SLC6A1基因突变致儿童失神发作1例并文献复习
2017-12-02张赟健周水珍
张赟健 周水珍
SLC6A1基因突变致儿童失神发作1例并文献复习
张赟健 周水珍


1 病例资料


入院体格检查:神志清,皮肤未见特殊色素斑,无特殊面容,颅神经检查未见异常,双侧瞳孔等大等圆,对光反应灵敏,伸舌居中,颈软,四肢肌力、肌张力正常,双侧膝腱反射可引出,病理征(-),无震颤,共济运动未见异常。
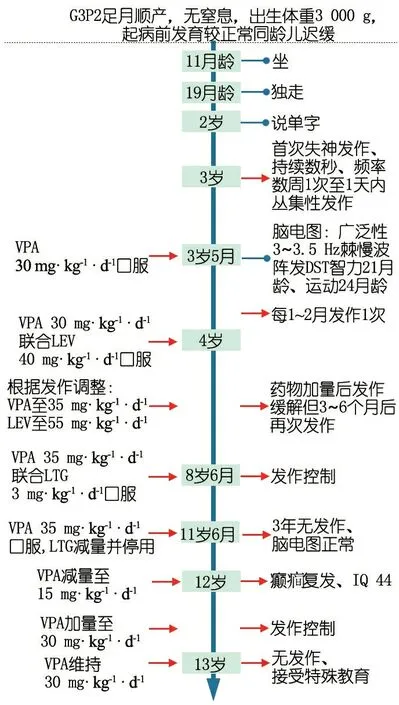
图1临床信息时间轴
注 VPA:丙戊酸钠;LEV:左乙拉西坦;LTG:拉莫三嗪
辅助检查:患儿10岁时头颅MRI未见明显异常(图2)。8岁7个月行视频脑电图(VEEG)检查,清醒安静闭眼时双侧枕区8Hz低至中波幅α节律,双侧基本对称,调节调幅欠佳,闪光刺激未见相关异常放电,醒睡各期左侧颞区尖慢波发放,醒睡广泛性3~3.5 Hz棘慢波阵发,并监测到清醒期多次典型失神发作(图3)。12岁行瑞文智力测试IQ为44。
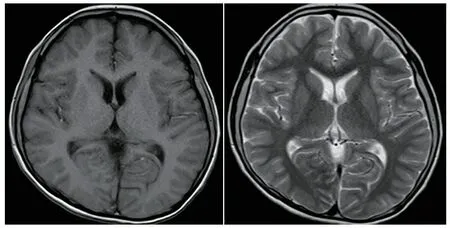
图2患儿10岁头颅MRI
注 头颅横断位T1(左)与T2(右)加权图像
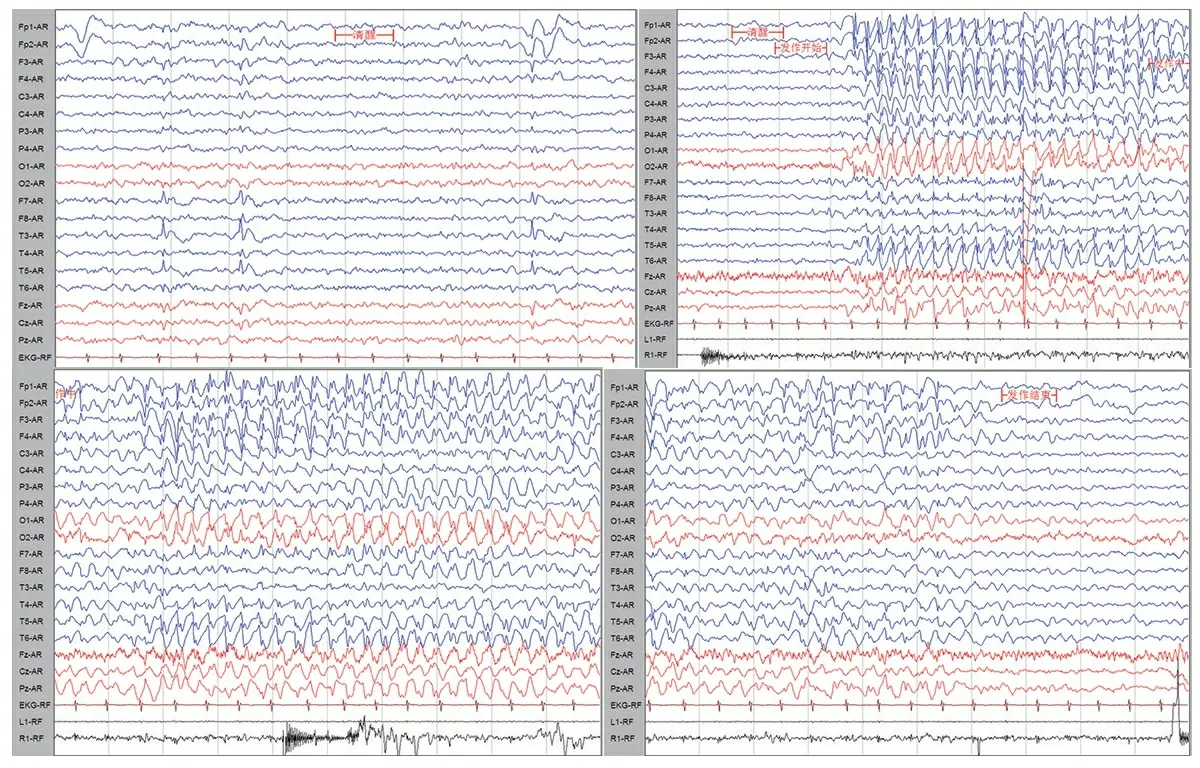
图3患儿8岁时视频脑电图皮肤表现
注 走纸速度30 mm·s-1,灵敏度100 μV·cm-1,高频滤波70 Hz
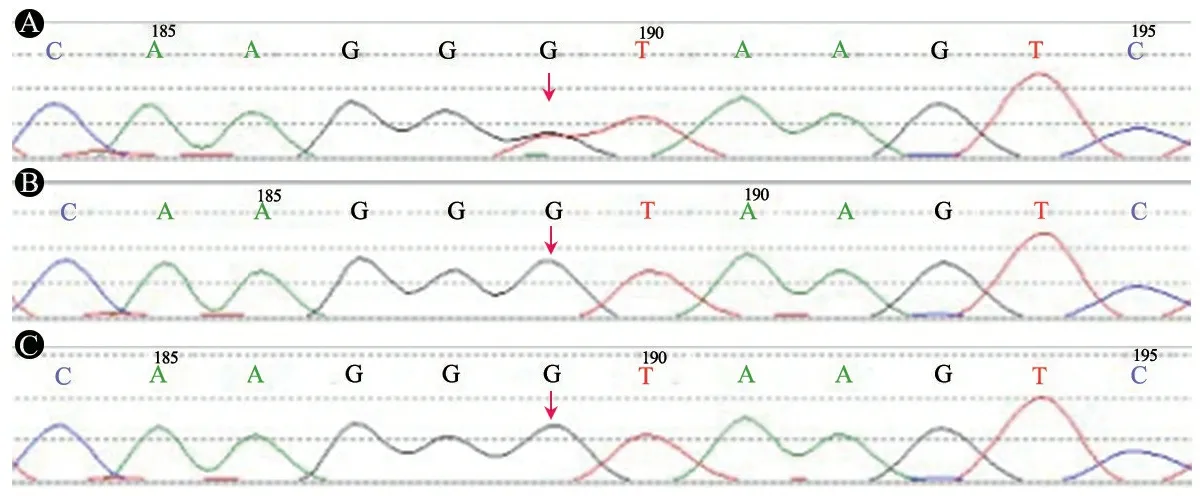
图4患儿及其父母SLC6A1基因测序图
注 A:患儿SLC6A1基因c.370+1Ggt;T杂合剪切突变(箭头);B和C:患儿父和母相同位置碱基未见突变(箭头)
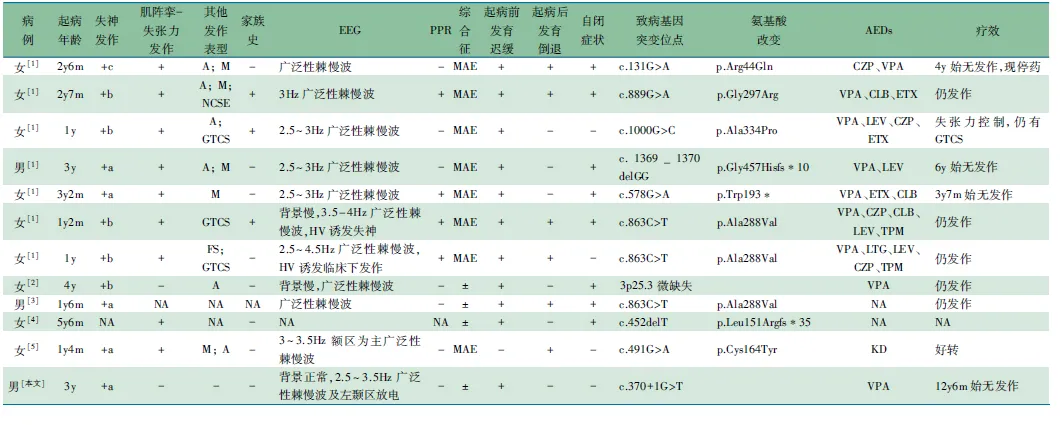
表1 12例SLC6A1基因突变者临床资料和基因突变位点

获得患儿及父母知情同意后,对患儿家系行全外显子检测(WES)并行Sanger验证,发现SLC6A1基因存在杂合剪切突变c.370+1Ggt;T,父母验证未检测到该变异,为患儿的新发变异(图4)。在人类基因突变数据库专业版(HGMD)、千人基因组数据库和ExAC数据集均无该位点突变及单核苷酸多态性(SNP)报道。对c.370+1Ggt;T进行致病性分析,该位点为罕见变异,位于经典的剪切位,应用MutationTaster预测为有害变异。
2 文献复习


3 讨论
GABA是哺乳动物中枢神经系统重要的抑制性神经递质,调节抑制性突触传递作用,从而防止脑内神经元过度兴奋[12]。神经突触间隙中的GABA主要通过突触前膜、神经胶质细胞膜或囊泡膜上的GABA 转运体(GAT)的摄取,使其在突触间隙中的浓度降低,从而减少或终止GABA的抑制性突触传递。GAT-1是电压依赖性GABA转运体中一种重要的亚型,由SLC6A1基因编码。
SLC6A1基因定位于染色体3p25.3[13],该基因在人类和其他动物发育期与成熟大脑中均广泛表达,其编码的GABA转运体GAT-1主要位于GABA能中间神经元的轴突和神经终端,GAT-1可从突触间隙重摄取GABA。Nelson等[14]最早于1990年对编码人类大脑神经递质GABA转运体的cDNA克隆并测序。该cDNA含有1个编码599个氨基酸的疏水蛋白的开放阅读框,具有12个跨膜片段。Hirunsatit等[15]报告,SLC6A1基因包含16个外显子,长度约46.5 kb。


动物研究发现,GABA转运体Gat1功能缺陷大鼠存在自发性脑电棘慢波发放及失神发作,Gat1敲除大鼠或使用Gat-1抑制剂的大鼠,脑电图均表现为类似SLC6A1基因突变患者失神发作的广泛性棘慢波异常放电[16]。因此,动物模型研究证实了SLC6A1突变者的临床表型。



[1] Carvill GL, Mcmahon JM, Schneider A, et al. Mutations in the GABA transporter SLC6A1 cause epilepsy with myoclonic-atonic seizures. Am J Hum Genet, 2015, 96(5): 808-815
[2] Dikow N, Maas B, Karch S, et al. 3p25. 3 microdeletion of GABA transporters SLC6A1 and SLC6A11 results in intellectual disability, epilepsy and stereotypic behavior. Am J Med Genet A, 2014, 164A(12): 3061-3068
[3] Sanders SJ, Murtha MT, Gupta AR, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature, 2012, 485(7397): 237-241
[4] Rauch A, Wieczorek D, Graf E, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet, 2012, 380(9854): 1674-1682
[5] Palmer S, Towne M C, Pearl P L, et al. SLC6A1 mutation and ketogenic diet in epilepsy with myoclonic-atonic seizures. Pediatr Neurol, 2016, 64: 77-79
[6] Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia, 2017, 58(4): 522-530
[7] Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia, 2017, 58(4): 512-521
[8] Yalcin O. Genes and molecular mechanisms involved in the epileptogenesis of idiopathic absence epilepsies. Seizure, 2012, 21(2): 79-86
[9] Everett K, Chioza B, Aicardi J, et al. Linkage and mutational analysis of CLCN2 in childhood absence epilepsy. Epilepsy Res, 2007, 75(2-3): 145-153
[10] Guo Y, Yan KP, Qu Q, et al. Common variants of KCNJ10 are associated with susceptibility and anti-epileptic drug resistance in Chinese genetic generalized epilepsies. PLoS One, 2015, 10(4): e124896
[11] Addis L, Rosch RE, Valentin A, et al. Analysis of rare copy number variation in absence epilepsies. Neurol Genet, 2016, 2(2): e56
[12] Scimemi A. Structure, function, and plasticity of GABA transporters. Front Cell Neurosci, 2014, 8: 161
[13] Huang F, Shi LJ, Heng HH, et al. Assignment of the human GABA transporter gene (GABATHG) locus to chromosome 3p24-p25. Genomics, 1995, 29(1): 302-304
[14] Nelson H, Mandiyan S, Nelson N. Cloning of the human brain GABA transporter. FEBS Lett, 1990, 269(1): 181-184
[15] Hirunsatit R, Ilomaki R, Malison R, et al. Sequence variation and linkage disequilibrium in the GABA transporter-1 gene (SLC6A1) in five populations: implications for pharmacogenetic research. BMC Genet, 2007, 8: 71
[16] Cope DW, Di Giovanni G, Fyson SJ, et al. Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med, 2009, 15(12): 1392-1398
[17] Whitlow RD, Sacher A, Loo DD, et al. The anticonvulsant valproate increases the turnover rate of gamma-aminobutyric acid transporters. J Biol Chem, 2003, 278(20): 17716-17726
[18] Salat K, Podkowa A, Malikowska N, et al. Novel, highly potent and in vivo active inhibitor of GABA transporter subtype 1 with anticonvulsant, anxiolytic, antidepressant and antinociceptive properties. Neuropharmacology, 2017, 113(Pt A): 331-342
[19] Schousboe A, Madsen KK, Barker-Haliski ML, et al. The GABA synapse as a target for antiepileptic drugs: a historical overview focused on GABA transporters. Neurochem Res, 2014, 39(10): 1980-1987
[20] Yuan FF, Gu X, Huang X, et al. SLC6A1 gene involvement in susceptibility to attention-deficit/hyperactivity disorder: A case-control study and gene- environment interaction. Prog Neuropsychopharmacol Biol Psychiatry, 2017, 77: 202-208
[21] Thoeringer CK, Ripke S, Unschuld PG, et al. The GABA transporter 1 (SLC6A1): a novel candidate gene for anxiety disorders. J Neural Transm (Vienna), 2009, 116(6): 649-657
2017-10-09
2017-10-15)
(本文编辑:张崇凡)
SLC6A1genemutationinachildwithabsenceseizuresandliteraturereview
ZHANGYun-jian,ZHOUShui-zhen
(DepartmentofNeurology,Children'sHospitalofFudanUniversity,Shanghai201102,China)
ZHOU Shui-zhen, E-mail: szzhou@shmu.edu.cn
ObjectiveTo explore the clinical features and genetic characteristics of patients withSLC6A1 gene mutations.MethodsThe clinical data of a patient withSLC6A1 gene mutation from Children's hospital of Fudan university were collected. The related literatures were searched from Wanfang Data Service Platform, China National Knowledge Infrastructure, National Center for Biotechnology Information and Pubmed (up to June 2017) by using search terms "SLC6A1" and "epilepsy". The clinical features, electroencephalogram and treatment of the patients withSLC6A1 gene mutations were studied.ResultsA boy with absence seizures and psychomotor retardation was followed up whose first attack happened at the age of 3 years. The seizures manifested as absence with eyelid flutter, with no limb convulsions and the duration varied from seconds to dozens of seconds. The seizure frequency ranged from once in several weeks to daily cluster seizures. Electroencephalogram was abnormal because of bursts of generalized 3 to 3.5 Hz spike-and-wave activity. Whole exome-sequencing study (trios) identified a de novo splicing mutation of c.370+1Ggt;T inSLC6A1. It was not previously reported in public database and predicted deleterious by Mutation Taster. And this was the first case ofSLC6A1 gene mutation in China. A total of twelve patients including the present case withSLC6A1 gene mutation were studied. Among them, 11 cases had absence seizures, including 5 typical absence and 5 absences with eyelid myoclonias, the other one case was atypical absence. Ten mutations were identified, including 5 missense mutations, 2 truncated mutations, 1 frameshift mutation, 1 splicing mutation and one with chromosome microdeletion.ConclusionSLC6A1 gene mutation is one of the causes of absence seizures with mental retardation or developmental regression.
Epilepsy; Absence; Gene;SLC6A1
上海市卫生和计划生育委员会科研课题:201640065;上海市申康新兴前沿项目:SHDC12015113
复旦大学附属儿科医院神经科 上海,201102
周水珍,E-mail:szzhou@shmu.edu.cn
10.3969/j.issn.1673-5501.2017.05.010
