Efficacy and metabolic influence on blood-glucose and serum lipid of ziprasidone in the treatment of elderly patients with first-episode schizophrenia
2017-11-29JingCHENXingenPANMincaiQIANShoukaiYANG
Jing CHEN, Xingen PAN, Mincai QIAN*, Shoukai YANG
•Original research article•
Efficacy and metabolic influence on blood-glucose and serum lipid of ziprasidone in the treatment of elderly patients with first-episode schizophrenia
Jing CHEN, Xingen PAN, Mincai QIAN*, Shoukai YANG
ziprasidone, late onset schizophrenia, curative effect, blood glucose, blood lipid
1. Introduction
According to a report published over 10 years ago,elderly patients with schizophrenia accounted for over half of hospitalized elderly patients with mental illness.[1]In recent years, with the aging of China’s population, the number of elderly patients with firstepisode schizophrenia is also gradually increasing.Because therapeutic drugs have pronounced effects on the physiology and biochemistry of elderly persons, it is important to select pharmacological interventions based on sufficient evidence. There is sparse research available on medication selection in this population,therefore we hope that this study will add to the growing body of evidence.
Schizophrenia is a serious mental illness effecting mostly young and middle aged people. For schizophrenia, atypical antipsychotics have been widely used, with reliable curative effects. However,some atypical antipsychotics were found to lead to weight gain in patients.[2,3]Generally speaking,taking atypical antipsychotics was a subjectively bad experience for patients as well, especially those who were already overweight. In addition, atypical antipsychotics also tended to increase blood sugar and blood lipids, heightening the risk of cardiovascular and cerebrovascular issues. This combination of side effects often resulted in low medication compliance, which in turn seriously impacted treatment effect. In order to study the efficacy and effects on blood pressure and blood lipid in elderly patients, we chose ziprasidone in the treatment of elderly patients with first-episode schizophrenia and carried out clinical observation.
2. Methods
2.1 General information
A total of 71 elderly patients were treated at our hospital from March 2012 to April 2016. Among them, 38 patients(21 men and 17 women) met the inclusion criteria and were included in this study. Their age ranged from 60 to 78 years with a mean age of 68.5 years. All patients were randomly divided into the ziprasidone treatment group(i.e. the study group) and the olanzapine treatment group(i.e. the control group), with 19 cases in each group. The study group consisted of 10 men and 9 women, with a mean age of 68.9 years. The control group consisted of 11 mean and 8 women, with a mean age of 68.2 years.There was no significant statistical difference between the two groups.
This study was approved by the Ethics Committee of Huzhou Third People’s Hospital of Zhejiang Province.
Inclusion criteria and exclusion criteria
Inclusion criteria were the following: 1) age ≥60 years old, 2) met the diagnostic criteria for schizophrenia according to the ICD-10 classification of mental and behavioral disorders,[4]with a PANSS total score≥60 points, 3) no issues taking ziprasidone or olanzapine,and 4) informed consent was provided by patients’legal guardians.
Exclusion criteria were the following: 1) having diabetes mellitus, hyperlipidemia, or severe liver and renal insufficiency, 2) presence of organic neurological disease such as epilepsy, intracranial infection, brain tumor as determined by MRI or EEG examination, 3)presence of systemic diseases such as systemic lupus erythematosus (SLE), and schizophrenia-like behavior caused by drug intoxication.
Patients were withdrawn from the study if any of the conditions were met: 1) serious complications occurred during the treatment, 2) serious adverse drug reaction, 3) informed consent was withdrawn by the patient’s legal guardian, or 4) our researchers collectively determined, based on the patient’s condition, that the patient should be withdrawn from the study.
2.2 Methods
In this study, randomized grouping was used with admission time as the compatibility factor, and every fourth patient (as determined by time of admission)were set as an interval for randomized grouping.As a result, 38 cases of elderly patients with firstepisode schizophrenia were randomly divided into the ziprasidone treatment group (study group) and olanzapine treatment group (control group), with 19 cases in each group. Single blind (patient blind) parallel controlled trial design was used.
2.3 Treatment method
The study group orally took Ziprasidone Hydrochloride Capsules (20 mg per capsule, Jiangsu Nhwa Pharmaceutical Co. Ltd.), with an initial dose of 40 mg/day (dose ranging from 40 to 120 mg/day, and a mean(sd) dose of 92.4 (4.5) mg/day). The control group orally took olanzapine tablets (5 mg/tablet, Jiangsu Hansoh Pharmaceutical Group. Co. Ltd.,), with an initial dose of 5mg/day (dose ranging 5 to 20 mg/day and a mean (sd)of 9.5 (4.3) mg/day). The two groups both completed a treatment period of 12 weeks without a single case dropping. During treatment, there was no combined use with other antipsychotics, antidepressants,mood stabilizers, or anticonvulsant drugs. In case of adverse reactions, benzodiazepines, trihexyphenidyl hydrochloride, or propranolol was appropriately used for treatment. Ordinary diet was given during the treatment process. Dosage was adjusted according to clinical efficacy and tolerance.
2.4 Observation index and curative effect standard
PANSS, with good reliability and validity,[6]was used for the evaluation of psychiatric symptoms at the time of admission (baseline), and at the end of 4th, 8th, and 12th weeks of treatment, respectively. The curative effect,based on PANSS score reduction rate, was assigned as follows: ≥75% was considered cured, 50% -74% was considered significant progress, 25% - 49% was considered progress, and < 25% was considered ineffective. TESS was used for the assessment of adverse reactions.[7]For each patient, fasting venous blood (4 ml) was collected in the morning for the measurement of FBS, TC, TG, and LDL-c. Liver and kidney functions, electrocardiogram,routine blood test, and routine urine test were inspected once every 2 weeks.
2.5 Statistical methods
SPSS 17.0 was used for the statistical analysis.Continuous variables between independent sampleswere compared using t-test. Repeated measure ANOVA was used to compare the differences between each variable of the two groups during treatment. Rank sum test was used to compare the difference in curative efficacy between the two groups. Statistical significance was set at p < 0.05.
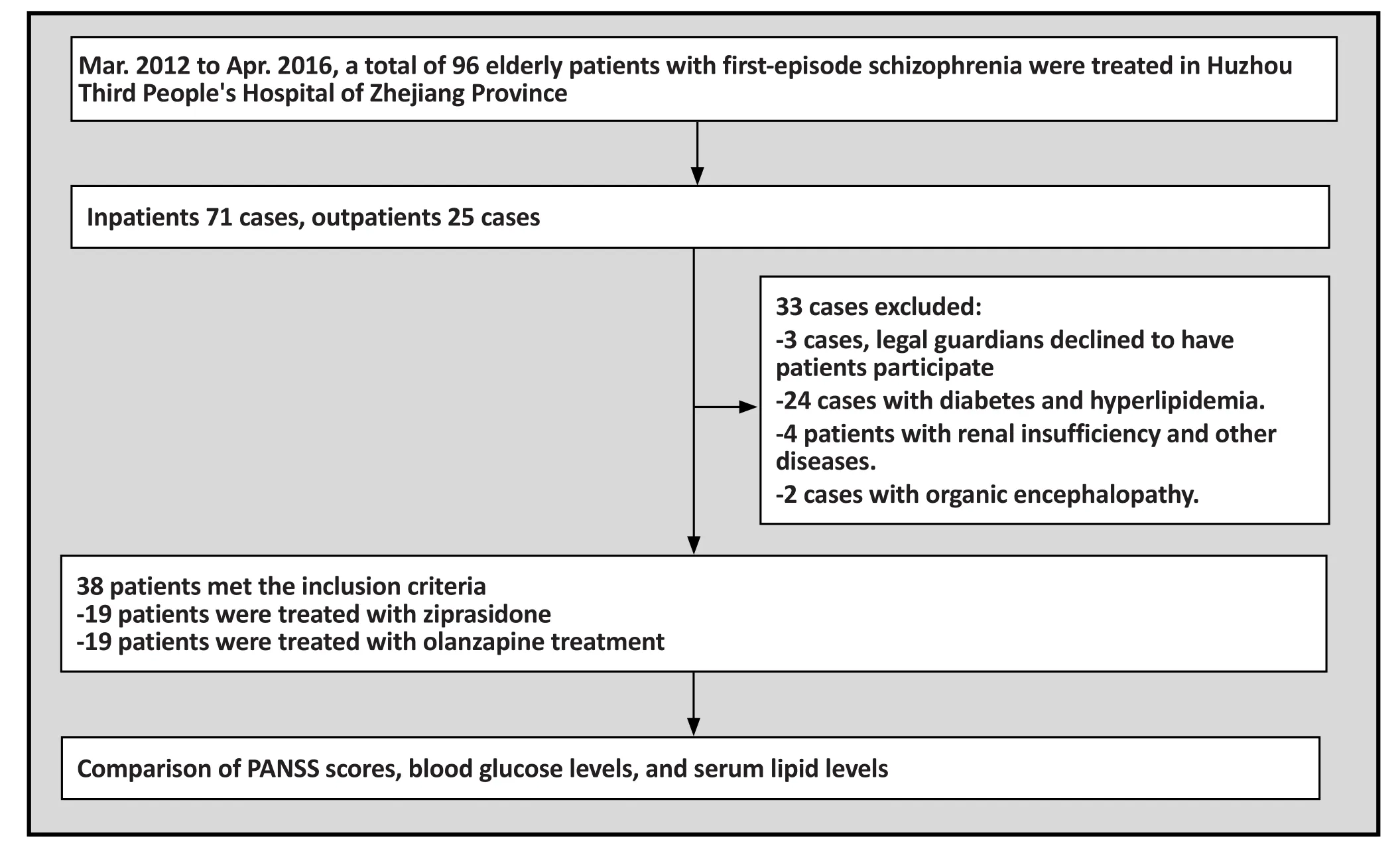
Figure 1. Flowchart of study
3. Results
3.1 Curative effect analysis
According to our criteria for PANSS score reduction,in the study group there were: 4 cured cases, 6 cases with significant progress, 5 cases with progress, and 4 ineffective cases. Our standard for effective treatment included the ‘cured’, ‘with significant progress’, and‘with progress’ designations. Hence, the effectiveness rate was 79.0%, with a cure rate of 21.0%. In the control group there were 3 cured cases, 8 cases with significant progress, 3 cases with progress, and 5 ineffective cases,with an effective rate of 73.7%, and a cure rate of 15.8%.The difference in curative effects of the two groups had no statistical significance. (Mann-Whitney test: Z=0.122, p=0.903).
3.2 PANSS scores
As shown in Table 1, PANSS scores of the two groups were significantly lower after treatment, with statistically significant differences. The total scores of PANSS decreased further with longer treatment duration.However, there was no statistically significant difference between the two groups in the change of total score of PANSS during the treatment (Ftime×group=0.009, p=0.958).
3.3 Comparison of blood glucose levels
As shown in Table 2, comparing the blood glucose levels between the two groups at each treatment stage,ziprasidone had no significant effect on fasting blood glucose, while fasting blood glucose values increased after treatment in the olanzapine group. The difference between the two groups in change of blood glucose levels during treatment was statistically significant(Ftime×group=7.539, p=0.001). At the end of 4th, 8th,and 12th weeks respectively, there were statistically significant differences in fasting blood glucose between the ziprasidone group and the olanzapine group.
3.4 Comparison of serum lipid levels
As shown in Table 3, comparing the serum lipid levels between the two groups at each treatment stage,the TC, TG and LDL-c levels of the ziprasidone group measured before treatment and at each treatment time point had no statistically significant difference. The TC,TG and LDL-c levels of the olanzapine group measured
before treatment and at each treatment time point had statistically significant difference. The difference in the change of each of the serum lipid index levels between the two groups during treatment was also statistically significant (TC: Ftime×group=32.194, p<0.001; TG:Ftime×group=488.312, p<0.001; LDL-C: Ftime×group=9.380,p<0.001). At the end of 4th, 8th, and 12thweek respectively, there were statistically significant differences in TC, TG and LDL-c levels between the ziprasidone group and the olanzapine group.

Table 1. Statistical comparison of PANSS total scores (mean[SD]) of the two groups in each treatment stage
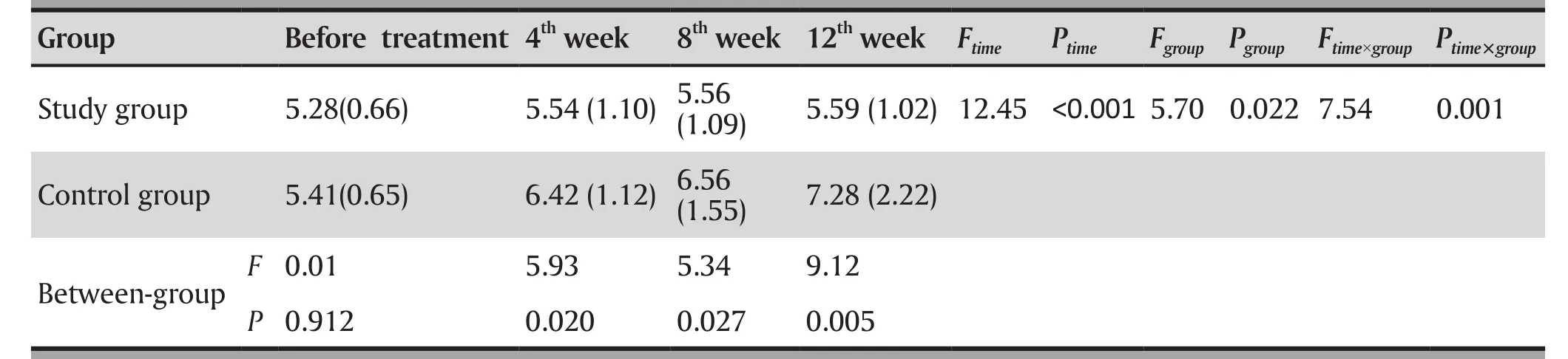
Table 2. Statistical comparison of blood glucose level (mean[SD]) mmol/L of the two groups in each treatment stage

Table 3. Comparison of blood lipids level (mean[SD]) mmol/L of the two groups in each treatment stage
3.5 Side effects
Till the end of the study, side effects in the ziprasidone group included 1 case of skin rash, 2 cases of nausea,1 case of constipation, 1 case of muscle rigidity, and 1 case of dry mouth. Side effects in the olanzapine group included 2 cases of sleepiness, 5 cases of significant body weight gain, 1 case of constipation, and 1 case of dry mouth. The control group had significantly more cases of sleepiness and significant body weight gain than the study group (accurate estimation p=0.045).There was no significant difference between the two groups in the overall side effect ratios. The changes in liver and renal functions, electrocardiogram, and routine blood and urine test results of the two groups of patients were within the normal range, without statistically significant differences compared with pretreatment conditions.
4. Discussion
4.1 Main findings
It was found in this study that ziprasidone and olanzapine had comparable curative effects in the treatment of schizophrenia in elderly patients. The ziprasidone group had an effectiveness rate of 79.0%, with a cure rate of 21.0%. The olanzapine group had an effectiveness rate of 73.7%, with a cure rate of 15.8%. Between the two groups, the PANSS scores in each treatment stage had no significant difference, indicating that ziprasidone and olanzapine had comparable curative effects in the treatment of elderly patients with first-episode schizophrenia. Meanwhile, there was no difference in onset time, which is consistent with the literature.[8,9]
Comparing the blood glucose and lipid levels in each treatment stage between the two groups, there were significant differences. Ziprasidone had less effect on patients’ blood glucose and serum lipid,which was consistent with the results from a study by Cui and colleagues.[10]Olanzapine had the function of raising blood glucose and serum lipid levels. This was consistent with results from Koike and colleagues,[11]which reported diabetes caused by olanzapine.
For the two groups, extrapyramidal side effects(such as muscle stiffness) had a relatively low incidence rate, which could be related to the higher 5-serotonin(5-HT) receptor affinity than the D2 receptor.[12]In this study, the intervention group and the control group had comparable ratios of curative effects and overall side effects. But the control group had fairly significant sleepiness and weight gain. Sleepiness and weight gain had reciprocal interactions, for which the root cause could be closely related to sugar and lipid metabolism.
4.2 Limitations
Metabolic syndrome, i.e. abnormal glucose metabolism(hyperglycemia), abnormal lipids metabolism(dyslipidemia), elevated blood pressure, and abdominal obesity are serious side effects from atypical antipsychotics that require attention. The advantage of ziprasidone differentiating it from other atypical antipsychotics is that it has almost no risk of causing metabolic syndrome. However, the mechanism of its effects on the metabolisms of carbohydrates and lipids is still not clear, requiring further study. In this study, the sample size was small, and a single blind randomized design was used, which could impact the results. Future studies should use a double-blind randomized study design with a larger sample size.
4.3 Implications
At present, the etiology of schizophrenia is not clear.The treatment of schizophrenia mainly involves the long-term use of drugs affecting the neurotransmitters of the central nervous system. Different drugs have different mechanisms, side effects and metabolic pathways, effecting the body in a variety of ways.
Elderly peoples’ various organ functions, such as in the digestive system, liver, pancreas, urinary system, nervous system and endocrine system, are significantly reduced. Therefore, impact on organ functioning must be considered when prescribing medications for schizophrenia in elderly patients.A personalized treatment plan, after carefully considering the effects of each medication, is ideal.For patients with schizophrenia, a well applied use of antipsychotic medication can play a crucial role in treatment. As schizophrenia usually persists and develops progressively, elderly patients with decreased metabolism and immunity require a longer time for treatment. In addition, it is common to see other chronic diseases in elderly patients such as cardiovascular and cerebrovascular diseases. Control of blood glucose and lipid levels in the treatment of schizophrenia may reduce the risk of cardiovascular and cerebrovascular accidents. Therefore it is of great clinical significance to select antipsychotic medications for elderly patients which have little effect on blood glucose and blood lipids.
Ziprasidone in the treatment of schizophrenia in elderly patients was reliably effective and had little effect on their glucose and lipid metabolism.Ziprasidone can reduce the risk of cardiovascular and cerebrovascular incidents, improve medication compliance in elderly patients and ensure long-term effectiveness of pharmacological treatment.
For patients with cardiovascular risks, especially elderly patients with schizophrenia who have comorbid metabolism related diseases, obesity, or family history of cardiovascular disease, ziprasidone is a potentially suitable medication choice.
Funding
This study was funded by the Zhejiang Province Huzhou 3rd People’s Hospital Public Welfare Application Research project (project # 2016GYB03)
Conflict of interest statement
The authors declare no conflict of interest related to this study.
Informed consent
Written informed consent was obtained from the guardians of all participants.
Ethical approval
This study was approved by the Ethics Committee of Huzhou Third People’s Hospital of Zhejiang Province.
Authors’ contributions
Jing Chen was responsible for the study design, data collection, writing and revision of the article.
Jing Chen, Xingen Pan, Mincai Qian, and Shoukai Yang were responsible for the management and treatment of patients.
1. Wang L. [A clinical control study of schizophrenia in old age and adolescence]. Sichuan Jing Shen Wei Sheng. 1998;11(3):197. Chinese
2. Russell JM, Mackell JA. Body weight gain associated with a typical antipsychotics: Epidemiology and therapeutic implications. CNS Drugs. 2001;15: 537-551
3. Daumit GL, Goff DC, Meyer JM, Davis VG, Nasrallah HA,McEvoy JP, et al.Antipsychotic effects on estimated 10 year coronary heart disease risk in the CATIE schizophrenia study. Schizophr Res. 2008;105(1-3): 175-187. doi: http://dx.doi.org/10.1016/j.schres.2008.07.006
4. Liu P, Wang XD, Yu X. [ICD-10 Classification of Mental and Behavioral Disorders]. Beijing: People’s Medical Publishing House; 1993. pp: 72-106. Chinese
5. He YL, Zhang MY. [Positive and negative symptom scale(PANSS) and its application]. Lin Chuang Jing Shen Yi Xue Za Zhi. 1998;7: 353. Chinese
6. Si TM, Yang JZ, Shu L, Wang XL, Kong QM, Zhou M, et al. [The reliability,validity of PANSS and its implication]. Zhongguo Xin Li Wei Sheng Za Zhi. 2004;18(1): 45-47. Chinese
7. Zhang MY. [Treatment Emergent Symptom Scale (TESS)].Shanghai Arch Psychiatry. 1984;2: 24-25. Chinese
8. Keefe RS, Sweeney JA, Gu H, Hamer RM, Perkins DO,McEvoy JP, et al.Effect of olanzapine, quetiapine, and risperidone on neurocognitivefunction in early psychosis:A randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164(7): 1061-1072. doi: http://dx.doi.org/10.1176/ajp.2007.164.7.1061
9. Zhu YP, Zhao YS, Su X, Dong LH, Tang J. [Comparative study on the effects of olanzapine and ziprasidone on the life quality of patients with schizophrenia]. Sichuan Jing Shen Wei Sheng. 2012;25(2): 90-93. Chinese
10. Cui KY, Liu LF, Yang LM. [Effects of ziprasidone and risperidone on serum prolactin, body weight, blood sugar and blood lipid of schizophrenic patients]. Jing Shen Yi Xue Za Zhi. 2010;23(1): 7-9. Chinese
11. Koike H, Sade T, Mizuno M. In vitro and in vivo pharmacology of olmesartan medoxomil, an angiotension:type ATI receptor antagonist. J Hypertens Suppl. 2001;19(1):S3-S14
12. Woodward ND, Purdon SE, Meltzer HY, Zald DH. A metaanalysis of neuropsychological change to clozapine,olanzapine,quetiapine, and risperidone in schizophrenia.Int J Neuropsychopharmacol. 2005;8(3): 457-472. doi: http://dx.doi.org/10.1017/S146114570500516X
齐拉西酮治疗老年期首发精神分裂症的疗效及对糖、脂代谢的影响
陈静,潘新根,钱敏才,杨守开
齐拉西酮;老年期首发精神分裂症;疗效;血糖;血脂
Background:As the age of the population in China rises, the occurrence of first-episode of schizophrenia in elderly persons is also gradually increasing. However, studies examining selection of therapeutic drugs for this population are relatively few.
Objective:To examine the therapeutic efficacy and metabolic influence on blood-glucose and serum lipid of ziprasidone in the treatment of elderly patients with first-episode schizophrenia.
Methods:Using randomized grouping, 38 elderly patients with first-episode schizophrenia were randomly divided into the ziprasidone treatment group (i.e. the study group) and the olanzapine treatment group(i.e. the control group), with 19 cases in either group respectively. The positive and negative symptoms scale (PANSS) was used to evaluate the efficacy, and adverse drug reaction scale (TESS) was used to evaluate adverse drug reactions, at the points prior to the treatment, at the end of 4th, 8th, and 12th weeks of treatment, respectively. Fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG), and low density lipoprotein (LDL-c) were also measured.
Results:There was no significant difference between the two groups in PANSS score at the end of week 4, week 8 and week 12. The curative effect on the two groups was similar. The results of repeated measure ANOVA showed that there were significant differences in FBG( Ftime×group=7.539, p=0.001),TC(Ftime×group=32.194, p<0.001), TG(Ftime×group=488.312, p<0.001), and LDL-c (Ftime×group=9.380, p<0.001)between the study group and the control group across the different time points.
Conclusion: Ziprasidone in the treatment of first episode schizophrenia in elderly patients has efficacy and less effect on blood-glucose and serum lipid metabolism.
[Shanghai Arch Psychiatry. 2017;29(2): 104-110.
http://dx.doi.org/10.11919/j.issn.1002-0829.217005]
The Third People’s Hospital of Huzhou, Huzhou, Zhejiang Province, China
*correspondence: Mincai Qian. Mailing address: Department of Geriatric Psychiatry, The Third People’s Hospital of Huzhou, Huzhou, Zhejiang Province,China. Postcode: 313000. E-Mail: 781703956@qq.com
背景:随着我国人口老龄化的出现,老年期首发精神分裂症有逐渐增多趋势,合理选择治疗药物成为一个值得关注的问题。
目的:探讨齐拉西酮治疗老年期首发精神分裂症的临床疗效及其对糖、脂代谢指标的影响。
方法:采用区组随机化分组,将38例老年期首发精神分裂症患者随机分为齐拉西酮治疗组(研究组)与奥氮平治疗组(对照组)各19例。分别于治疗前、4周末、8周末、12周末采用阳性与阴性症状量表(PANSS)评定疗效,副反应量表(TESS)评定药物不良反应。同时测量空腹血糖(FBG)及总胆固醇(TC)、甘油三酯(TG)、低密度脂蛋白(LDL-c)。
结果:两组PANSS评分4周、8周、12周末均无显著差异,两组疗效相当。重复测量方差分析显示治疗期间研究组与对照组的空腹血糖(FBG)( Ftime×group=7.539, p=0.001)及总胆固醇(TC)(Ftime×group=32.194, p<0.001)、甘油三酯(TG)(Ftime×group=488.312, p<0.001)、低密度脂蛋白(LDL-c)(Ftime×group=9.380, p<0.001)均有显著性差异。结论:齐拉西酮治疗老年期精神分裂症疗效确切,对糖、脂代谢影响较小,适合老年患者长期服用。

Jing Chen obtained her bachelor’s degree in clinical medicine from the Yun Yang Medical School, Hubei Province in 2001. Since 2004, she has been working in the Geriatric Psychiatry Department of the Third People’s Hospital of Huzhou City as an attending doctor. Her main research interests are in geriatric psychology and stress disorders in elderly patients.
Notice for the 2017 China Mood Disorders Conference
“The 2017 China Mood Disorders Conference” will be held at Kunming Medical University in Kunming, Yunnan Province (southwest China) from August 3rdto the 5th, 2017. The conference is being hosted by the Shanghai Jiaotong University School of Medicine – Shanghai Mental Health Center and is being co-sponsored by the China Mood Disorders and Psychiatry Society, and the Clinical Branch of the China Neuroscience Society.
The society welcomes paper submissions and conference participations. The conference affairs group will be accepting abstracts (i.e. objectives, methods, results and conclusions) of under 1000 words. These can be e-mailed to: ccad2017@163.com. The academic committee for this conference will review papers and select high quality reports for presentation at the conference. We look forward to your participation and support! The deadline for paper submission is 30thJune, 2017.
Dates: 3rdAugust to 5thAugust, 2017 (check in on 3rdAugust)
Address:Flower City Hotel, Kunming (Flower City, 8188 Jin Wa RD, Dong Bai Sha He, Kuming, Yunnan)
Cost arrangement: The registration fee, travel fee and accommodation fee are at your own expense. The registration fee is 800 yuan (Yunnan representatives and graduate students with student IDs can pay half of the registration fee). The accommodation fee during the conference is 400 yuan per standard room.
Contact: Chuan Li 18321950868, Tao Yang 15800579832
E-Mail: ccad2017@163.com
March 2017
猜你喜欢
杂志排行
上海精神医学的其它文章
- Efficacy towards negative symptoms and safety of repetitive transcranial magnetic stimulation treatment for patients with schizophrenia: a systematic review
- Factors related to acute anxiety and depression in inpatients with accidental orthopedic injuries
- Placement instability among young people removed from their original family and the likely mental health implications
- A study of the characteristics of alexithymia and emotion regulation in patients with depression
- The current situations and needs of mental health in China
- Factitious disorder - A rare cause for unexplained epistaxis
