A dinuclear Ni(II) complex with a mixed ligand system: synthesis, structure and fluorescence properties
2017-12-11RUANYajieZOUShukaiDUGegeLIUBaolin
RUAN Yajie, ZOU Shukai, DU Gege, LIU Baolin
(Henan Key Laboratory of Polyoxometalate Chemistry, School of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, Henan, China)
AdinuclearNi(II)complexwithamixedligandsystem:synthesis,structureandfluorescenceproperties
RUAN Yajie, ZOU Shukai, DU Gege, LIU Baolin*
(HenanKeyLaboratoryofPolyoxometalateChemistry,SchoolofChemistryandChemicalEngineering,HenanUniversity,Kaifeng475004,Henan,China)
A dinuclear complex Ni2H3LHL1·CH3OH (Ⅰ) was sythesized and characterized by IR, elemental analysis, UV-vis and fluorescence spectra, thermal gravimetric analysis and single crystal X-ray diffraction. Complex Ⅰ crystallizes in the triclinic system, space groupPī, witha= 1.229 5(1) nm,b= 1.236 8(1) nm,c= 1.642 9(2) nm,α= 69.083(2)°,β= 82.337(2)°,γ= 86.421(2)°,V= 2.312(4) nm3,Z= 2, C45H52NiN8O13,M= 1 030.37,ρc= 1.480 g/cm3,μ(MoKα) = 0.888 mm-1,F(000) = 1 076,GOOF= 0.908,Z= 2, the finalR1= 0.058 3 andwR2= 0.100 7 forIgt; 2σ(I). The structure of complex Ⅰ consists of dinuclear units. The complex exhibits a tetranuclear hydrogen bonding framework through intermolecular O-H…O and N-H…O interactions.
Ni(II); fluorescence spectra; hydrogen bonding interaction; crystal structure
1 Experimental
All reagents were purchased from commercial sources and used without further purification. The ligand H6L were prepared as described in the literature[32]. Elemental analyses for carbon, hydrogen and nitrogen were performed on a Perkin-Elmer 2400II elemental analyzer. The infrared spectra were recorded on an Avatar-360 spectrometer using KBr pellets in the range of 400-4 000 cm-1. The UV-vis spectra were recorded on a UV-550 spectrometer in a range of 400-800 nm. The fluorescence spectra were measured on a F-7000 Fluorometer. Thermogravimetric analysis was carried out on an TGA/SDTA851e analyzer in a nitrogen atmosphere, and the complex were heated to 1 000 ℃ at a heating rate of 10 ℃ min-1.
1.1 Synthesis of Complex Ⅰ
An aqueous solution of NaOH (0.16 g, 4 mmol) was added to the Schiff base ligand H6L (0.590 g, 1 mmol) dissolved in 10 mL of water. The reaction mixture was stirred for 5 min. Then, a solution of Ni(OAc)2·4H2O (0.25 g, 1 mmol) in methanol (30 mL) was added, and the mixture was kept stirring for another 2 h at room temperature. The resulting green solution was filtered and allowed to stand at room temperature. Green block single crystals, suitable for X-ray diffraction analysis, were formed after two weeks. The yield was 0.036 g (66%). IR (KBr, cm-1): 1 644 vs, 1 567 s, 1 479 vs, 1 229 m, 1 107 w, 867 s, 757 s, 729 s.
For C45H52NiN8O13
Anal. calcd, %: C 52.46, H 5.09, N 10.88.
Found, %: C 51.39, H 5.13, N 10.83.
1.2 X-ray structure determination
2 Results and discussion
The results have revealed that the Ni2+ion and the pH of the solutions play an important role in the hydrolytic behaviour of the Schiff base ligand. The reaction of nickel acetate alone with ligand H6L in a mixture of water and methanol in a 1∶3 molar ratio in the presence of NaOH yielded the binuclear Complex Ⅰ. In this complex, two imine groups of the ligand H6L have been degraded into NH2groups, giving the new ligand (HL1)-[H2L1= 3-((2-(bis(2-aminoethyl)amino)ethylimino)methyl)-2- hydroxybenzoic acid]. The result indicates that the acidic pH facilitates the hydrolysis of the ligand with similar to findings reported in the literature[30].
2.1 Crystal structure of Complex Ⅰ
Single-crystal X-Ray diffraction analysis has revealed that Complex Ⅰ crystallize in the space groupPī. The structure consists of a binuclear unit and a methanol molecule of crystallization. A perspective view of the binuclear portion is depicted in Fig.1. Two Ni ions exist in dissimilar environments. The Ni1 ion is six-coordinated with a N3O3environment, and the coordination polyhedron can be viewed as distorted octahedron (Fig.3). Two nitrogen atoms (N2, N4) and two oxygen atoms (O4, O7) build the basal plane, whereas the apical positions are occupied by a nitrogen atom (N3) and an oxygen atom (O1) from the ligands. The Ni1-O and Ni1-N bond lengths cover ranges 0.203 6(3)-0.205 5(2) nm and 0.207 1(3)-0.210 2(3) nm, which are similar to the values reported in the literature[32]. The Ni ion lies about 0.013 13 nm above the average basal plane. The dihedral angle formed by Ni1-N4-N2 and Ni1-O4-O7 is 169.349(13)°. The Ni2 ion adopts a slightly distorted octahedral geometry with an N4O2donor set. The equatorial positions of Ni2 are occupied by NO3donor atoms from the (HL1)-, and the axial positions are occupied by an oxygen atom of the (HL1)-and a carboxylic oxygen atom from the (H3L)3-. The Ni2-O distances are in the range 0.200 3(3)-0.237 9(4) nm, the Ni2-N distances in the range 0.198 8(4)-0.210 1(4) nm. The Ni2 ion lies about 0.008 24 nm above the equatorial plane. The dihedral angle formed by Ni2-N8-O10 and Ni2-N5-N6 is 164.911 (18)°. The value of the Ni1…Ni2 separation is 0.777 3 (1) nm.

Table 1 Crystallographic data and refinement parameters for complexe Ⅰ
*R1= ∑||Fo| - |Fc||/∑|Fo|,wR2= [∑w(|Fo|2- |Fc|2)2/∑w(Fo2)2]1/2.
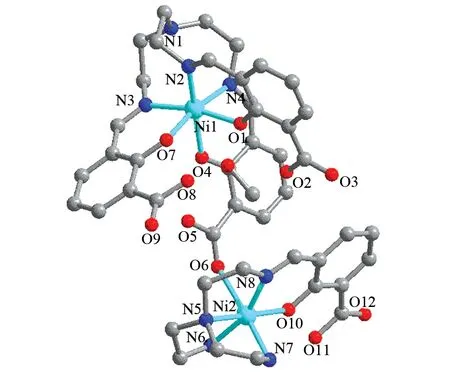
Fig.1 Molecular structure of complex Ⅰ, the methanol molecule and all hydrogen atoms are omitted for clarity
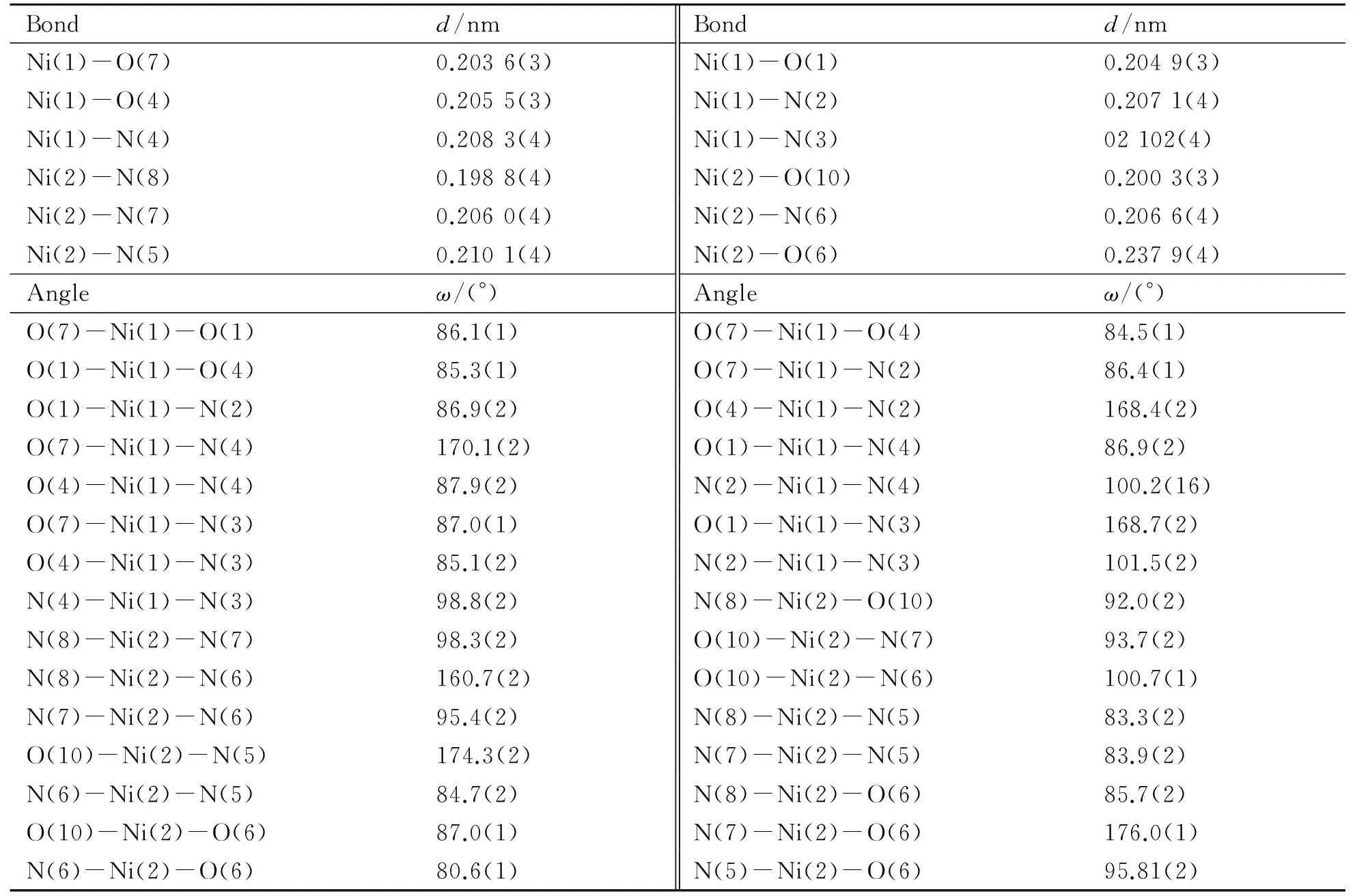
Bondd/nmBondd/nmNi(1)-O(7)0.2036(3)Ni(1)-O(1)0.2049(3)Ni(1)-O(4)0.2055(3)Ni(1)-N(2)0.2071(4)Ni(1)-N(4)0.2083(4)Ni(1)-N(3)02102(4)Ni(2)-N(8)0.1988(4)Ni(2)-O(10)0.2003(3)Ni(2)-N(7)0.2060(4)Ni(2)-N(6)0.2066(4)Ni(2)-N(5)0.2101(4)Ni(2)-O(6)0.2379(4)Angleω/(°)Angleω/(°)O(7)-Ni(1)-O(1)86.1(1)O(7)-Ni(1)-O(4)84.5(1)O(1)-Ni(1)-O(4)85.3(1)O(7)-Ni(1)-N(2)86.4(1)O(1)-Ni(1)-N(2)86.9(2)O(4)-Ni(1)-N(2)168.4(2)O(7)-Ni(1)-N(4)170.1(2)O(1)-Ni(1)-N(4)86.9(2)O(4)-Ni(1)-N(4)87.9(2)N(2)-Ni(1)-N(4)100.2(16)O(7)-Ni(1)-N(3)87.0(1)O(1)-Ni(1)-N(3)168.7(2)O(4)-Ni(1)-N(3)85.1(2)N(2)-Ni(1)-N(3)101.5(2)N(4)-Ni(1)-N(3)98.8(2)N(8)-Ni(2)-O(10)92.0(2)N(8)-Ni(2)-N(7)98.3(2)O(10)-Ni(2)-N(7)93.7(2)N(8)-Ni(2)-N(6)160.7(2)O(10)-Ni(2)-N(6)100.7(1)N(7)-Ni(2)-N(6)95.4(2)N(8)-Ni(2)-N(5)83.3(2)O(10)-Ni(2)-N(5)174.3(2)N(7)-Ni(2)-N(5)83.9(2)N(6)-Ni(2)-N(5)84.7(2)N(8)-Ni(2)-O(6)85.7(2)O(10)-Ni(2)-O(6)87.0(1)N(7)-Ni(2)-O(6)176.0(1)N(6)-Ni(2)-O(6)80.6(1)N(5)-Ni(2)-O(6)95.81(2)
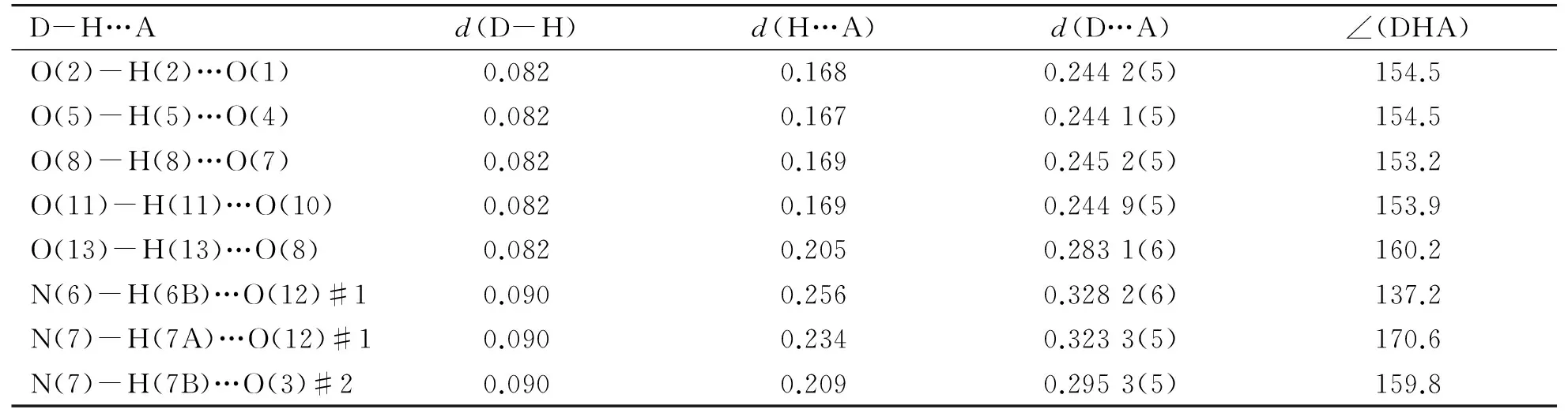
Table 3 Hydrogen bonding in crystal of complex Ⅰ (nm and °)
Symmetry codes: #1 -x+2, -y+2, -z; #2 -x+2, -y+1, -z.
There is a rich hydrogen bonding system in the crystal which strengthens the connection of two single-core fragments of nickel and increase the stability of the dinuclear unit. Carboxyl oxygen atoms from the ligand and the methanol molecules also connected by hydrogen bonding (Table 3) to form a tetranuclear structure, as shown in Fig.2.
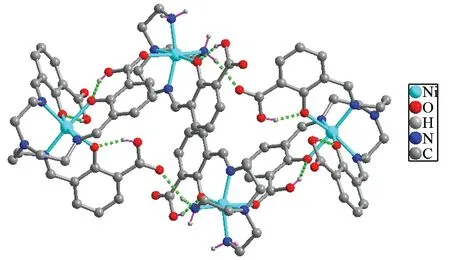
Fig.2 Hydrogen bonded framework of complex Ⅰ
2.2 Fluorescence analysis of Ⅰ
The luminescence spectrum of the Complex Ⅰ was studied at room temperature in the solid state. When excited at 395 nm, the ligand H6L exhibits a broad strong emission around 489 nm. The band of Ⅰ was red shifted to 481 nm, which may be assigned to ligand-metal charge transfer effects[35-36](Fig.3).
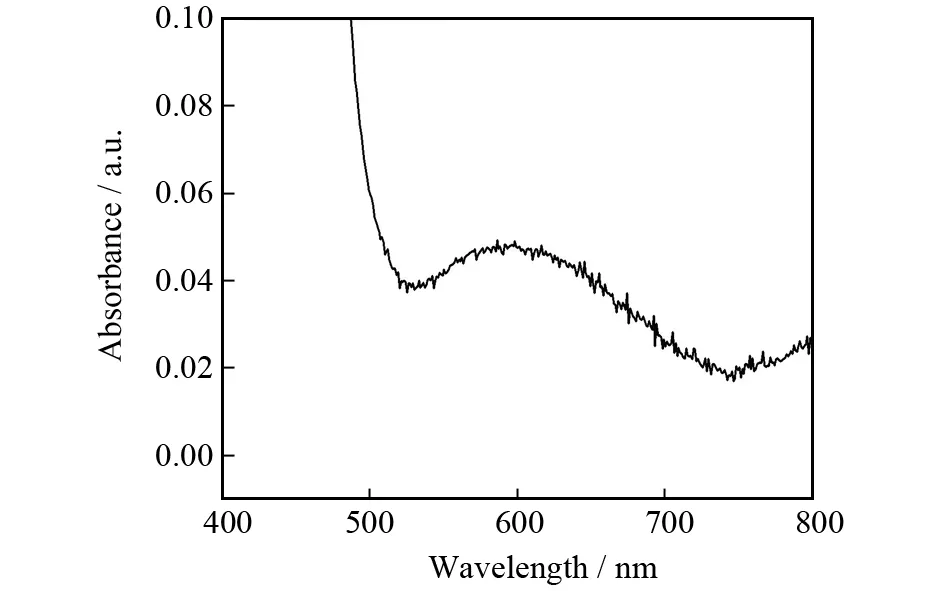
Fig.3 Fluorescence spectra of the ligand H6L and complex Ⅰ
2.3 Thermal gravimetric analysis of Ⅰ
The thermo gravimetric analysis curve of Complex Ⅰ is given in Fig.4. The weight loss of 3.01% between 25 ℃ and 230 ℃ corresponds to the loss of methanol molecule (calcd 3.11%). The weight loss of 85.13% in the range of 230-445 ℃ corresponds to the loss of the organic ligands (calcd 85.50%).
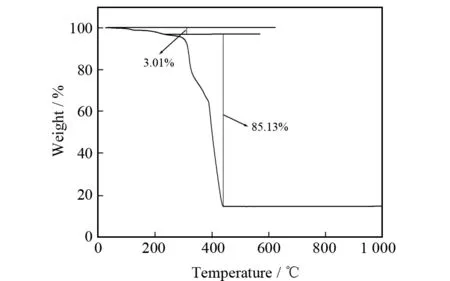
Fig.4 TGA curve of complexⅠ
3 Conclusion
In this paper, we used a tripodal Schiff-base ligand H6L. The results have reveals that one imine groups in the ligand H6L has been degraded into a NH2group, giving the new ligand. We have focused our attention to explore the hydrolytic behaviour of the Schiff base ligand H6L with nickel (II) salts. The result indicates that the acidic pH facilitates the hydrolysis of the ligand.
[1] RIBAS J, ESCUER A, MONFORT M, et al. Polynuclear NiII and MnII azido bridging complexes. Structural trends and magnetic behavior [J]. Coordination Chemistry Reviews, 1999, 193/195(4): 1027-1068.
[2] SOLER M, WERNSDORFER W, FOLTING K, et al. Single-molecule magnets: A large Mn30 molecular nanomagnet exhibiting quantum tunneling of magnetization [J]. Journal of the American Chemical Society, 2004, 126(7): 2156-2165.
[3] GRUENWALD K R, KIRILLOV A M, HAUKKA M, et al. Mono-, di- and polynuclear copper(II) compounds derived from N-butyldiethanolamine: structural features, magnetism and catalytic activity for the mild peroxidative oxidation of cyclohexane [J]. Dalton Transactions, 2009, 12(12): 2109-2120.
[4] HAUKKA H J, KUWABARA J, KIM J H, et al. Allosteric supramolecular triple-layer catalysts [J]. Science, 2010, 330(6000): 66-69.
[5] ZHANG Y Z, WERNSDORFER W, PAN F, et al. An azido-bridged disc-like heptanuclear cobalt(II) cluster: towards a single-molecule magnet [J]. Chemistry Communications, 2006, 1(31): 3302-3304.
[6] RINCK J, NOVITCAN G, VAN DEN HEUVEL W, et al. An octanuclear [CrIII4DyIII4] 3d-4f single-molecule magnet [J]. Angewandte Chemie International Edition, 2010, 49(41): 7583-7587.
[7] LIU T F, LIU Y, XUAN W M, et al. Chiral nanoscale metal-organic tetrahedral cages:diastereoselective self-assembly and enantioselective separation [J]. Angewandte Chemie International Edition, 2010, 49(24): 4121-4124.
[8] YUAN G Z, ZHU C F, XUAN W M, et al. Enantioselective recognition and separation by a homochiral porous lamellar solid based on unsymmetrical schiff base metal complexes [J]. Chemistry, 2009, 15(26): 6428-6434.
[9] MURRIE M. Cobalt(II) single-molecule magnets [J]. Chemical Society Reviews, 2010, 39(6): 1986-1995.
[10] JIANG S D, WANG B W, SUN H L, et al. An organo-metallic single-ion magnet [J]. Journal of the American Chemical Society, 2011, 133(13): 4730-4733.
[11] LONG J R M, HABIB F, LIN P H, et al. Single-molecule magnet behavior for an antiferromagnetically superexchange-coupled dinuclear dysprosium(III) complex [J]. Journal of the American Chemical Society, 2011, 133(14): 5319-5328.
[12] ERXLEBEN A. Structures and properties of Zn(II) coordination polymers [J]. Coordination Chemistry Reviews, 2003, 246(1/2): 203-228.
[13] HILLJ P, PALZA H, ALAM S, et al. Decomposition of dinuclear manganese complexes for the preparation of nanostructured oxide materials [J]. Inorganic Chemistry, 2008, 47(18): 8306-8014.
[14] NIU J Y, HUA J A, MA X, et al. Temperature-controlled assembly of a series of inorganic-organic hybrid arsenomolybdates [J]. Crystengcomm, 2012, 14(11): 4060-4067.
[15] XI X B, FANG Y, DOG T W, et al. Bottom-up assembly from a helicate to homochiral micro- and mesoporous metal-organic frameworks [J]. Angewandte Chemie International Edition, 2011, 50(5): 1154-1158.
减少环境应激原,以保持宝宝良好的稳定情绪,去除诱发因素,如花粉、灰尘、纤维、宠物、动物皮屑、室外污染物如汽车尾气,经常打扫暗角,晒洗被褥。
[16] KATSUKI T. Catalytic asymmetric oxidations using optically active (salen) manganese(III) complexes as catalysts [J]. Cheminform, 1995, 26(32): 189-214.
[17] POSPISIL P J, CARSTEN D H, JACOBSEN E N. X-Ray structural studies of highly enantioselective Mn(salen) epoxidation catalysts [J]. Chemistry-A European Journal, 2010, 2(8): 974-980.
[18] NEUMANN R, DAHAN M. A ruthenium-substituted polyoxometalate as an inorganic dioxygenase for activation of molecular oxygen [J]. Nature, 1997, 388(6640): 353-355.
[19] CANALI L, SHERRINGTON D C. Utilization of homogeneous and supported chiral metal(salen) complexes in asymmetric catalysis [J]. Cheminform, 1999, 30(21): 85-93.
[20] FANGH C, GG Y Y, JIA H Y, et al. Construction of three high-dimensional supramolecular networks from temperature-driven conformational isomers [J]. Crystengcomm, 2010, 13(1): 67-71.
[21] KARMAKAR R, CHOUDHURY C R, HUGHES D L, et al. Two new end-to-end single dicyanamide bridged Cu(II) complexes with schiff base ligands: Structural, electrochemical and magnetic properties [J]. Inorganica Chimica Acta, 2006, 359(4): 1184-1192.
[22] YAMADA S. Advancement in stereochemical aspects of schiff base metal complexes [J]. Coordination Chemistry Reviews, 1999, 190/192(5): 537-555.
[23] ZHANG W, LOEBACH J L, WILSON S R, et al. Enantioselective epoxidation of unfunctionalized olefins catalyzed by salen manganese complexes [J]. Cheminform, 1990, 21(32): 2801-2803.
[25] LI G B, FANG H C, CAI Y P, et al. Construction of a novel Zn-Ni trinuclear schiff base and a Ni2+chemosensor [J]. Inorganic Chemistry, 2010, 49(16): 7241-7243.
[26] ZHOU X X, FANG H C, GG Y Y, et al. The maltreatment syndrome in children by vincent j. fontana [J]. Crystal Growth Design, 2010, 10(9): 4014-4022.
[27] LIU Y Y, MA G F, BAI H Y, et al. Effect of anions on the self-assembly of Cd(II)-containing coordination polymers based on a novel flexible tetrakis(imidazole) ligand [J]. Crystal Growth Design, 2010, 10(2): 995-1015.
[28] MASSOUD S S, BPEN M, HAQ Z K, et al. Self-assembly of an azido-bridged [NiII6] cluster featuring four fused defective cubanes [J]. Inorganic Chemistry, 2008, 47(9): 3465.
[29] SARKAR B, RAY M S, DREW M G B, et al. Trinuclear Cu(II) complexes containing peripheral ketonic oxygen bridges and aμ3-OH core: steric influence on their structures and existence [J]. Polyhedron, 2006, 25(16): 3084-3094.
[30] DONG Y B, ZHAO X, HUANG R Q. New Ag(I)-containing coordination polymers generated from multidentate schiff-base ligands [J].Inorganic Chemistry, 2004, 43(18): 5603-5612.
[31] MUKHERJEE P, DREW M G B, GHOAH A. Anion-directed template synthesis and hydrolysis of mono-condensed schiff base of 1,3-pentanediamine and o-hydroxyacetophenone in Ni(II) and Cu(II) complexes [J]. European Journal of Inorganic Chemistry, 2008, 2008(21): 3372-3381.
[32] LIU B L, LIU Q X, XIAO H P, et al. Family of dumbbell Ni4Ln2(Ln = Pr, Sm, Eu, Gd, Tb, Ho, Er) complexes: syntheses, structures, luminescent and magnetic properties [J]. Dalton Transactions, 2013, 42: 5047-5055.
[33] BLESSING R H. Experimental electron density in crystalline H3PO4[J]. Acta Crystallographica, 1995, 51(5): 661-668.
[34] SHELDRICK G M. SHELXTL [CP]. Version 5.1, Bruker Analytical X-ray Systems Inc, 1997.
[35] GANG D B, GAO H, AN B, et al. Synthesis, crystal structure and luminescent properties of a nickel complex [Ni(NCS)2(phen)2] with mixed ligands [J]. Chemical Research, 2009, 20(4): 62-64.
[36] JONH A, KATIYAR V, PANG K, et al. Ni(II) and Cu(II) complexes of phenoxy-ketimine ligands: Synthesis, structures and their utility in bulk ring-opening polymerization (ROP) ofL-lactide [J]. Polyhedron, 2007, 26(15): 4033-4044.
[责任编辑:吴文鹏]
混和配体体系双核Ni(II)配合物的合成、结构和荧光性质
阮亚杰,邹淑凯,杜各各,刘宝林*
(河南省多酸化学重点实验室,河南大学 化学化工学院,河南 开封 475004)
合成了一双核化合物Ni2H3LHL1·CH3OH(Ⅰ),并通过IR,元素分析,UV-vis和荧光光谱,热重分析和单晶X射线衍射进行了表征. 该化合物属于Pī空间群,三斜晶系. 晶胞参数:a= 1.229 5(1) nm,b= 1.236 8(1) nm,c= 1.642 9(2) nm,α= 69.083(2)°,β= 82.337(2)°,γ= 86.421(2)°,V= 2.312(4) nm3,Z= 2, C45H52NiN8O13,M= 1 030.37,ρc= 1.480 g/cm3,μ(MoKα) = 0.888 mm-1,F(000) = 1 076,GOOF= 0.908,Z= 2,R1= 0.058 3,wR2= 0.100 7,Igt; 2σ(I). 化合物Ⅰ 的结构由双核单元组成. 通过不同分子间O-H…O和N-H…O相互作用显示出四核氢键合框架.
Ni(II);荧光光谱;氢键;晶体结构
O614.3DocumentcodeA
1008-1011(2017)06-0709-06
date: 2017-09-11.
National Natural Science Foundation of China (U1304301), Foundation of Science and Technology Department of Henan Province (152102210054), Foundation for Young-backbone Teachers of Higher Education Institutions in Henan Province (2014GGJS-023) and Foundation of Educational Department of Henan Province (13A150069).
Biography: RUAN Yajie (1991-), female, majoring in funcation coordination compounds.*
, E-mail:blliu@henu.edu.cn.
Recently, great interest has been focused on polynuclear complexes and coordination polymers, not only because of their intriguing structural motifs, but also their interesting properties and potential applications in catalysis, optics, magnetism, and enantioselective recognition and separation[1-11]. Various approaches have been developed to synthesize the polynuclear complexes[12-15]. The design and synthesis of polydentate ligands with chelating groups has been proved to be one of the most efficient approaches. Schiff bases have been widely used to synthesize homo/hetero polynuclear complexes. Multi-dentate Schiff base ligands are capable of forming complexes with many metal ions which exhibit unusual coordination, high thermodynamic stability, good fluorescent properties, biological activities, etc[16-21]. Owing to the facile preparation, structural varieties and diverse chemical properties, these multidentate Schiff-base ligands have been widely applied in constructing polynuclear complexes[22-27]. One of the major problems encountered during the synthesis of the complexes with these ligands is the hydrolysis of the Schiff base leading to the formation of several new products[28-31]. It is observed that the hydrolysis is dependent on several factors such as the metal ions[28-29], the pH value[30], the size of the chelate rings[28-29], the coordination ability of the counterion etc[31].
In a previous work, we have reported a new tripodal Schiff-base ligand H6L. By using this Schiff base, a new family of heterometallic hexanuclear Ni4Ln2clusters was obtained[32]. The results have reveals that one imine groups in the ligand H6L has been degraded into a NH2group, giving the new ligand. Coordination and therefore deprotonation of one arm of the ligand H6L to Ni2+possibly forms small quantities of H3O+NO-3in situ, which catalyses the hydrolysis of the remaining unbound 3-carboxylsalicylidene arms back to amine groups and then binds to the metal centre. As a continuation of this work, in the present paper, we focused our attention to explore the hydrolytic behaviour of the Schiff base ligand H6L with nickel (II) salts. A dinuclear complex Ni2H3LHL1·CH3OH was synthesized and characterized by IR, UV-vis and fluorescence spectra, elemental analysis, single crystal X-ray diffraction and thermal gravimetric analysis.
