Fe(II)活化过一硫酸盐氧化调理剩余活性污泥
2017-11-07刘昌庚谢四才
刘昌庚,伍 斌,谢四才
Fe(II)活化过一硫酸盐氧化调理剩余活性污泥
刘昌庚*,伍 斌,谢四才
(攀枝花学院资源与环境工程学院,四川攀枝花 617000)
采用Fe(II)活化过一硫酸盐(Fe(II)-PMS)氧化对剩余活性污泥进行调理研究.结果表明,Fe(II)-PMS氧化能有效改善污泥的脱水性能;在优化实验条件下(pH为6.7,Fe(II)和PMS投量分别为60和120mg/gTSS),标准化毛细吸附时间(SCST=CST0/CST)和毛细吸附时间(CST)减少率分别为11.28和91.13%.Fe(II)-PMS氧化有助于污泥稳定性提高和组分溶出,处理后VSS减少率为15.74%、上清液中TN和TOC的含量较初始值分别增加6.21和9.13倍.此外,研究表明Fe(II)-PMS氧化能有效破解和降解胞外聚合物(EPS)(特别是蛋白质),释放出EPS结合水,进而显著改善污泥脱水性能.
过一硫酸盐;脱水性能;剩余活性污泥;毛细吸附时间
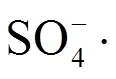
1 材料与方法
1.1 实验材料
牛血清蛋白为生化试剂,其他试剂均为分析纯试剂.供试污泥取自攀枝花市某污水处理厂的污泥浓缩池,取回后自然沉淀12h,去掉上清液后将污泥存放于4℃的冰箱中,其基本性质如表1.
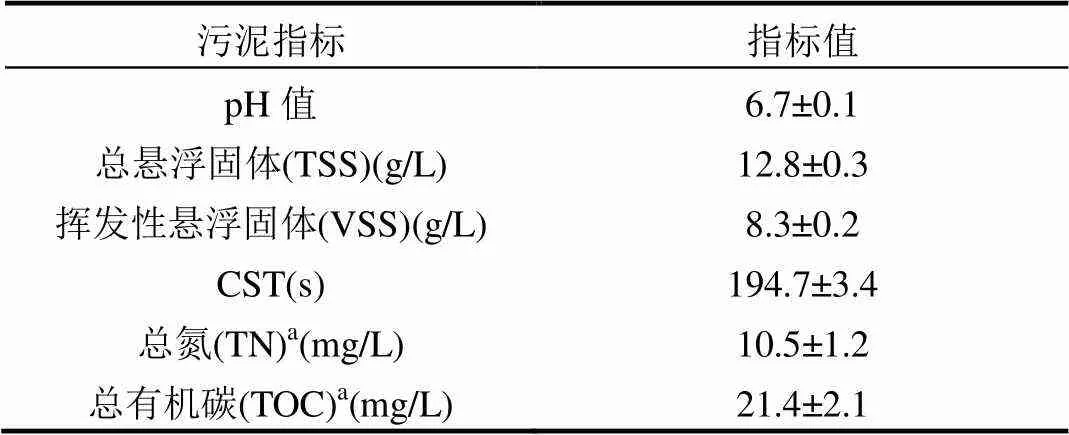
表1 供试剩余活性污泥的基本性质
注:a为污泥上清液中浓度.
1.2 实验方法
取200mL供试剩余活性污泥于500mL锥形瓶中,然后预热至室温(~20℃);随后考察不同初始pH、Fe(II)及PMS投量对污泥脱水性能的影响.pH采用浓度为2mol/L的硫酸和氢氧化钠调节,待pH调至设置值后,同时加入Fe(II)及PMS进行氧化调理,并以120r/min的速度搅拌至结束.在处理过程中,按预先设定时间取样5mL用于测定毛细吸附时间(CST).为保证实验数据可靠性,每组实验重复操作3次.
1.3 分析方法
pH值和毛细吸附时间分别利用酸度计(雷磁,PHS-3C)和CST测定仪(304B,英国Triton公司)测定;总悬浮固体(TSS)和挥发性悬浮固体(VSS)采用重量法测定;EPS分层方法详见文献[13],多糖和蛋白质浓度分别采用硫酸-蒽酮法和考马斯亮蓝G-250法测定[13,16];上清液中总氮(TN)和总有机碳(TOC)含量利用TOC/TN分析仪测定(TOC-L,日本Shimadzu公司).标准化毛细吸附时间(SCST)用于表征污泥的脱水性能,SCST值越大,污泥脱水性能越好,其计算式如下:
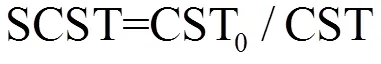
式中:CST0和CST分别表示污泥处理前后的毛细吸附时间.
2 结果与讨论
2.1 pH对污泥脱水性能的影响
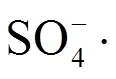
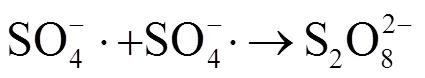
相比Fenton氧化,Fe(II)-PMS氧化调理污泥受初始pH值影响较小,可在污泥原始pH值条件下取得较好的脱水效率;而Fenton氧化则需在酸性条件才能获得较好的污泥脱水效率,但是在极酸性条件下,其氧化效率会因[Fe(H2O2)]2+的形成而严重受到抑制[20].由图1可知,在反应20min后,pH值为3、5、6.7、9时的SCST值分别为6.32、8.95、11.18、3.24,对应CST减少率分别为84.18%、88.83%、91.06%、69.14%.因此,可推断出pH值并非Fe(II)-PMS氧化改善污泥脱水性能的主要影响因素.该研究结果与前期Zhen等[7]报道的结论相似,他们指出Fe(II)活化过硫酸盐(Fe(II)-PDS)氧化能在较广pH范围内(3.0~8.5)显著改善污泥的脱水性能.由以上研究结果可知,Fe(II)-PMS氧化改善污泥脱水性能在成本控制和处理效率方面均具有优势.
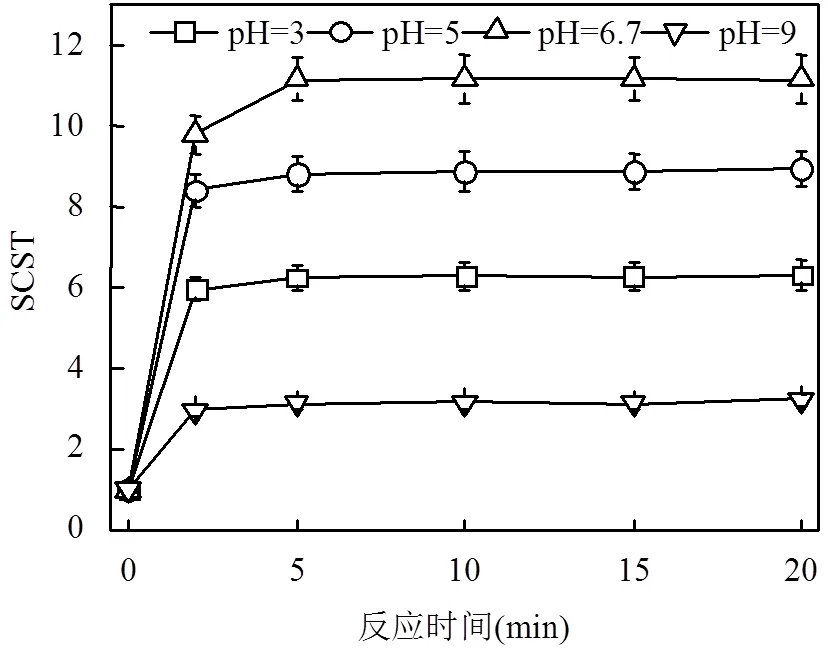
图1 pH值对污泥脱水性能的影响
2.2 Fe(II)和PMS投量对污泥脱水性能的影响
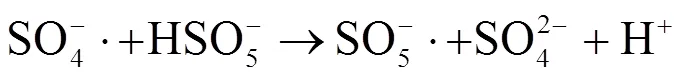
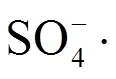
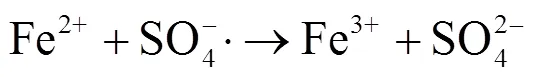
2.3 Fe(II)-PMS氧化对污泥组分溶出的影响
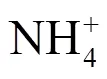
本研究还探究了Fe(II)-PMS氧化对污泥稳定性的改善效果.在优化条件下,污泥经Fe(II)- PMS氧化处理后,VSS减少率为15.74%.VSS减少率是污泥稳定性的重要指标,VSS减少率越高,污泥稳定性能越好[26].因此,Fe(II)-PMS氧化有利于提高污泥稳定性.最近,Kim等[25]指出污泥经热活化PMS和PDS处理后,VSS减少率分别为4.9%~15.4%和4.1%~7.7%.此外,Chen等[14]同样指出PMS较PDS氧化更能有效的降解有机物.因此,活化PMS氧化较活化PDS氧化可能更有助于提高污泥的稳定性.
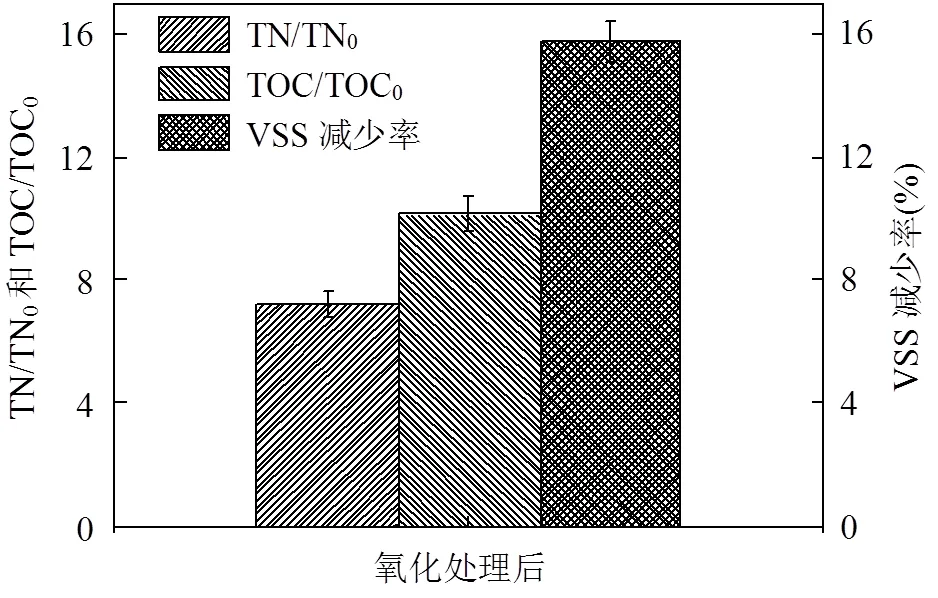
图4 Fe(II)-PMS氧化处理后VSS减少率、上清液中TN和TOC的浓度
2.4 Fe(II)-PMS氧化对EPS的影响
EPS是污泥的重要组成部分,可分为黏液层EPS(S-EPS)、松散结合EPS(LB-EPS)和紧密结合EPS(TB-EPS)[13].EPS主要由蛋白质和多糖组成,这两者可占其总质量的70%~80%,是影响污泥脱水性能的主要因素[7,27].本研究为考察Fe(II)-PMS氧化对污泥EPS破解的影响,在优化条件下氧化处理前后EPS各层中蛋白质和多糖的变化如图5所示.由图可知,原始污泥蛋白质和多糖含量分别为413.34和60.47mg/L,且主要存在于S-EPS和TB-EPS层中.经Fe(II)-PMS氧化后,蛋白质含量迅速下降至91.46mg/L;而多糖含量则轻微增加至65.14mg/L.鉴于蛋白质含量大量减少的同时污泥脱水性能得到显著改善,因此可推测出EPS中蛋白质含量对污泥的脱水性能的改善具有负面的影响.
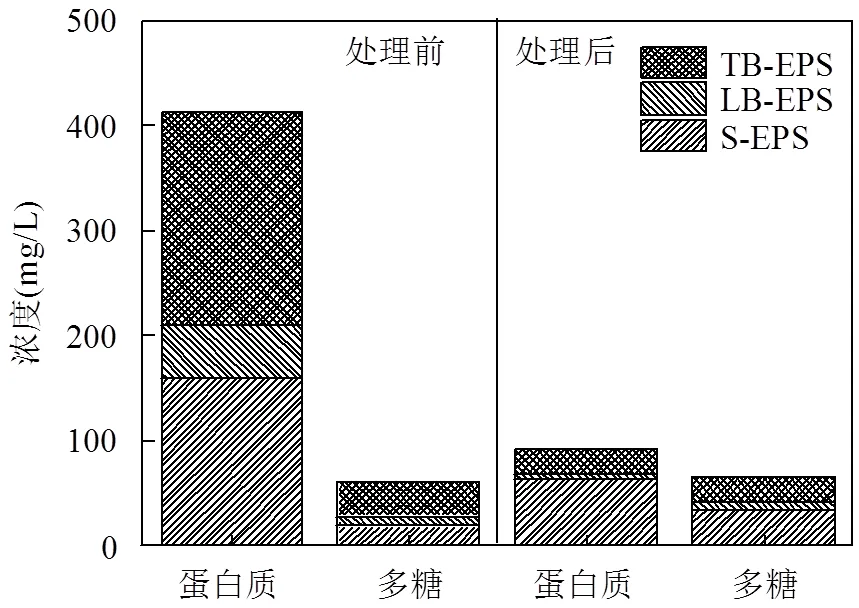
图5 Fe(II)-PMS氧化处理后蛋白质和多糖在EPS各层中的浓度
3 结论
3.1 Fe(II)-PMS氧化能显著改善污泥的脱水性能.优化实验条件:pH为6.7,Fe(II)和PMS投量分别为60和120mg/gTSS.此时,SCST值为11.28,CST减少率为91.13%.
3.2 Fe(II)-PMS氧化有助于污泥稳定性提高和组分溶出.在优化条件下,VSS减少率为15.74%、上清液中TN和TOC的含量较初始值分别增加6.21和9.13倍.
3.3 Fe(II)-PMS氧化处理污泥能有效破解和降解EPS(特别是蛋白质),释放出EPS结合水,进而显著改善污泥脱水性能.
[1] 徐 鑫,濮文虹,时亚飞,等.活化过硫酸盐对市政污泥调理效果的影响 [J]. 环境科学, 2015,36(11):4202-4207.
[3] 邢 奕,王志强,洪 晨,等.芬顿试剂与DDBAC联合调理污泥的工艺优化 [J]. 中国环境科学, 2015,35(4):1164-1172.
[4] Wu C, Jin L Y, Zhang P Y, et al. Effects of potassium ferrate oxidation on sludge disintegration, dewaterability and anaerobic biodegradation [J]. International Biodeterioration & Biodegradation, 2015,102:137-142.
[5] Liu J B, Yu D W, Zhang J, et al. Rheological properties of sewage sludge during enhanced anaerobic digestion with microwave- H2O2pretreatment [J]. Water Research, 2016,98:98-108.
[6] Oncu N B, Balcioglu I A. Microwave-assisted chemical oxidation of biological waste sludge: Simultaneous micropollutant degradation and sludge solubilization [J]. Bioresource Technology, 2013,146(10):126-134.
[7] Zhen G Y, Lu X Q, Zhao Y C, et al. Enhanced dewaterability of sewage sludge in the presence of Fe(II)-activated persulfate oxidation [J]. Bioresource Technology, 2012,116(4):259-265.
[8] 朱思瑞,高乃云,鲁 仙,等.热激活过硫酸盐氧化降解水中双酚A [J].中国环境科学, 2017,37(1):188-194.
[9] 高 磊,顾小钢,吕树光,等.热活化过硫酸钠耦合甲酸技术研究—处理水溶液中四氯化碳与六价铬污染[J].中国环境科学, 2016,36(9):2645-2649.
[10] 郭佑罗,关小红,高乃云,等.紫外/过硫酸盐工艺降解水中氯贝酸的研究[J]. 中国环境科学, 2016,36(7):2014-2019.
硫化钠的过量添加会造成砷滤饼发生量的增大,提高硫化钠的利用效率会减少硫化钠的使用量,目前硫化钠的添加方式是从硫化钠添加槽通过泵接在反应槽底部添加的方式,此方式可改为硫化氢直接吸入式,提高利用率。通过岗位操作人员对硫化ORP值(氧化还原电位)稳定控制,均衡硫化钠控制量,从而达到减少砷滤饼发生量的目的。
[11] Anipsitakis G P, Dionysiou D D. Degradation of organic contaminants in water with sulfate radicals generated by the conjunction of peroxymonosulfate with cobalt [J]. Environmental Science & Technology, 2003,37(20):4790-4797.
[12] Ren W C, Zhou Z, Zhu Y Y, et al. Effect of sulfate radical oxidation on disintegration of waste activated sludge [J]. International Biodeterioration & Biodegradation, 2015,104: 384-390.
[13] Zhou X, Wang Q L, Jiang G M, et al. A novel conditioning process for enhancing dewaterability of waste activated sludge by combination of zero-valent iron and persulfate [J]. Bioresource Technology, 2015,185:416-420.
[14] Chen X Y, Wang W P, Xiao H, et al. Accelerated TiO2photocatalytic degradation of acid orange 7under visible light mediated by peroxymonosulfate [J]. Chemical Engineering Journal, 2012,193(12):290-295.
[15] Rodríguez-Chueca J, Amor C, Silva T, et al. Treatment of winery wastewater by sulphate radicals: HSO5-/transition metal/UV-ALEDs [J]. Chemical Engineering Journal, 2017,310:473-483.
[16] 武 辰.高锰酸钾/高铁酸钾破解剩余污泥研究 [D].北京:北京林业大学, 2014.
[17] Buxton G V, Bydder M, Salmon G A. The reactivity of chlorine atoms in aqueous solution. Part II. The equilibrium SO4-+ Cl-reversible arrow Cl· + SO42-[J]. Physical Chemistry Chemical Physics, 1999,1(2):269-273.
[18] Rastogi A, Ai-Abed S R, Dionysiou D D. Sulfate radical-based ferrous-peroxymonosulfate oxidative system for PCBs degradation in aqueous and sediment systems [J]. Applied Catalysis B-Environmental, 2009,85(3/4):171-179.
[19] Guan Y H, Ma J, Li X C, et al. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system [J]. Environmental Science & Technology, 2011,45(21): 9308-9314.
[20] Figueroa S, Vazquez L, Alvarez-Gallegos A. Decolorizing textile wastewater with Fenton’s reagent electrogenerated with a solar photovoltaic cell [J]. Water Research, 2009,43(2):283-294.
[21] Cai C, Zhang Z, Zhong X, et al. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15catalyst for the degradation of Orange II in water [J]. Journal of Hazardous Materials, 2015,283:70-79.
[22] Yu W B, Yang J K, Shi Y F, et al. Roles of iron species and pH optimization on sewage sludge conditioning with Fenton’s reagent and lime [J]. Water Research, 2016,95:124-133.
[23] Chen K F, Kao C M, Wu L C, et al. Methyl tert-butyl ether (MTBE) degradation by ferrous ion-activated persulfate oxidation: Feasibility and kinetics studies [J]. Water Environment Research, 2009,81(7):687-694.
[24] Liang C J, Bruell C J, Michael C M, et al. Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate–thiosulfate redox couple [J]. Chemosphere, 2004,55(9):1213-1223.
[25] Kim M S, Lee K M, Kim H E, et al. Disintegration of waste activated sludge by thermally-activated persulfates for enhanced dewaterability [J]. Environmental Science & Technology, 2016,50(13):7106-7115.
[26] Pathak A, Dastidar M G, Sreekrishnan T R. Bioleaching of heavy metals from sewage sludge: A review [J]. Journal of Environmental Management, 2009,90(8):2343-2353.
[27] Zhou X, Jiang G M, Wang Q L, et al. A review on sludge conditioning by sludge pretreatment with a focus on advanced oxidation [J]. RSC Advances, 2014,4(92):50644-50652.
[28] Liu T, Chen Z L, Yu W Z, et al. Characterization of organic membrane foulants in a submerged membrane bioreactor with pre-ozonation using three dimensional excitation-emission matrix fluorescence spectroscopy [J]. Water Research, 2011, 45(3):2111-2121.
[29] Zhen G Y, Lu X Q, Wang B Y, et al. Synergetic pretreatment of waste activated sludge by Fe(II)-activated persulfate oxidation under mild temperature for enhanced dewaterability [J]. Bioresource Technology, 2012,124(9):29-36.
Conditioning of excess activate sludge by Fe(II)-activated peroxymonosulfate oxidation.
LIU Chang-geng*, WU Bin, XIE Si-cai
(School of Resources and Environmental Engineering, Panzhihua University, Panzhihua 617000, China)., 2017,37(10):3794~3799
Fe(II)-activated peroxymonosulfate (Fe(II)-PMS) oxidation applied to condition excess activated sludge was investigated in this work. The results showed that Fe(II)-PMS oxidation could effectively improve sludge dewaterability. The optimal pH and dosages of Fe(II) and PMS were 6.7, 60mg/gTSS, and 120mg/gTSS, respectively, under which the standardized-capillary suction time (SCST=CST0/CST) and CST reduction were 11.28 and 91.13%, respectively.Fe(II)-PMS oxidation was also favor of sludge solubilization and enhanced stabilization. Under the optimum experimental conditions, VSS reduction was 15.74% and the concentrations of TN and TOC in the supernatant increased 6.21 and 9.13-fold compared to their initial values, respectively. In addition, Fe(II)-PMS oxidation was beneficial to destroy and degrade extracellular polymeric substances (EPS) (especially for proteins), which resulted in the release of EPS-bound water and subsequently improved sludge dewaterability significantly.
peroxymonosulfate;dewaterability;excess activated sludge;capillary suction time
X703.1
A
1000-6923(2017)10-3794-06
刘昌庚(1985-),男,四川宜宾人,副教授,博士,主要从事高级氧化技术及大气环境化学研究.发表论文20余篇.
2017-03-17
国家自然科学基金资助项目(21607088);攀枝花学院博士基金资助项目(0210600022);攀枝花市科技计划资助项目(2015TX-8);干热河谷特色生物资源开发四川省高校重点实验室开放基金资助项目(GR-2017-E-04)
* 责任作者, 副教授, changwyx@163.com
