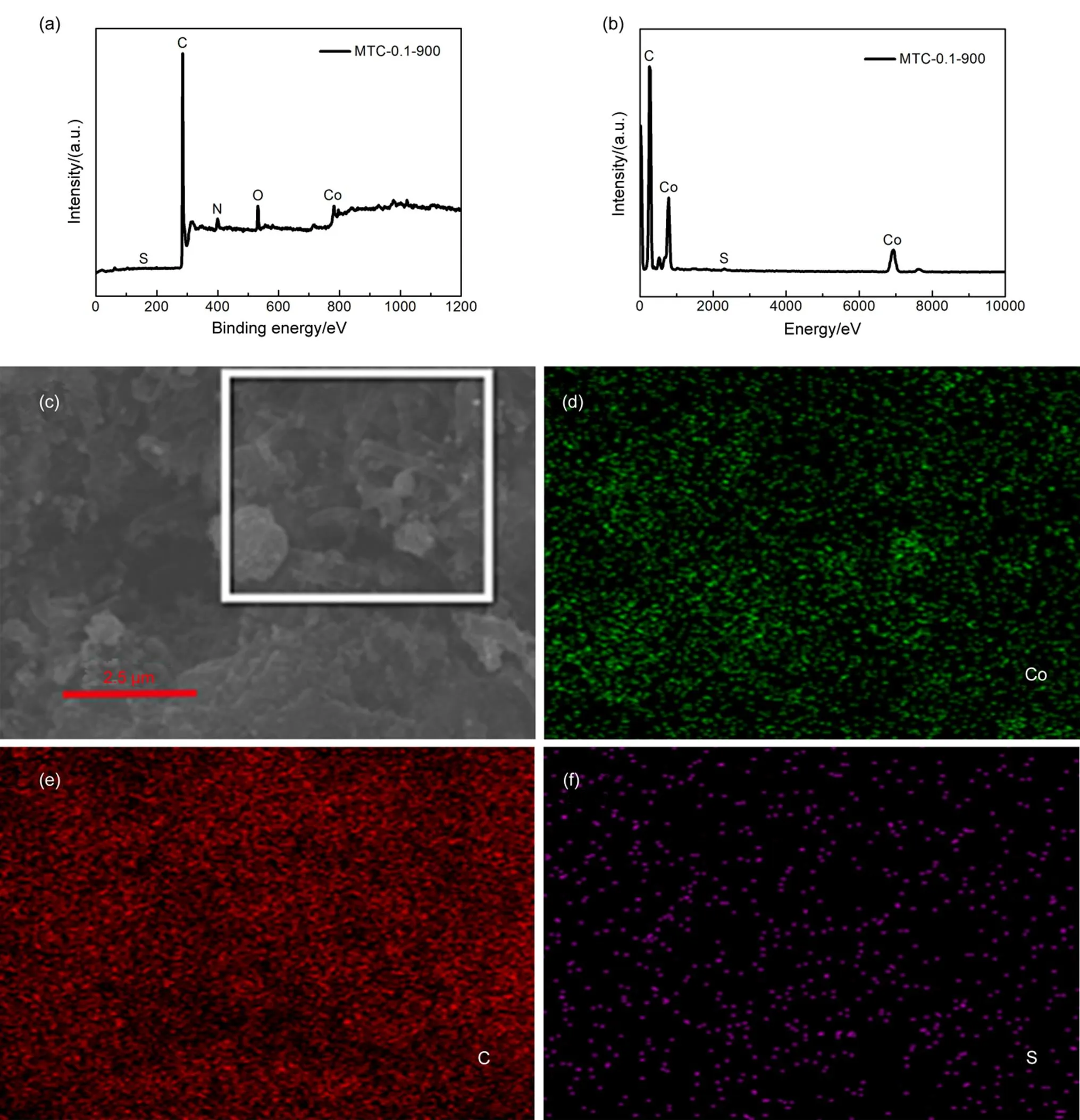
Fig.4 (a) XPS spectra of MTC-0.1-900, (b) energy dispersive spectrometer analysis, (c−f) the corresponding elemental mappings of MTC-0.1-900.
Durability of MTC-0.1-900 and Pt/C were also evaluated by chronoamperometric current () measurements at a rotation speed of 1600 r·min−1(Fig.7(b)). After operation at −0.3 V for 12000 s, there is only 2.9% current decay for MTC-0.1-900, which is more stable in comparison with Pt/C (23.3% decay after 12000 s operation). The methanol tolerance of MTC-0.1-900 and Pt/C catalyst was also investigated in the presence of 3 mol∙L−1methanol. There is almost no change about the current density for MTC-0.1-900 with the addition of methanol (Fig.8). Unlike MTC-0.1-900, the Pt/C obviously decreases after the addition of methanol at 300 s. It can be concluded that the MTC-0.1-900 shows better methanol tolerance compared to Pt/C. The high stability is resulted from the graphite based materials with graphitic carbon encapsulated structure, preventing the catalyst directly contact with the electrolyte and O224. The results indicate that the MTC-0.1-900 is more stable than Pt/C catalyst in alkaline solutions.

Fig.5 (a) CV curves of Pt/C and all as-prepared samples at the scan rate of 50 mV·s−1 in O2 saturated 0.1 mol∙L−1 KOH, (b) LSV curves of Pt/C and all as-prepared catalysts (inset is the zoom-in of the LSV curves) at the scan rate of 5 mV·s−1 (1600 r·min−1) in O2 saturated 0.1 mol∙L−1 KOH, (c) LSV curves of MTC-0.1-900 at different rotation rates in O2 saturated 0.1 mol∙L−1 KOH, (d) Tafel slopes of Pt/C and all as-prepared catalysts.

Fig.6 (a) LSV curves of MTC-0.1-900 after subducting background current , (b) LSV curves of MTC-0.1-900 at different rotation speeds in N2 saturated 0.1 mol∙L−1 KOH (background current), (c) K-L plots of MTC-0.1-900 before subducting background current, (d) K-L plots of MTC-0.1-900 after subducting background current.

Fig.7 (a) CV curves of MTC-0.1-900 in N2 or O2 saturated 0.1 mol∙L−1 KOH, (b) chronoamperometric responses of Pt/C and MTC-0.1-900 at −0.3 V (1600 r·min−1).
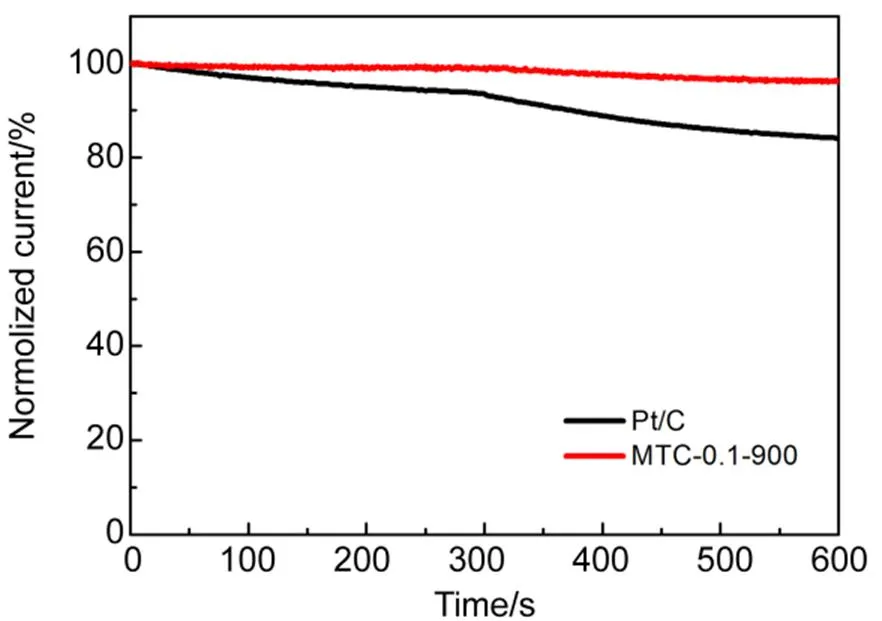
Fig.8 Chronoamperometric responses of MTC-0.1-900 and Pt/C upon addition of 3 mol∙L−1 methanol into O2-saturated 0.1 mol∙L−1 KOH at −0.3 V (1600 r·min−1).
4 Conclusions
To summarize, we have developed a facile method to fabricate a catalyst which shows high ORR activity with graphitic carbon encapsulated Co@Co2C core-shell structure. Graphitic carbon, cobalt and cobalt carbide structure are formed during the pyrolysis progress. And the existence of cobalt nitrate in the precursor facilitates the formation of carbon and graphitic carbon core-shell structure under high temperature. RDE measurements illustrate a 4e−transferred progress in 0.1 mol∙L−1KOH solutions. The nitrogen and sulfur atoms result in the redistribution of spin and charge densities, leading to more carbon atoms active sites for ORR in the catalysts. In addition, the high durability and methanol tolerance are attributed to the carbon shell, which prevents the catalyst directly contact with the electrolyte and O2. This research opens a facile and commercial strategy for large-scale production of ORR electrocatalysts in the new energy devices.
Supporting Information: available free of chargethe internet at http://www.whxb.pku.edu.cn.
(1) Cui, Y.; Kuang, Y. J.; Zhang, X. H.; Liu, B.; Chen, J. H.2013,(5), 989. [崔 颖, 匡尹杰, 张小华, 刘 博, 陈金华. 物理化学学报, 2013,(5), 989.] doi: 10.3866/PKU.WHXB201303121
(2) Du, C.; Gao, X.; Chen, W.2016,(7), 1049. doi: 10.1016/S1872-2067(15)61059-2
(3) Adina, M.; Bruno, J.; Serge, P.2011,(4), 1238. doi: 10.1039/c0ee00601g
(4) Melke, J.; Peter, B.; Habereder, A.; Ziegler, J.; Fasel, C.; Nefedov, A.; Sezen, H.; Woll, C.; Ehrenberg, H.; Roth, C.2016,(1), 82. doi: 10.1021/acsami.5b06225
(5) Zhu, Z.; Zhai, Y.; Dong, S.2014,(19), 16721. doi: 10.1021/am503689t
(6) Kuttiyiel, K. A.; Choi, Y. M.; Hwang, S. M.; Park, G. G.; Yang, T. H.; Su, D.; Sasaki, K.; Liu, P.; Adzic, R. R.2015,,442. doi: 10.1016/j.nanoen.2015.03.007
(7) Lee, S.; Kwak, D. H.; Han, S. B.; Hwang, E. T.; Kim, M. C.; Lee, J. Y.; Lee, Y. W.; Park, K. W.2016,,805. doi: 10.1016/j.electacta.2016.01.135
(8) Xiao, M.; Zhu, J.; Feng, L.; Liu, C.; Xing, W.2015,(15), 2521. doi: 10.1002/adma.201500262
(9) Gu, L.; Jiang, L.; Jin, J.; Liu, J.; Sun, G.2015,,572. doi: 10.1016/j.carbon.2014.11.010
(10) Liu, M.; Zhang, R.; Chen, W.2014,(10), 5117. doi: 10.1021/cr400523y
(11) Du, R.; Zhang, N.; Zhu, J.; Wang, Y.; Xu, C.; Hu, Y.; Mao, N.; Xu, H.; Duan, W.; Zhuang, L.; Qu, L.; Hou, Y.; Zhang, J.2015,(32), 3903. doi: 10.1002/smll.201500587
(12) Zheng, Y.; Jiao, Y.; Chen, J.; Liu, J.; Liang, J.; Du, A.; Zhang, W.; Zhu, Z.; Smith, S. C.; Jaroniec, M., LU; Qing, G.; Qiao, S.2011,(50), 20116. doi: 10.1021/ja209206c
(13) Qu, K.; Zheng, Y.; Dai, S.; Qiao, S.2016,,373. doi: 10.1016/j.nanoen.2015.11.027
(14) Gong, Y.; Fei, H.; Zou, X.; Zhou, W.; Yang, S.; Ye, G.; Liu, Z.; Peng, Z.; Lou, J.; Vajtai, R.; Yakobson, B. I.; Tour, J.; Ajayan, P. M.2015,(4), 1181. doi: 10.1021/cm5037502
(15) Gong, K.; Du, F.; Xia, Z.; DurstocK, M.; Dai, L.2009,(5915), 760. doi: 10.1126/science.1168049
(16) Wu, H.; Shi, L.; Lei, J.; Liu, D.; Qu, D.; Xie, Z.; Du, X.; Yang, P.; Hu, X.; Li, J.; Tang, H.2016,,90. doi: 10.1016/j.jpowsour.2016.05.044
(17) Dou, S.; Shen, A.; Ma, Z.; Wu, J.; Tao, L.; Wang, S.2015,,21. doi: 10.1016/j.jelechem.2015.05.013
(18) Zhang, G.; Lu, W.; Cao, F.; Xiao, Z.; Zheng, X.2016,,114. doi: 10.1016/j.jpowsour.2015.10.055
(19) Wen, Z.; Ci, S.; Zhang, F.; Feng, X.; Cui, S.; Mao, S.; Luo, S.; He, Z.; Chen, J.2012,(11), 1399. doi: 10.1002/adma.201104392
(20) Xia, W.; Zou, R.; An, L.; Xia, D.; Guo, S.2015,(2), 568. doi: 10.1039/C4EE02281E
(21) Yu, J.; Chen, G.; Sunarso, J.; Zhu, Y.; Ran, R.; Zhu, Z.; Zhou, Z.2016,(9), 1600060. doi: 10.1002/advs.201600060
(22) Deng, D.; Yu, L.; Chen, X.; Wang, G.; Jin, L.; Pan, X.; Sun, J.; Deng, G.; Bao, X.2013,(1), 371. doi: 10.1002/anie.201204958
(23) Wang, Y.; Wang, X.; Antonietti, M.2012,(1), 68. doi: 10.1002/anie.201101182
(24) Lee, J.; Park, G.; Kim, S.; Liu, M.; Cho, J.2013,(3), 1060. doi: 10.1002/ange.201207193
(25) Wang, Y.; Liu, Q.; Zhang, L.; Hu, T.; Liu, W.; Liu, N.; Du, F.; Li, Q.; Wang, Y.2016,(47), 22547. doi: 10.1016/j.ijhydene.2016.05.287
(26) Ratha, S.; Samantara, A. K.; Rout, C. S.; Jena, B. K.2015,(1), 285. doi: 10.1007/s10008-015-3035-0
(27) Yang, Z.; Lin, L.; Liu, Y.; Zhou, X.; Yuan, C.; Xu, A.2016,(58), 52937. doi: 10.1039/C6RA05523K
(28) Kong, A.; Mao, C.; Lin, Q.; Wei, X.; Bu, X.; Feng, P.2015,(15), 6748. doi: 10.1039/c4dt03726j
(29) Hu, H.; Han, L.; Yu, M.; Wang, Z.; Lou, X.2016,(1), 107. doi: 10.1039/c5ee02903a
(30) Wu, Z.; Chen, P.; Wu, Q.; Yang, L.; Pan, Z.; Wang, Q.2014,,118. doi: 10.1016/j.nanoen.2014.05.019
(31) Han, C.; Bo, X.; Zhang, Y.; Li, M.; Nsabimana, A.; Guo, L.2015,(13), 5607. doi: 10.1039/C4NR07571D
(32) Qiao, X.; Liao, S.; Wang, G.; Zheng, R.; Song, H.; Li, X.2016,,272. doi: 10.1016/j.carbon.2015.12.034
(33) Zhong, H.; Wang, J.; Zhang, Y.; Xu, W.; Xing, W.; Xu, D.; Zhang, Y.; Zhang, X.2014,(51), 14235. doi: 10.1002/anie.201408990
(34) Wu, Z.; Xu, X.; Hu, B.; Liang, H.; Lin, Y.; Chen, L.; Yu, S.2015,(28), 8179. doi: 10.1002/anie.201502173
(35) Jin, H.; Zhang, H.; Zhong, H.; Zhang, J.2011,(9), 3389. doi: 10.1039/C1EE01437D
(36) Yazdi, A. Z.; Roberts, E. P. L.; Sundararaj, U.2016,,99. doi: 10.1016/j.carbon.2015.12.096
(37) Zhang, R.; Chen, W.2015,(5), 522. doi: 10.1007/s11434-015-0740-0
(38) Gao, X.; Lu, Y.; Zhang, R.; He, S.; Ju, J.; Liu, M.; Li, L.; Chen, W.2015,(10), 2302. doi: 10.1039/C4TC02582B
(39) Tian, C. X.; Yang, J.; Li, L.; Zhang, X. H.; Chen, J. H.2016,(6), 1473. [田春霞, 杨军帅, 李 丽, 张小华, 陈金华. 物理化学学报, 2016,(6), 1473.] doi: 10.3866/PKU.WHXB201603112
(40) Zhang, X. H.; Zhong, J. D.; Yu, Y. M.; Zhang, Y. S.; Liu, B.; Chen, J. H.2013,(6), 1297. [张小华, 钟金娣, 于亚明, 张云松, 刘 博, 陈金华. 物理化学学报, 2013,(6), 1297.] doi: 10.3866/PKU.WHXB201304011
(41) Liu, Y.; Li, J.; Li, W.; Li, Y.; Chen, Q.; Zhan, F.2015,,492. doi: 10.1016/j.jpowsour.2015.09.042
(42) Sun, T.; Xu, L.; Li, S.; Chai, W.; Huang, Y.; Yan, Y.; Chen, J.2016,, 1. doi: 10.1016/j.apcatb.2016.04.006
(43) Zhang, R.; Zhang, C.; Chen, W.2016,(48), 18723. doi: 10.1039/C6TA08363C
(44) Sun, H.; Sun, X.; Hu, T.; Yu, M.; Lu, F.; Lian, J.2014,(5), 2263. doi: 10.1021/jp408021m
(45) Li, J.; Zhou, Z.; Liu, K.; Li, F.; Peng, Z.; Tang, Y.; Wang, H.2017,,30. doi: 10.1016/j.jpowsour.2017.01.018
(46) Ji, G. B.; Tang, S. L.; Ren, S. K.; Zhang, F. M.; Gu, B. X.; Du, Y. W.2004,(1−2), 156. doi: 10.1016/j.jcrysgro.2004.06.025
(47) Yuan, H.; Wang, Y.; Zhou, S.; Liu, L.; Chen, X.; Lou, S.; Yuan, R.; Hao, Y.; Li, N.2010,(11), 1817. doi: 10.1007/s11671-010-9718-7
(48) Khassin, A. A.; Yurieva, T. M.; Kaichev, V. V.; Bukhtiyarov, V. I.; Budneva, A. A.; Paukshtis, E. A.; Parmon, V. N.2001,(1), 189. doi: 10.1016/S1381-1169(01)00216-3
(49) Luo, Z.; Irtem, E.; Ibáñez, M.; Nafria, R.; Martı́-Sánchez, S.; GenÇ, A.; De, L. M. M.; Liu, Y.; Cadavid, D.; Llorca, J.2016,(27), 17435. doi: 10.1021/acsami.6b02786
(50) Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S. Z.2012,(46), 11640. doi: 10.1002/anie.201206720
(51) Wu, Z.; Wang, J.; Han, L.; Lin, R.; Liu, H.; Xin, H. L.; Wang, D.2016,(8), 4681. doi: 10.1039/c5nr07929b
(52) Zhang, S.; Zhang, H.; Liu, Q.; Chen, S.2013,(10), 3302. doi: 10.1039/c2ta01351g
氮、硫共掺杂的碳负载的钴@碳化钴:一种高效的非贵金属氧还原电催化剂
申海波1江 浩1刘易斯1,3郝佳瑜1李文章1,2,4,*李 洁1,*
(1中南大学化学化工学院,长沙 410083;2中南大学湖南省锰资源高效清洁利用重点实验室,长沙 410083;3加拿大西安大略大学机械与材料工程系,安大略 N6A5B9;4有色金属成矿预测与地质环境监测教育部重点实验室(中南大学),长沙 410083)
规模化生产低成本和高效率的氧还原电催化剂在当今仍然是严峻的挑战。本文以三聚氰胺、三聚硫氰酸和硝酸钴作为原料,通过两步热解合成了一种新的氮、硫共掺杂的碳负载的钴@碳化钴(标记为MTC-0.1-900)氧还原催化剂。利用扫描电子显微镜、透射电子显微镜、X射线衍射、拉曼光谱、比表面分析和X射线光电子能谱分析对该催化剂进行了表征,并采用循环伏安和线性扫描伏安曲线等方法测试其在0.1 mol∙L−1KOH中的氧还原性能。结果显示,与商业Pt/C催化剂相比,MTC-0.1-900的起始电位和半波电位分别高出了29和5 mV。在−0.3 V (Ag/AgCl)电位下工作12000 s后,MTC-0.1-900催化剂的电流可达到起始电流的97.1%,高于Pt/C催化剂的76.7%,显示出该催化剂具有更稳定的性能。抗甲醇实验表明,MTC-0.1-900的对氧还原的选择性也要优于Pt/C。该催化剂优良的性能为金属空气电池阴极材料提供了一种减少或者取代Pt的新选择。
氮、硫共掺杂;钴@碳化钴;石墨化碳;核壳结构;氧还原
O646
10.3866/PKU.WHXB201704264
February 20, 2017;
April 15, 2017;
April 26, 2017.
LI Wen-Zhang, Email: liwenzhang@csu.edu.cn. LI Jie, Email: lijieliu@csu.edu.cn; Tel./Fax: +86-731-88879616.
The project was supported by the National Nature Science Foundation of China (51474255), the Hunan Provincial Science and Technology Plan Project, China (2016TP1007), the Open Research Fund Program of Key Laboratory of Metallogenic Prediction of Nonferrous Metals and Geological Environment Monitoring, China (Central South University), Ministry of Education, the Fundamental Research Funds for the Central Universities of Central South University, China.
国家自然科学基金(51474255), 湖南省科技计划项目(2016TP1007), 中南大学有色金属成矿预测与地质环境监测教育部重点实验室开放基金和中南大学中央高校基本科研业务费专项资金资助
猜你喜欢
杂志排行
物理化学学报的其它文章
- Development and Validation of a Reduced Chemical Kinetic Mechanism for HCCI Engine of Biodiesel Surrogate
- Efficient Synthesis of Sulfur and Nitrogen Co-Doped Porous Carbon by Microwave-Assisted Pyrolysis of Ionic Liquid
- OL负载Cu催化剂及其催化氧化CO及乙酸乙酯性能
- Combined Effects of the Hole and Twin Boundary on the Deformation of Ag Nanowires: a Molecular Dynamics Simulation Study
- First-Principles Study: the Structural Stability and Sulfur Anion Redox of Li1−xNiO2−ySy
- S掺杂促进Fe/N/C催化剂氧还原活性的实验与理论研究
