姜黄素对N-甲基亚硝基脲诱发膀胱癌大鼠化学干预作用及机制分析
2017-11-01吴金生王清明郑传秋纪萌孙立江
吴金生,王清明,郑传秋,纪萌,孙立江*
(1. 青岛大学, 山东青岛市 266071; 2. 潍坊医学院,山东潍坊市 261053)
研究报告
姜黄素对N-甲基亚硝基脲诱发膀胱癌大鼠化学干预作用及机制分析
吴金生1,王清明2,郑传秋2,纪萌2,孙立江1*
(1. 青岛大学, 山东青岛市 266071; 2. 潍坊医学院,山东潍坊市 261053)
目的分析姜黄素对N-甲基亚硝基脲(MNU)诱导的膀胱癌大鼠模型的化学干预作用及作用机制。方法将100只SD大鼠随机分为四组,对照组(10只)、模型组(10只)、干预组(40只)和治疗组(40只),对照组等时等量的膀胱灌注生理盐水,其他三组均对大鼠进行膀胱灌注MNU,诱发SD大鼠形成膀胱癌模型(将浓度为1 mg/mL的 MNU溶液灌注入膀胱内,MNU灌注时间为第2、4、6和8 周,每次2 mg,每2周1次,共4次),模型组在诱发大鼠膀胱癌时膀胱灌注蒸馏水,干预组在膀胱灌注MNU时灌注姜黄素溶液(400 μmol/L),即第1、3、5、7和9周膀胱灌注,第10周安乐死大鼠;治疗组在诱发大鼠膀胱癌模型后膀胱灌注姜黄素溶液(400 μmol/L),即在第10、12、14、16、18周时间内持续膀胱灌注,在第19周时处死大鼠,获得的膀胱组织依次通过苏木精-伊红(HE)染色,观察病理变化;TUNEL末端标记法测定肿瘤组织中细胞凋亡情况;Western blot检测凋亡相关蛋白表达。结果模型组在第10周时膀胱癌的发生率为90% (9/10),干预组在第10周时大鼠膀胱癌的发生率为12.5%(5/40),治疗组第10周时膀胱癌的发生率为92.5% (37/40),比较干预组与模型组大鼠膀胱癌的发生率差异有显著性(P<0.05),说明姜黄素对MUN诱发膀胱癌大鼠有明显的化学干预作用;在治疗组膀胱癌形成后给予姜黄素治疗,第19周膀胱癌发生率为78.4%(30/37),与治疗前的第10周比较说明姜黄素对膀胱癌有治疗作用,可以延缓膀胱癌的恶化。TUNEL实验证实姜黄素显著促进膀胱癌细胞的凋亡,抑制膀胱癌细胞的增殖。Western blot结果发现,姜黄素抑制NF-κB的激活,有效下调NF-κB调节的基因产物的表达。结论姜黄素对MNU诱导的膀胱癌大鼠模型有明显的的化学干预作用,且作用机制可能是通过抑制NF-κB的激活并且有效下调NF-κB调节的基因产物,来调节膀胱癌中相关蛋白的表达机制,即抑制增殖,诱导凋亡,进一步发挥抗癌的化学干预作用以及预防膀胱癌的复发。
姜黄素;N-甲基亚硝基脲;膀胱癌;化学干预;作用机制;大鼠
膀胱癌是现今人类泌尿系的高发性肿瘤疾病,虽经膀胱灌注的治疗方法取得一定的治疗效果,但复发是该病治疗的难题[1,2],为患者及社会带来巨大的经济压力。近年来研究发现,从姜黄科植物中提取得到的成分-姜黄素,主要存在姜黄、莪术等药用植物的根或茎中,能抑制淋巴癌、卵巢癌等诸多癌症细胞的生长,是广谱的抗癌化合物,且毒副作用小[3]。但对姜黄素对MNU诱导的膀胱癌大鼠的化学干预作用及作用机制的分析的报道相对较少,数据不完全,是临床膀胱癌治疗研究的缺口。笔者通过研究姜黄素对MNU诱导的膀胱癌大鼠的化学干预作用及作用机制的分析,以期为人类膀胱癌的研究提供实验依据,为临床膀胱癌的治疗提供思路。
1 材料与方法
1.1材料
1.1.1 实验动物
选取SPF级SD大鼠100只,雌雄不限,体重均在210~230 g,甴济南朋悦实验动物繁育有限公司提供【SCXK(鲁)2014-0007】。随机分为对照组(10只)、模型组(10只)、干预组(40只)和治疗组(40只);实验所用MNU在实验前一天放置于冰箱过夜,两组SD大鼠的基本资料对比差异无显著性,有可比性。
1.1.2 试剂
N-甲基亚硝基脲及姜黄素溶液均购自美国Sigma公司。
1.2方法
SD大鼠进行1周的适应性喂养,对照组仅等时等量膀胱灌注生理盐水,其他三组均对大鼠进行膀胱灌注MNU,诱发形成膀胱癌模型(即,将浓度为1 mg/mL的 MNU溶液灌注入膀胱内,MNU灌注时间为第2、4、6和8周,每次2 mg,每2周1次),模型组在诱发大鼠膀胱癌时膀胱灌注生理盐水,干预组膀胱灌注姜黄素溶液400 μmol/L,即第1、3、5、7和9周膀胱灌注,干预组第10周处死大鼠;治疗组在诱发大鼠膀胱癌模型后膀胱灌注姜黄素溶液(400 μmol/L),即第10周开始持续膀胱灌注8周后(灌注时间第10、12、14、16、18周)安乐死大鼠;对照组、模型组及干预组第10周处死大鼠,治疗组在第 19 周时处死大鼠,各脏器放氮气储存,取膀胱组织依次通过苏木精-伊红(HE) 染色,置显微镜下观察病理变化;TUNEL末端标记法[4]测定肿瘤组织中细胞凋亡情况;Western blot检测凋亡相关蛋白表达。
1.3统计学方法
2 结果
2.1各组SD大鼠的膀胱癌大鼠发病率情况
模型组在第10周时膀胱癌的发生率为90% (9/10),干预组在第10周时大鼠膀胱癌的发生率为12.5% (1/8),治疗组第10周时膀胱癌的发生率为 92.5% (37/40),比较干预组与模型组大鼠膀胱癌的发生率差异有显著性(P<0.05),说明姜黄素对MUN诱发膀胱癌大鼠有明显的化学干预作用;在治疗组膀胱癌形成后给予姜黄素治疗,第19周膀胱癌发生率为78.4%(30/37),与治疗前的第10周比较说明姜黄素对膀胱癌有治疗作用,可以延缓膀胱癌的复发。详见表1。
2.2姜黄素对细胞增殖指数的影响
在第10周,检测细胞增殖指数治疗组明显增加,治疗组较模型组对比差异无显著性(P>0.05),干预组较对照组或治疗组明显降低,差异有显著性(P<0.05);第19周,治疗组19周较治疗组10周亦有降低,差异有显著性(P<0.05),显示姜黄素可有效抑制细胞的增殖能力,详见表2。
2.3姜黄素对细胞凋亡的影响
第10周,干预组细胞凋亡较模型组增加明显,两组对比差异有显著性(P<0.05),治疗组较模型组凋亡细胞无明显变化,治疗组第19周较治疗组10周差异有显著性(P<0.05),说明姜黄素对膀胱癌细胞有促进凋亡的作用,详见表2、图1。
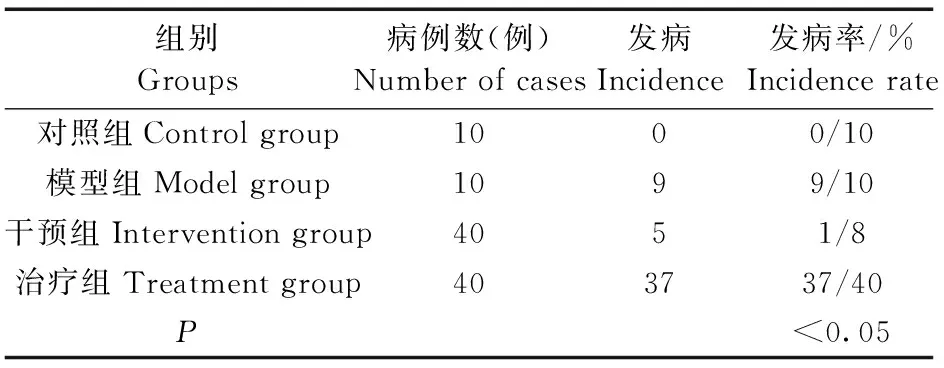
表1 各组SD大鼠的膀胱癌发病率对比情况Tab.1 Comparison of incidence rates of bladder cancer between the SD rats in each group
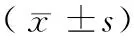
表2 姜黄素对细胞增殖指数及细胞凋亡的影响Tab.2 Effects of curcumin on cell proliferation index and apoptosis index in the rats

注:A. 模型组; B.干预组; C.治疗组第10周; D. 治疗组第19周。图1 TUNEL末端标记法测定肿瘤组织中细胞凋亡情况(bar=50 μm)Note.A. Model group. B. Intervention group. C. Treatment group at 10th week. D. Treatment group at 19th week.Fig.1 Apoptosis in the tumor tissues detected by TUNEL staining assay
2.4各组病理切片分析
对照组、模型组及干预组第10周处死大鼠,治疗组在第19周时处死大鼠,各脏器放液氮储存,取膀胱组织依次通过苏木精-伊红(HE)染色,置显微镜下观察病理变化,详见图2。除对照组外,其他三组均有不同程度的癌变,图中可见被覆的移行上皮增厚,细胞层次较多,细胞核大小不一。模型组与干预组的病理变化对比,干预组的细胞层次减少,说明在MNU诱发大鼠膀胱癌阶段,姜黄素有抑制细胞癌变的作用;在治疗组第19周,可见细胞形态较规则,呈不典型增生,说明姜黄素能减缓膀胱癌症恶化。详见图2。
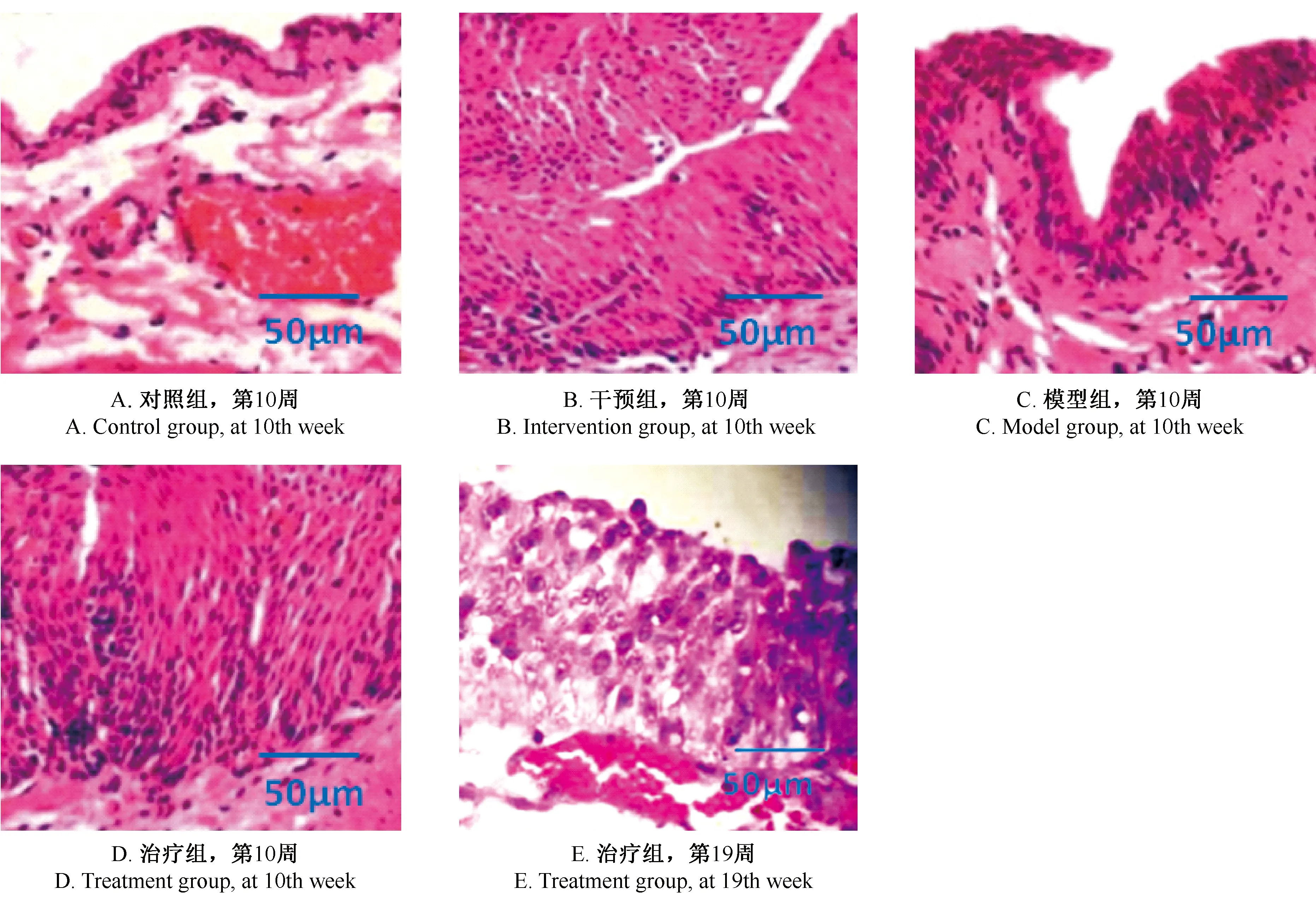
图2 各组病理切片情况Fig.2 Pathological changes in the bladder tissues of the rats(HE staing)
2.5Westernblot检测凋亡相关蛋白表达结果
有图3结果推测,姜黄素对Bcl-2和Bax在mRNA表达水平的影响是姜黄素能调节T24细胞Bcl-2和Bax mRNA的表达水平(以GAPDH为内参),不断提高姜黄素浓度,使得Bcl-2的表达水平下降,Bax的表达水平有所上调。
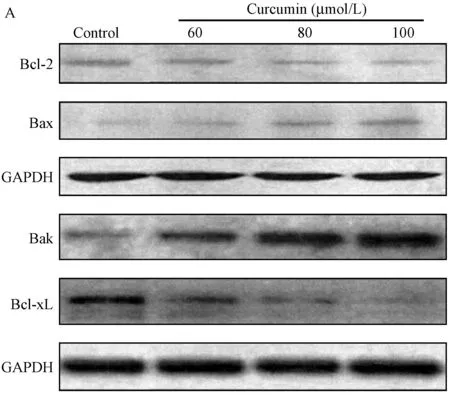
注:内参选用GAPDH基因的表达水平。三次重复实验的数值表示为均数士标准差。*P<0.05, **P<0.01对照组和实验组有统计学意义。图3 姜黄素作用于T24细胞持续48 h, 提取蛋白分析姜黄素对Bcl-2、Bcl-xl和Bax、Bak mRNA的表达水平的影响Note. The expression levels of gene GAPDH are as the internal reference. The results of three repeated tests are expressed as ±s. The differences of the control and experimental groups are statistically significant (P<0.05 and P<0.01, respectively).Fig.3 T24 cells are treated with curcumin for 48 hours. The effects on the expression level of Bcl-2, Bcl-xl, Bax and Bak mRNA are analyzed by protein extraction
3 讨论
膀胱癌是现在人类泌尿系的高发肿瘤疾病,膀胱癌的发病机制尚不明确,现在临床治疗膀胱癌大多采取切除膀胱以及膀胱灌注卡介苗[5],但是膀胱癌的复发以及膀胱灌注后的不良反应是该治疗方法的限制[6-8],为了解决该难题,近年来向中医药方向探索寻求低毒高效的药物,姜黄科植物中的姜黄素类化合物,调查研究发现,其是毒副作用小的广谱类抗癌化合物能广泛抑制淋巴癌、乳腺癌、前列腺癌等诸多癌症细胞的生长[9-11]。本文通过研究姜黄素对MNU诱导的膀胱癌大鼠的化学干预作用及作用机制的分析,为人类膀胱癌的治愈提供实验依据,开发有效的治疗手段,为临床膀胱癌治疗研究找到突破口[12,13]。
本文研究发现,模型组在第10周时膀胱癌的发生率为90% (9/10),干预组在第10周时大鼠膀胱癌的发生率为12.5% (5/40),治疗组第10周时膀胱癌的发生率为 92.5% (37/40),比较干预组与模型组大鼠膀胱癌的发生率差异有显著性(P<0.05),说明姜黄素对MUN诱发膀胱癌大鼠有明显的化学干预作用;在治疗组膀胱癌形成后给予姜黄素治疗,第19周膀胱癌发生率为78.4%(30/37),与治疗前的第10周比较说明姜黄素对膀胱癌有治疗作用,可以延缓膀胱癌的复发。TUNEL实验证实姜黄素显著促进膀胱癌细胞的凋亡,能有效抑制膀胱癌细胞的生成和增加膀胱癌细胞的凋亡,达到膀胱癌的治疗作用以及降低膀胱癌的复发。Western blot 结果发现,姜黄素抑制NF-κB的激活,有效下调NF-κB调节的基因产物的表达。本文研究显示,MUN诱发膀胱癌大鼠经过姜黄素治疗后膀胱癌的发病率有下降,说明姜黄素对MNU诱导膀胱癌大鼠的化学干预作用明显,有治疗作用。
综上所述,姜黄素对MNU诱导膀胱癌大鼠有明显的化学干预作用,其作用机制是增加膀胱癌细胞的凋亡,抑制膀胱癌细胞的生成,抑制NF-κB的激活并且有效下调NF-κB和Bcl-2的表达调节的基因产物,来调节膀胱癌中相关蛋白的表达机制,进一步发挥抗癌的干预作用,降低复发率。
[1] Logan C, Brown M, Hayne D. Intravesical therapies for bladder cancer — indications and limitations [J]. BJU Int, 2012, 110(Suppl 4): 12-21.
[2] Coenen MJ, Ploeg M, Schijvenaars MM, et al. Allelic imbalance analysis using a single-nucleotide polymorphism microarray for the detection of bladder cancer recurrence [J]. Clin Cancer Res. 2008, 14(24): 8198-8204.
[3] Cheng AL, Hsu CH, Lin JK, et al. Phase Ⅰ clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions [J]. Anticancer Res, 2001, 21(4B): 2895-2900.
[4] Liang Z, Xie W, Wu R, et al. Inhibition of tobacco smoke-induced bladder MAPK activation and epithelial-mesenchymal transition in mice by curcumin [J]. Int J Clin Exp Pathol, 2015, 8(5): 4503-4510.
[5] Böhle A, Brandau S. Immune mechanisms in bacillus Calmette-Guerin immunotherapy for superficial bladder cancer [J]. J Urol, 2003, 170(3): 964-969.
[6] Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of non-muscle invasive bladder cancer (stages Ta,T1,and Tis): 2007 update [J]. J Urol, 2007, 178(6): 2314-2330.
[7] Lamm DL, Vandermeijden AP, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer [J]. J Urol, 1992, 147(3,1): 596-600.
[8] Koga H, Kuroda M, Kudo S, et al. Adverse drug reactions of intravesical bacillus Calmette-Guerin instillation and risk factors of the development of adverse drug reactions in superficial cancer and carcinoma in situ of the bladder [J]. Int J Urol,2005,12(2): 145-151.
[9] Zhang CL, Li BQ, Zhang X, et al. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients’PBMCs: potential role for stat-3 and NF-κB signaling [J]. J Invest Dermatol, 2010, 130(8): 2110-2119.
[10] Robert BM, Kwiatkowski F, Leheurteur M, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer [J]. Cancer Biol Ther, 2010,9(1): 8-14.
[11] Ide H, Tokiwa S, Sakamaki K, et al. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate specific antigen [J]. Prostate, 2010, 70(10): 1127-1133.
[12] Aggarwal S, Ichikawa H, Takada Y, et al. Curcumin (diferuloymethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IκBα kinase and Akt activation [J]. Mol Pharmacol, 2006, 69(1): 195-206.
[13] Tharakan ST, Inamoto T, Sung B, et al. Curcumin potentiates the antitumor effects of gemcitabine in an orthotopic model of human bladder cancer through suppression of proliferative and angiogenic biomarkers [J]. Biochem Pharmacol, 2010, 79(2): 218-228.
EffectofcurcuminonchemicalinterventionandmechanismofMNU-inducedbladdercancerinrats
WU Jin-sheng1, WANG Qing-ming2, ZHENG Chuan-qiu2, JI Meng2, SUN Li-jiang1*
(1. Qingdao University, Qingdao 266071, China; 2. Weifang Medical College, Weifang 261053)
ObjectiveTo study the effect of curcumin on rat model of N-methylnitrosourea (MNU)-induced bladder cancer and its mechanism.MethodsOne hundred SD rats were randomly divided into four groups: control group (n=10), model group (n=10), intervention group (n=40) and treatment group (n=40). Rats in the control group
intravesical infusion of distilled water. Rats in the other three groups were given MNU (1 mg/mL) in 2 mL saline at 2nd, 4th, 6th and 8th weeks to induce bladder cancer. In the model group, the rats were injected with distilled water in the bladder. The rats in the intervention group received 2 mL curcumin solution (400 μmol/L) at the 1st, 3rd, 5th, 7th and 9th weeks, and were sacrificed at the 11th week. In the model group, the rats were injected with distilled water in the bladder. In the treatment group, the rats had intravesical instillation of curcumin in the bladder (400 μmol/L, 2 mL) at 10, 12, 14, 16, and 18 weeks, and sacrificed at the 19th week. Bladder tissue samples were taken for pathological examination using hematoxylin and eosin (HE) staining. TUNEL staining assay was used to detect the apoptosis in tumor tissue. The expression of apoptosis-related proteins was detected by Western blot.ResultsThe incidence of bladder cancer was 90% (9/10) in the model group, 12.5% (5/40) in the intervention group and 92.5% (37/40) in the treatment group at the 10th week, showing a significant difference between the intervention group and model group (P<0.05), indicating an obvious interventional effect of curcumin on the bladder cancer. The incidence rate of bladder cancer in the treatment group was 78.4% (30/37) at the 19th week, and compared with the 10th week before treatment, showing that curcumin can delay the recurrence of bladder cancer. TUNEL staining assay confirmed that curcumin significantly promoted the apoptosis in bladder cancer cells and inhibited their proliferation. The Western blot analysis showed that curcumin inhibited the activation of NF-κB and effectively down-regulated the expression of NF-κB-regulated gene product.ConclusionsCurcumin has a significant interventional effect on MNU-induced bladder cancer in the rat models. The mechanism may be through inhibition of NF-κB activation and effective down-regulated NF-κB regulation of the gene products, and to regulate the expression of related proteins in bladder cancer, i.e., inhibition of proliferation, induction of apoptosis, and further play a role of anti-cancer intervention and prevention of bladder cancer recurrence.
Curcumin; N-methylnitrosourea; Bladder cancer; Chemical intervention; Mechanism of action; Rats
SUN Li-jiang,E-mail: sywang@ahmu.edu.cn
Q95-33
A
1005-4847(2017) 05-0567-05
10.3969/j.issn.1005-4847.2017.05.018
2017-02-15
吴金生(1975年-),男,副教授,青岛大学在读博士生,研究方向:泌尿外科。 Email:wjswfmc@163.com
孙立江(1963年-),男,教授,博士生导师。
