瑞巴派特对双抗致大鼠胃黏膜损伤的保护作用及机制
2017-12-28刘晨晨徐兆军韩维维张振玉王劲松黄文斌
刘晨晨,李 超,徐兆军,韩维维,张振玉,王劲松,黄文斌
1.南京医科大学附属南京医院(南京市第一医院)消化科,江苏 南京 210006; 2.济宁市第一人民医院消化科;3.南京医科大学附属南京医院(南京市第一医院)病理科
瑞巴派特对双抗致大鼠胃黏膜损伤的保护作用及机制
刘晨晨1,2,李 超1,徐兆军1,韩维维1,张振玉1,王劲松3,黄文斌3
1.南京医科大学附属南京医院(南京市第一医院)消化科,江苏 南京 210006; 2.济宁市第一人民医院消化科;3.南京医科大学附属南京医院(南京市第一医院)病理科
目的探讨瑞巴派特对阿司匹林联合氯吡格雷(双抗)引起的胃黏膜损伤的影响及机制。方法将30只雄性SD大鼠随机分为对照组、双抗损伤组和瑞巴派特保护组。双抗损伤组大鼠给予阿司匹林(10.41 mg·kg-1·d-1)联合氯吡格雷(7.81 mg·kg-1·d-1)灌胃,瑞巴派特保护组大鼠在每次行双抗灌胃前1 h用瑞巴派特100 mg·kg-1·d-1)对大鼠进行灌胃预处理,对照组给予等量的生理盐水灌胃,连续14 d;实验第15天处死所有大鼠,评估胃黏膜大体及病理损伤情况,应用免疫组化法检测胃黏膜中VEGF的表达,应用Western blotting检测胃黏膜中EGF的表达,应用TUNEL法检测胃黏膜中细胞的凋亡。结果与对照组相比,双抗损伤组大鼠胃黏膜大体及病理损伤明显增加,胃黏膜中EGF、VEGF表达明显减少(P<0.05),胃黏膜细胞凋亡显著增加(P<0.05);与双抗损伤组相比,瑞巴派特保护组胃黏膜损伤明显减轻,胃黏膜中EGF、VEGF表达明显增加(P<0.05),胃黏膜细胞凋亡显著降低(P<0.05)。结论瑞巴派特对双抗引起的胃黏膜损伤有一定的保护作用,其机制可能通过增加胃黏膜中EGF、VEGF的表达、降低细胞凋亡而促进损伤黏膜的修复。
瑞巴派特;阿司匹林;氯吡格雷;胃黏膜损伤;细胞凋亡
抗血小板药物在治疗缺血性心脑血管疾病中起重要作用,目前已经成为治疗心脑血管疾病的基础用药。氯吡格雷作为新型噻吩吡啶类药物,是一种二磷酸腺苷受体拮抗剂,具有较好的抗血小板功效,可显著减少各类心脑血管疾病缺血事件的发生[1-2]。临床中氯吡格雷与阿司匹林合用即双抗,用于防治经皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)患者术后支架内血栓形成和减少缺血事件已经成为PCI术后的标准治疗方案。而临床研究显示,长期单独服用小剂量氯吡格雷(75 mg/d)出现胃肠道不良反应的风险与小剂量阿司匹林(100 mg/d)相似[3], 而二者联用引起胃肠道损伤的危险更大,出现严重上消化道出血的风险增加7.4倍[4-5]。本实验旨在研究瑞巴派特对大鼠阿司匹林联合氯吡格雷引起的胃黏膜损伤影响,并探讨其潜在的机制。
1 材料与方法
1.1实验动物雄性Sprague-Dawley大鼠30只,2个月龄,体质量180~220 g,由南京医科大学附属南京医院动物实验中心提供,动物使用许可证号:SYXK(苏)2009-0015,饲养于南京医科大学附属南京医院动物实验中心:温度(24±2)℃,湿度(55±5)%,噪音<50 db,12 h一次白天-黑夜循环,平衡饲料分笼饲养,每笼6只,自由饮水。
1.2药物与试剂瑞巴派特购自湖北康宝泰精细化工有限公司,阿司匹林原料药购自山东新华制药股份有限公司,硫酸氯吡格雷购自北京诺德恒信化工技术有限公司,以上药物均溶于生理盐水中,经过超声乳化后制成悬浊液;TUNEL凋亡检测试剂盒购自德国Roche公司;VEGF、EGF抗体均购自Santa Cruz公司。
1.3实验方法
1.3.1 动物分组:将30只大鼠随机分为3组:对照组、双抗损伤组、瑞巴派特保护组,每组10只。大鼠适应性观察1周后开始实验,双抗损伤组给予阿司匹林(10.41 mg·kg-1·d-1)联合氯吡格雷(7.81 mg·kg-1·d-1)灌胃(灌胃剂量参照人类长期口服剂量折算成大鼠口服剂量),瑞巴派特保护组先给予瑞巴派特灌胃进行预处理,1 h后再给予双抗进行灌胃,对照组大鼠给予等量的生理盐水进行灌胃,每日清晨1次,持续14 d;除禁食期间,大鼠均给予正常饮食。所有大鼠于末次灌胃后,禁食不禁水18 h后在质量浓度为100 g/L的水合氯醛腹腔麻醉下处死,取出胃组织,观察胃黏膜损伤情况,采集标本,进行各项指标的检测。
1.3.2 标本处理:打开腹腔分离胃周围的血管及组织,在食管末端及十二指肠上端将胃结扎后取出,之后沿胃大弯切开,用冷生理盐水冲洗并用滤纸擦干,观察胃黏膜损伤情况,留取大体照片;将一部分胃黏膜用质量浓度为40 g/L的甲醛溶液固定后行石蜡包埋切片、HE染色;将另一部分胃黏膜组织冻存于-80 ℃保存待用。
1.3.3 胃黏膜损伤指数判定:根据GUTH等[6]标准累积计算损伤指数(lesion index,LI):斑点样损伤,1分;损伤直径(长度)<1 mm,2分;损伤直径(长度)1~2 mm,3分;损伤直径(长度)2~4 mm,4分;损伤直径(长度)>4 mm,5分;宽度>2 mm的损伤积分值×2。
1.3.4 病理学观察:胃组织经质量浓度为40 g/L中性甲醛固定24 h,经常规脱水、透明、石蜡包埋切片,HE染色,光镜下进行观察;光镜下胃黏膜损伤的程度判断[7],按照黏膜损伤的程度分为0~Ⅳ级,0级:黏膜组织完好无缺损,偶见极少数上皮细胞脱落;Ι级:黏膜上皮细胞损伤,胞质有空泡,肿胀,核固缩或细胞破碎,有部分上皮细胞脱落,但胃小凹无损伤;Ⅱ级,除了黏膜上皮细胞广泛损伤外,胃小凹也有破坏,损伤区附件可有毛细血管充血,但胃腺细胞无损伤;Ⅲ级,胃腺细胞损伤,可见腺细胞的胞质有空泡,核固缩,或有整层的坏死上皮细胞脱落,使固有层和腺细胞直接暴露于胃腔,并可见轻微出血;Ⅳ级,损伤深入腺体,并有部分腺体坏死脱落,整个胃黏膜广泛出血。
1.3.5 原位末端标记(TUNEL)法检测胃黏膜上皮细胞凋亡:石蜡切片经常规脱蜡、水化,蛋白酶K、37 ℃消化30 min,质量浓度为3 g/L的H2O2-甲醇溶液室温下作用10 min,质量浓度为1 g/L的Triton X-100,冰上作用2 min,滴加50 μl反应混合液,37 ℃孵育60 min (此时可在荧光显微镜下观察,拍照记录),滴加50 μl过氧化物酶(POD),37 ℃作用30 min,二氨基联苯胺(DAB)室温显色10 min,常规脱水、透明、中性树胶封片。每例观察5个视野共500个细胞核,以每百个有核细胞中的阳性细胞数比例作为凋亡指数,TUNEL染色阳性物质显示为棕黄色[8]。
1.3.6 VEGF免疫组化染色:采用Envision法,石蜡切片脱蜡至水,蛋白酶E 37 ℃ 10 min消化,PBS冲洗,加VEGF抗体4 ℃过夜,PBS冲洗,加Envision二抗37 ℃ 30 min,PBS冲洗,DAB显色,镜下控制显色时间,苏木精复染、脱水、透明、树胶封片。染色的结果判断参照BROWN等[8]的方法并稍作修改,在200倍镜下观察5个视野,每个视野随机观察100个细胞,染色强度:无着色,0分;淡黄色,1分;棕黄色,2分;棕褐色,3分;染色细胞阳性数:<5个/视野,0分;5~25个/视野,1分;26~50个/视野,2分;>50个/视野,3分;染色强度和阳性细胞数两项得分相加,0分,阴性(-);1~3分,弱阳性(+);4~5分,中度阳性(++);6分,强阳性(+++)。每张切片由2名病理医师分别计数,计数差10%以上则重新计数。
1.3.7 Western blotting分析:取约100 mg胃黏膜组织,加入蛋白裂解液,4 ℃裂解,14 000 r/min离心15 min,取上清为全蛋白提取物;将蛋白提取物与蛋白上样缓冲液混合,煮沸5 min,分装保存于-80 ℃冰箱;进行SDS-PAGE凝胶电泳、转膜,封闭后加入一抗4 ℃过夜;再加入辣根过氧化物酶标记的二抗,孵育后ECL法显色于X光片后照相,使用Image J 1.44p软件分析处理。
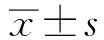
2 结果
2.1大体观察实验过程中双抗损伤组及瑞巴派特保护组大鼠各死亡1只,解剖后发现气管穿孔,对照组无大鼠意外死亡,对照组大鼠胃黏膜均未见明显损伤,损伤指数为0;双抗损伤组胃黏膜损伤明显,可见胃黏膜多发条索状充血糜烂,并可见新鲜出血灶,散在浅溃疡伴糜烂,损伤指数2.778±1.093;瑞巴派特保护组大体情况明显好转,可见黏膜轻微红肿,散在糜烂点,偶见浅溃疡,损伤指数1.000±0.707。与对照组相比,双抗损伤组大鼠大体评分明显升高(P<0.05);给予瑞巴派特处理后,大体评分明显降低(P<0.05,见图1)。
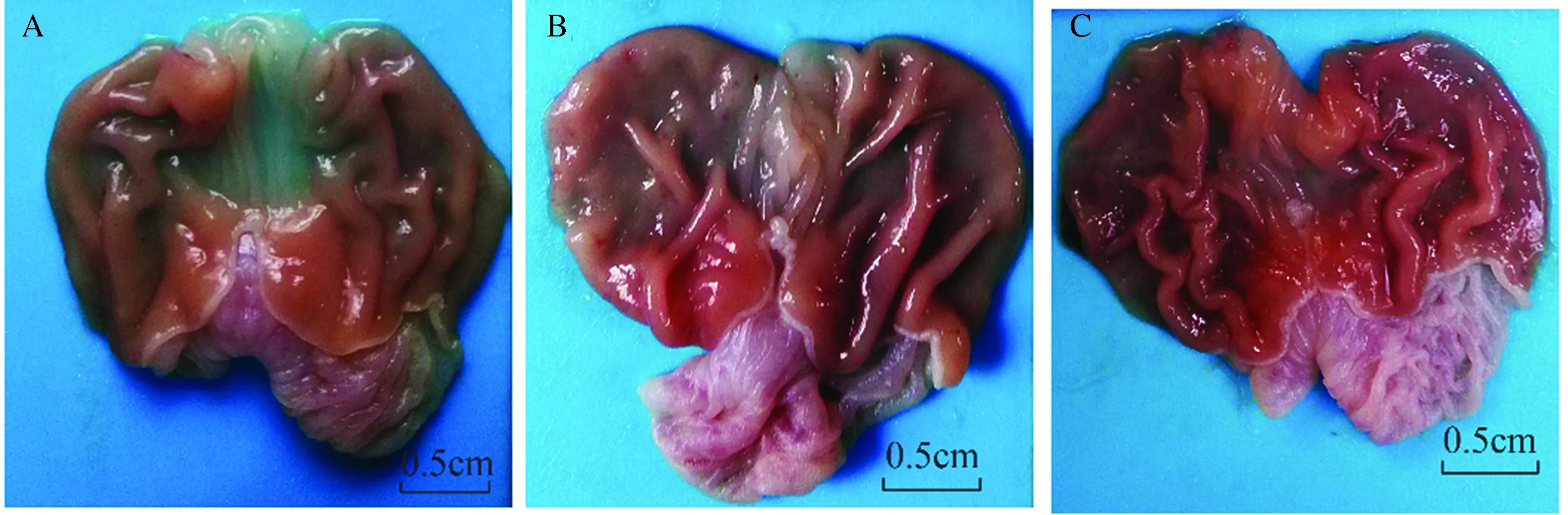
图1 各组大鼠胃黏膜大体情况 A:对照组;B:双抗损伤组;C:瑞巴派特保护组Fig 1 Gastric mucosal lesions in each group A: control group; B: Aspirin combined with Clopidogrel injury group; C: Rabamipide pretreatment group
2.2胃黏膜病理学变化对照组大鼠胃黏膜上皮结构完整,腺体排列紧密,形态正常,未见溃疡及糜烂等损伤,病理评级为0级;双抗损伤组胃黏膜部分上皮细胞脱落,组织结构损伤明显,可见胃腺体坏死,可见大量炎性细胞浸润,病理评级2.667±1.000;瑞巴派特保护组大鼠胃黏膜损伤明显减轻,可见黏膜散在少量点状充血灶,偶见点状糜烂,偶见少量胃腺体细胞坏死,可见少量炎性细胞浸润,病理评级1.000±0.707。与对照组相比,双抗损伤组病理学评级明显升高,差异有统计学意义(P<0.05,见图2);给予瑞巴派特处理后,病理评级明显降低,差异有统计学意义(P<0.05,见图2)。

图2 各组大鼠胃黏膜镜下形态 (HE 100×) A:对照组;B:双抗损伤组;C:瑞巴派特保护组Fig 2 Histological changes in each group (HE 100×) A: control group; B: Aspirin combined with Clopidogrel injury group; C: Rabamipide pretreatment group
2.3VEGF蛋白免疫组织化学染色根据免疫组化的结果,VEGF蛋白的阳性物质主要在细胞质内,呈粗细不等、深浅不一的棕色颗粒,对照组中呈现高表达,强阳性率为60.0%(6/10),表达评分5.30±1.059;双抗损伤组胃黏膜中少量表达,强阳性率为22.22%(2/9),表达评分3.33±1.732;瑞巴派特保护组中呈弱表达,强阳性率44.44%(4/9),表达评分5.00±1.118;双抗损伤组表达均低于对照组,差异有统计学意义(P<0.05),瑞巴派特保护组表达高于双抗损伤组,差异有统计学意义(P<0.05)(见图3)。
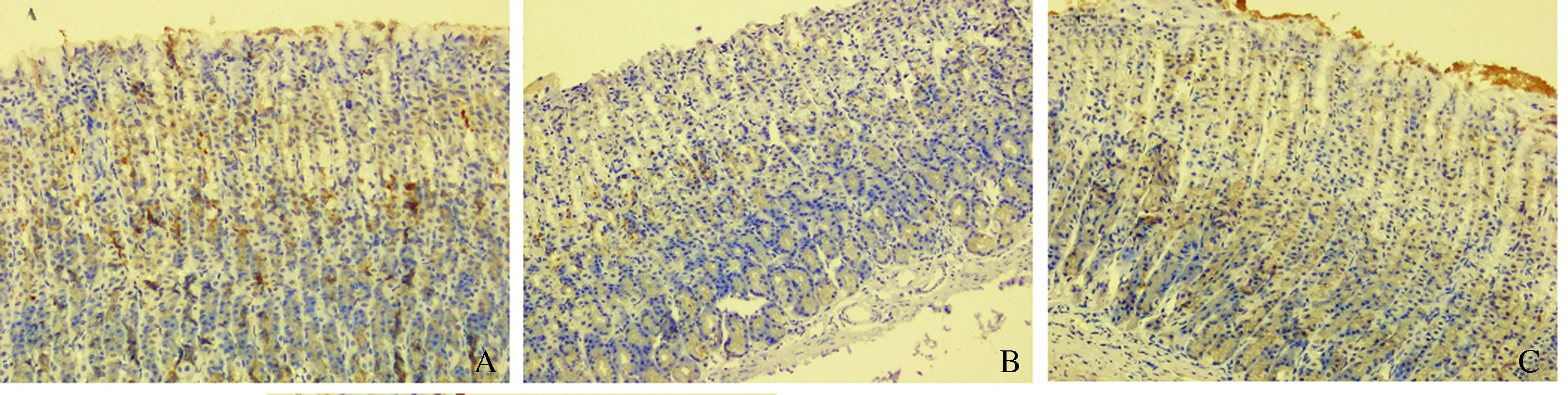
图3 各组大鼠胃黏膜VEGF的表达(200×) A:对照组;B:双抗损伤组;C:瑞巴派特保护组Fig 3 The expression of VEGF detected by immunohistoche-mistry method (200×) A: control group; B: Aspirin combined with Clopidogrel injury group; C: Rabamipide pretreatment group
2.4TUNEL检测各组大鼠胃黏膜上皮细胞凋亡凋亡细胞中棕黄色阳性物质主要位于细胞核内,表现为浓缩的核质紧贴于核膜或核质呈均匀染色。对照组大鼠胃黏膜中凋亡细胞较少,主要位于表面上皮内,呈散在分布,凋亡指数为6.00±1.826;双抗损伤组中大鼠胃黏膜中凋亡细胞较多,黏膜细胞全层均有显色,呈弥漫分布,凋亡指数24.44±4.876;瑞巴派特保护组大鼠胃黏膜中有少量凋亡细胞,在黏膜全层均可见到,多数为弥漫性分布,少数为散在分布,凋亡指数为18.11±1.833;双抗损伤组凋亡指数显著高于对照组,差异均有统计学意义(P<0.05),瑞巴派特保护组凋亡指数低于双抗损伤组,差异有统计学意义(P<0.05,见图4)。

图4 各组大鼠胃黏膜上皮细胞凋亡情况(200×) A:对照组;B:双抗损伤组;C:瑞巴派特保护组Fig 4 The gastric epithelial cell apoptosis of the rats tested by TUNEL assay (200×) A: control group; B: Aspirin combined with Clopidogrel injury group; C: Rabamipide pretreatment group
2.5Westernblotting检测大鼠胃黏膜中EGF的表达与对照组相比,双抗损伤组EGF表达量均显著减少(P<0.05),而瑞巴派特保护组中EGF的表达明显高于双抗损伤组(P<0.05,见图5~6)。
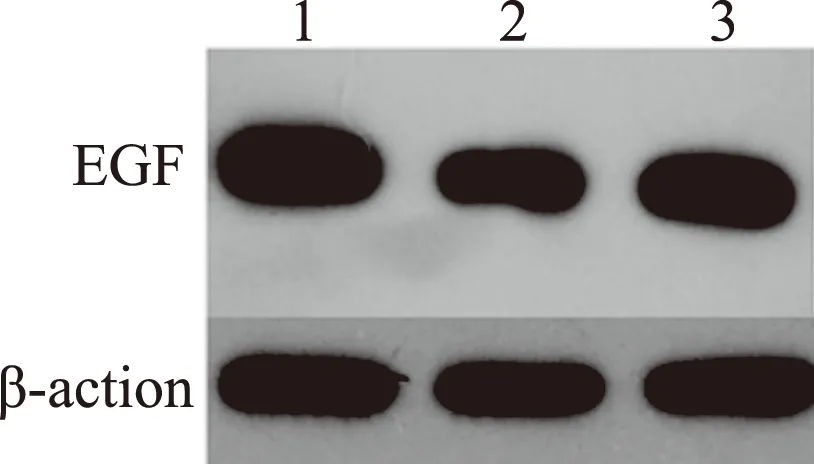
图5 各组大鼠胃黏膜EGF的表达 1:对照组;2:双抗损伤组;3:瑞巴派特保护组Fig 5 Expression of EGF in gastric tissues of rats detected by Western blotting 1: control group; 2: Aspirin combined with Clopidogrel injury group; 3: Rabamipide pretreatment group
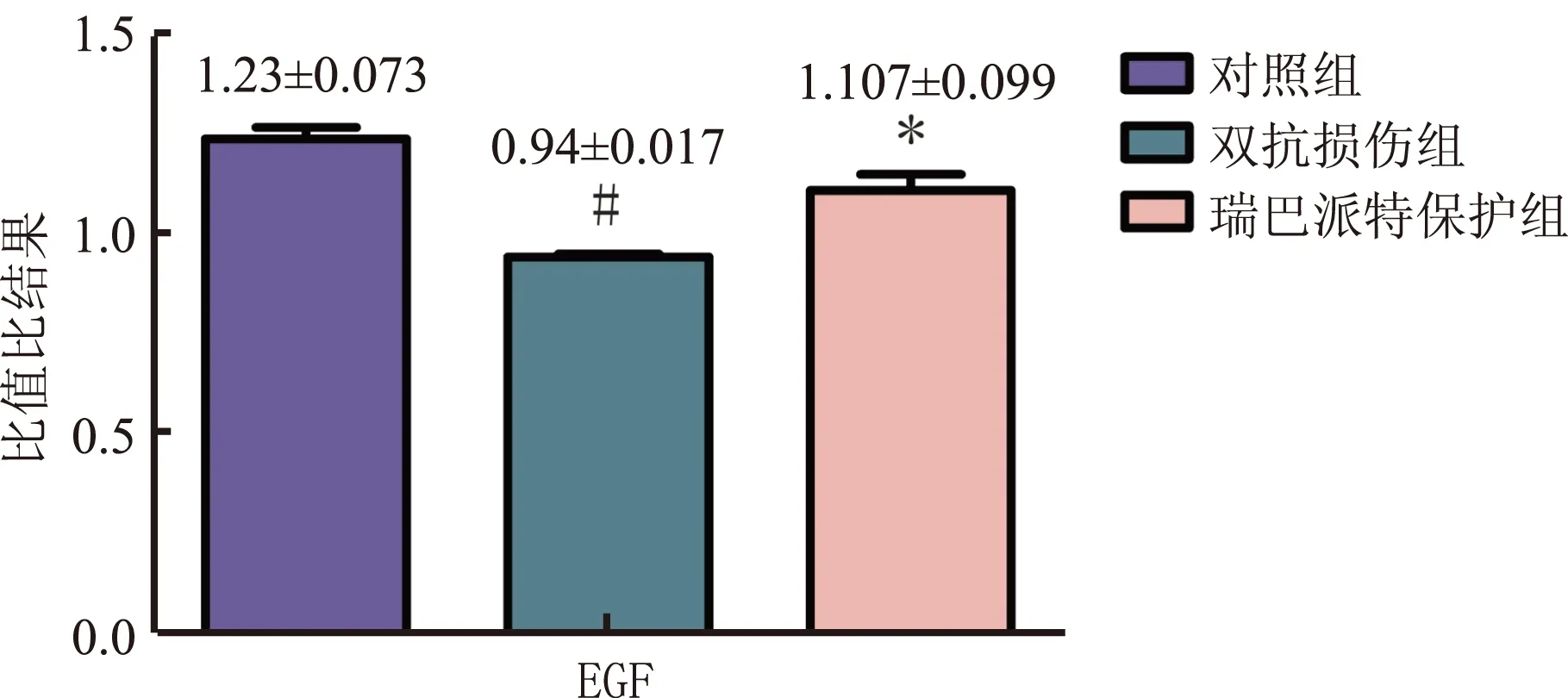
注:与双抗损伤组比较,*P<0.05;与对照组比较,#P<0.05。图6 各组EGF的表达情况Fig 6 EGF expression/β-actin in each group
3 讨论
自1997年美国FDA批准氯吡格雷用于新发中风、心梗、周围血管病已10余年,氯吡格雷在防治血栓事件中有很好的疗效。阿司匹林具有良好的解热、镇痛、抗炎、抗风湿、抗血小板等功效,一直广泛应用于临床,但长期服用阿司匹林会引起明显的胃肠道损伤。研究显示,单独服用氯吡格雷(75 mg/d)与单独服用小剂量阿司匹林(100 mg/d)引起的胃肠道损伤相似[3],而二者联用引起胃肠道损伤的风险明显增加,出现严重上消化道出血的风险增加7.4倍[4-5]。PPI和H2RA对NSAIDs引起的胃和十二指肠损伤效果明显,但长期服用PPI或H2RA可引起小肠菌群的过度生长(SIBO)和胆汁酸代谢的紊乱等消化道改变,进而加重肠道损伤[9-11],目前临床中尚无理想的防治双抗引起的胃黏膜损伤的药物。
瑞巴派特是常用的胃黏膜保护剂,可以促进消化道溃疡的愈合、提高黏膜愈合质量、减少溃疡复发,临床中常与抑酸剂联用来治疗胃和十二指肠溃疡、胃炎等;瑞巴派特主要通过降低超氧化物产量、抑制髓过氧化物酶的活性等而产生作用,可增加黏液分泌、刺激前列腺素的产生,具有抗炎、清除氧自由基的作用[12]。FUJIMORI等[13]与NIWA等[14]在临床研究中发现瑞巴派特对双氯芬酸引起的小肠损伤有一定的防治作用,可以显著抑制小肠黏膜的炎症反应;研究显示,云母可以防治大鼠中双氯芬酸引起的小肠损伤,可能是通过增强了黏膜屏障和增加了EGF的表达[15]。
本研究参照了张秋瓒等[16]的造模方法,实验过程中双抗损伤组及保护组大鼠因灌胃时误入气管各死亡1只,解剖后发现大鼠气管穿孔,对照组大鼠未出现意外死亡;实验结果显示,双抗损伤组大鼠胃黏膜大体及病理损伤明显,而保护组大鼠胃黏膜大体及病理损伤明显好转。因此,我们认为,双抗对大鼠胃黏膜有损伤作用,而瑞巴派特则可以显著改善这种损伤。
正常情况下,胃黏膜细胞凋亡与增殖处于动态平衡,当这一平衡紊乱时会引起胃黏膜完整性破坏从而导致疾病发生[17],研究[18-20]提示,多种原因引起的胃黏膜细胞凋亡是胃溃疡等损伤的基本病理改变。本研究采用TUNEL检测各组大鼠胃黏膜凋亡指数,以评价胃黏膜上皮细胞的凋亡情况,结果显示,双抗损伤组大鼠胃黏膜上皮细胞凋亡指数较对照组明显升高,差异有统计学意义,而瑞巴派特保护组较损伤组,胃黏膜上皮细胞凋亡指数明显降低,差异有统计学意义;因此,我们认为,阿司匹林联合氯吡格雷会引起大鼠胃黏膜上皮细胞的凋亡,而瑞巴派特则可以减缓胃黏膜上皮细胞的凋亡。
EGF是一种可以促进细胞增殖、分化和黏膜修复的重要因子,在维持黏膜完整性方面具有重要作用[21]。EGF通过促进DNA、RNA及蛋白质的合成而加速组织损伤的修复过程;VEGF又称血管渗透因子,可以通过特异性受体作用于血管内皮细胞,具有促进血管再生、黏膜上皮细胞及肉芽组织增生,维持黏膜完整性的作用,是目前已知的作用最强和特异性最高的血管生成促进因子。VEGF 主要通过调节各种胃黏膜保护因子和破坏因子之间的平衡而修复黏膜[22]。VEGF在胃黏膜保护中起双重作用,既通过增加微血管通透性稀释胃内有害物质,又可以通过刺激腺体和血管生成促进黏膜损伤的修复。本实验中,应用双抗灌胃后,采用Western blotting检测胃黏膜中EGF的表达,采用免疫组化检测VEGF的表达;结果显示,与对照组相比,双抗损伤组EGF、VEGF表达明显减少,瑞巴派特保护组EGF、VEGF表达较双抗损伤组增加,差异有统计学意义。因此,我们认为双抗引起的胃黏膜损伤部分通过抑制EGF及VEGF的表达而实现,而瑞巴派特则可以明显增加EGF及VEGF的表达,进而促进了胃黏膜损伤的修复。
基于此,本实验认为,瑞巴派特对阿司匹林联合氯吡格雷引起的胃黏膜损伤有一定的保护作用,至少部分是通过抑制胃黏膜上皮细胞的凋亡、增加EGF及VEGF的表达而促进双抗引起的胃黏膜损伤修复;本研究仅对瑞巴派特对阿司匹林联合氯吡格雷引起的胃黏膜损伤的保护作用进行了初步探讨,其具体的、更加深入的机制仍需要进一步的研究来证实。
[1] YUSUF S, ZHAO F, MEHTA S R, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation [J]. N Engl J Med, 2001, 345(7): 494-502.
[2] STEINHUBL S R, BERGER P B, MANN J T, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial [J]. JAMA, 2002, 288(19): 2411-2420.
[3] SEREBRUANY V L, MALININ A I, EISERT R M, et al. Risk of bleeding complications with antiplatelet agents: meta-analysis of 338,191 patients enrolled in 50 randomized controlled trials [J]. Am J Hematol, 2004, 75(1): 40-47.
[4] PETERS R J, MEHTA S R, FOX K A, et al. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study [J]. Circulation, 2003, 108(14): 1682-1687.
[5] HALLAS J, DALL M, ANDRIES A, et al. Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case-control study [J]. BMJ, 2006, 333(7571): 726.
[6] GUTH P H, AURES D, PAULSEN G. Topical aspirin plus HCl gastric lesions in the rat. Cytoprotective effect of prostaglandin, cimetidine, and probanthine [J]. J Gastroenterol, 1979, 76(1): 88-93.
[7] CAMPO R, MONTSERRAT A, BRULLET E. Transnasal gastroscopy compared to conventional gastroscopy:a randomized study of feasibility,safety and tolerance [J]. Endoscopy, 1998, 30(5): 448-452.
[8] BROWN R S, WAHL R L. Overexpression of Glut-1 glucose transporter in human breast cancer. An immunohistochemical study [J]. Cancer, 1993, 72(10): 2979-2985.
[9] SHINDO K, FUKUMURA M. Effect of H2-receptor antagonists on bile acid metabolism [J]. J Investig Med, 1995, 43(2): 170-177.
[10] 徐进, 杨洁, 王斌, 等. PPI联合瑞巴派特促进内镜黏膜下剥离术术后溃疡愈合的疗效分析[J]. 胃肠病学和肝病学杂志, 2016, 25(7): 745-748.
XU J, YANG J, WANG B, et al. The efficacy and safety of PPI plus Rebamipide for endoscopic submucosal dissection-induced ulcers: a Meta-analysis [J]. Chin J Gastroenterol Hepatol, 2016, 25(7): 745-748.
[11] LOMBARDO L, FOTI M, RUGGIA O, et al. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy [J]. Clin Gastroenterol Hepatol, 2010, 8(6): 504-508.
[12] NAITO Y, YOSHIKAWA T, TANIGAWA T, et al. Hydroxyl radical scavenging by rebamipide and related compounds: electron paramagnetic resonance study [J]. Free Radic Biol Med, 1995, 18(1): 117-123.
[13] FUJIMORI S, TAKAHASHI Y, GUDIS K, et al. Rebamipide has the potential to reduce the intensity of NSAID-induced small intestinal injury: a double-blind, randomized, controlled trial evaluated by capsule endoscopy [J]. J Gastroenterol, 2011, 46 (1): 57-64.
[14] NIWA Y, NAKAMURA M, OHMIYA N, et al. Efficacy of rebamipide for diclofenac-induced small-intestinal mucosal injuries in healthy subjects: a prospective, randomized, double-blinded, placebo-controlled, cross-over study [J]. J Gastroenterol, 2008, 43(4): 270-276.
[15] CHAO G, ZHANG S. Therapeutic effects of muscovite to non-steroidal anti-inflammatory drugs-induced small intestinal disease [J]. Int J Pharm, 2012, 436(1-2): 154-160.
[16] 张秋瓒, 王邦茂, 杨倩, 等. 氯吡格雷联合阿司匹林对大鼠胃黏膜的影响及TNF-α、VEGF表达研究[J]. 中国现代医学杂志, 2014, 24(5): 45-49.
ZHANG Q Z, WANG B M, YANG Q, et al. Study of the expression of TNF-α and VEGF and the effect of gastric mucosa in rats induced by Clopidogrel combined Aspiri [J]. China Journal of Modern Medicine, 2014, 24(5): 45-49.
[17] THOMPSON C B. Apoptosis in the pathogenesis and treatment of disease [J]. Science, 1995, 267(5203): 1456-1462.
[18] YAGUCHI T, SAITO M, YASUDA Y, et al. Caspase-4 activation in association with decreased adenosine deaminase activity may be a factor for gastric ulcer [J]. Digestion, 2010, 81(1): 62-67.
[19] 刘婧, 李兆申, 许国铭, 等. 细胞凋亡和增殖在大鼠应激性溃疡发病中的作用[J]. 中华消化杂志, 2003, 23(10): 595-598.
LIU J, LI Z S, XU G M, et al. The effects of apoptosis and proliferation on the pathogenesis of rat stress ulcer [J]. Chin J Dig, 2003, 23(10): 595-598.
[20] CHIOU S K, HODGES A, HOA N. Suppression of growth arrest and DNA damage-inducible 45alpha expression confers resistance to sulindac and indomethacin-induced gastric mucosal injury [J]. J Pharmacol Exp Ther, 2010, 334(3): 693-702.
[21] KONTUREK P C, KONTUREK S J, BRZOZOWSKI T, et al. Epidermal growth factor and transforming growth factor-alpha: role in protection and healins of gastric mucosal lesions [J]. Eur J Gastrcentcrol Hepatol, 1995, 7(10): 933-937.
[22] FERRARA N, DAVIS-SMYTH T. The biology of vascular endothelial growth factor [J]. Endocr Rev, 1997, 18(1): 4-25.
ProtectiveeffectandmechanismofRabamipideondualantiplateletdrugsinducedgastricinjuryinrats
LIU Chenchen1,2, LI Chao1, XU Zhaojun1, HAN Weiwei1, ZHANG Zhenyu1, WANG Jinsong3, HUANG Wenbin3
1.Department of Gastroenterology, Nanjing First Hospital Affiliated to Nanjing Medical University, Nanjing 210006; 2.Department of Gastroenterology, Jining First People’s Hospital; 3.Department of Pathology, Nanjing First Hospital Affiliated to Nanjing Medical University, China
ObjectiveTo investigate the effect and possible mechanism of Rabamipide on gastric injury induced by Aspirin combined with Clopidogrel in rats.MethodsThirty male SD rats were randomized into three groups: control group, Aspirin combined with Clopidogrel injury group and Rabamipide pretreatment group. Rats of Aspirin combined with Clopidogrel injury group were gavaged with Aspirin (10.41 mg·kg-1·d-1) and Clopidogrel (7.81 mg·kg-1·d-1) once daily for a continuous period of 14 days. Rats of rabamipide pretreatment group were gavaged with Rabamipide 1 hour once daily before given the same administration with the injury group. After 14 days administration, all rats were executed, and gastric mucosal lesion and histological changes were evaluated. Immunohistochemistry was used to detect the distribution and expression of VEGF. The gastric epithelial cell apoptosis of the rats was tested by TUNEL assay. The expression of EGF were determined by Western blotting.ResultsGastric mucosal lesions and histological changes in Aspirin combined with Clopidogrel injury group were significantly increased compared with control group, and gastric mucosal lesions and histological changes in Rabamipide pretreatment group were all significantly decreased compared with injury group (P<0.05). The expressions of EGF, VEGF in Aspirin combined with Clopidogrel injury group were all significantly reduced (P<0.05) and cell apoptosis was significantly increased compared with control group (P<0.05); expressions of EGF, VEGF were significantly increased in Rabamipide pretreatment group and cell apoptosis was significantly reduced compared with injury group.ConclusionRabamipide has a protecitve effect on gastric injury induced by Aspirin combined with Clopidogrel in rats, possibly through increasing the expressions of EGF, VEGF and inhibiting cell apoptosis, and thus collectively promotes the healing process of gastric injury.
Rabamipide; Aspirin; Clopidogrel; Gastric injuy; Cell apoptosis
南京市医学科技发展项目(YKK13103)
刘晨晨,硕士研究生,研究方向:胃肠道黏膜损伤与修复。E-mail:njliucc@163.com
张振玉,主任医师,硕士生导师,研究方向:胃肠道黏膜损伤与修复。E-mail:njzzy808@163.com
10.3969/j.issn.1006-5709.2017.12.016
R573
A
1006-5709(2017)12-1389-06
2017-01-14
李 健)
