全氟和多氟化合物替代品的研究进展
2017-10-13周秀鹃盛南王建设戴家银
周秀鹃,盛南,王建设,戴家银
中国科学院动物研究所,中国科学院动物生态与保护生物学重点实验室,北京 100101
全氟和多氟化合物替代品的研究进展
周秀鹃,盛南,王建设,戴家银*
中国科学院动物研究所,中国科学院动物生态与保护生物学重点实验室,北京 100101
全氟和多氟化合物(per-and polyfluoroalkyl substances, PFASs)是一类新型持久性有机污染物(POPs),广泛应用于工业和人类日常生活用品中。此类化合物具有高能量的C-F共价键,因此具有优良的理化特性和生物稳定性。由于存在持久性、生物累积性、长距离迁移以及毒性等问题,长链PFASs(C>7)已经成为全世界关注的焦点之一,寻找能够替代PFASs的新型化合物具有重要意义。本文介绍了几种可能替代PFASs的新型氟化替代品,PFASs替代品在各类环境介质中的分布、持久性、人体暴露及毒性等几个方面进行了综述,特别对目前存在的问题及今后的研究方向进行了讨论和展望,以期为PFASs替代品的环境污染及风险评估提供参考。
全氟和多氟化合物;PFASs替代品;环境行为;人体暴露;毒性
Received18 June 2016accepted22 August 2016
Abstract: Per-and polyfluoroalkyl substances (PFASs) are an emerging kind of persistent organic pollutant currently widely used in industrial and daily life supplies. With high-energy C-F bonds, PFASs have excellent physical, chemical properties and biological stability and have drawn the attention of researchers all over the world due to their high persistence, bioaccumulation potential, toxicity and ubiquitous distribution in the environment, biota and humans. It is of vital importance to look for fluorinated alternatives to long-chain PFASs. This paper summarizes hot issues about PFASs alternatives, such as environmental distribution and behavior, persistence, human exposure and toxicity. The emphasis is laid on existing problems and future research perspectives so as to provide evidence for the investigation of existing problems and future research directions.
Keywords: per-and polyfluoroalkyl substances; PFASs alternatives; environmental behavior; human exposure; toxicity
全氟和多氟化合物(per-and polyfluoroalkyl substances, PFASs)是一类高度氟化的脂肪族物质,即除官能团中的氢原子外,碳骨架上的氢原子全部或部分被氟原子替代的人工合成有机化合物[1]。由于PFASs的理化特性极其特殊,包括较强稳定性、疏水、疏油性等,被广泛地应用于地毯、皮革、纺织、包装、灭火泡沫、洗发香波、地板打磨、电镀等工业和民用领域。目前环境中存在的PFASs主要有全氟烷基羧酸(PFCAs)、全氟烷基磺酸(PFSAs)、全氟烷基磺酰胺(FOSAs)、氟化调聚醇(FTOHs)、全氟磷酸(膦酸)及其酯等,其中全氟辛烷羧酸(perfluorooctanoic acid, PFOA)和全氟辛烷磺酸(perfluorooctane sulfonic acid, PFOS)是目前最受关注和应用最广泛的2种典型全氟有机化合物。由于结构中含有高能量的C-F化学键(C-F: 485.3 kJmol-1),该类化合物普遍具有很高的稳定性,难以水解、光解和被微生物降解,因此许多PFASs具有环境持久性及高生物累积性[2]。大量研究表明PFASs已在各种环境介质和生物体中广泛检出,包括表层水[3-5]、污泥[6-7]、沉积物[8]、灰尘[9-10]、海洋生物[11-12]等,甚至人体中也检出多种PFASs[13-15]。流行病学研究发现PFASs的人体暴露与一些生化和生理指标的改变存在一定的正相关,包括肾癌和睾丸癌[16]、溃疡性结肠炎[17]、孕期高血压[18]、高胆固醇[19-20]、甲状腺机能减退[21-22]、免疫系统疾病[23-24]、胎儿生长迟缓[25-27]、妊娠期糖尿病[28]等。此外,毒理学研究也发现PFASs具有肝毒性[29]、神经毒性[30]、生殖发育毒性[29,31]、免疫毒性[29]、致癌性[32]、干扰脂肪代谢[33]以及内分泌干扰效应[34-35]等多种毒性。
由于PFASs具有持久性、累积性、长距离迁移以及高毒性等特性,严重地威胁了生态环境和人体健康,2001年国际社会共同签署了《关于持久性有机污染物(Persistent Organic Pollutants,简称POPs)的斯德哥尔摩公约》,开启了保护环境和人类健康免受有毒污染物危害的全球行动。3M公司于2002年停止生产PFOS及其相关产品。2009年5月9日联合国环境规划署正式将PFOS及全氟辛基磺酰氟(PASF)等列为新的持久性有机污染物,同意减少并最终禁止使用该类物质。最近,欧洲化学品管理局(ECHA)公布提案,建议将PFOA、全氟辛酸铵(APFO)以及C11~C14 PFCAs等化学品列为高度关注物质[36]。
长链PFASs的安全问题已经引发全球研发者和使用者的高度关注。淘汰部分长链PFASs,虽然减少了含氟聚合物生产过程中某些长链PFASs的使用量及排放量,但仍然无法从根本上解决长链PFASs的环境问题。为了适应全球化的发展,保护自身环境,研发无潜在生物蓄积性、低毒性的高性能化合物已迫在眉睫,寻找和研发新的PFASs替代品成为近年来科研工作者研究的热点之一。本文在2篇已有综述的基础上[37-38],概述了几种可能替代PFASs的新型化合物及其持久性、人体暴露及毒性等,对目前存在的问题及今后的研究方向进行了讨论和展望,以期为PFASs替代品的环境污染及风险评估提供参考。
1 长链PFASs替代品分类(The classification of long chain PFASs alternatives)
美国环境保护署(USEPA)提出禁用PFOA以后,其国内外就开展了PFASs替代品研究并取得了实质进展。迄今为止,3M、大金、杜邦、旭硝子、阿科玛和苏威在内的国际氟化工生产商已经向USEPA上报了50余种PFASs替代品以待评估。如3M公司研发的PFOS替代品全氟丁基磺酸(PFBS)无明显生物积累性,短时间内可随人体新陈代谢排出体外。由杜邦等公司利用调聚反应生产的全氟烷基C6基产品,由于没有C8基成分,没有PFOS及其衍生物,也不产生PFOA。这些调聚物基含氟表面活性剂很可能降解为C6F13CH2CH2SO3H(6:2 FTSA)或C6F13CH2CH2COOH(6:2 FTCA),而不是PFOS或PFOA,其毒性较C8小。国内外针对PFASs替代品的研发一般分为2类:(1)使用C4、C6结构的短链全氟烷基羧酸或磺酸盐,如PFBA、PFHxA、PFBS。(2)含功能官能团的全氟聚醚(PFPEs),尤其是全氟聚醚羧酸和磺酸(PFESAs和PFECAs),如6:2 FTCA、GenX、F-53B、6:2 FTSA。各化合物的结构和名称如表1所示。
2 PFASs替代品的环境含量以及应用(The content, and application of PFASs alternative in environment)
一些研究表明作为C8全氟化合物替代物的C4全氟化合物在环境中的浓度不断升高。Zhou等[39]在武汉汤逊湖氟化学工厂附近的水样检测到高浓度的PFBA和PFBS等短链全氟化合物,其浓度分别为3 660 ngL-1、4 770 ngL-1。6:2 FTSA作为PFOS的一种替代物,近年来被越来越多地使用在装饰性电镀行业和泡沫灭火器的生产过程中,如杜邦公司以6:2 FTSA合成了Forafac®1176。此外,6:2 FTSA还是Capstone®FS-17的主要成分[40]。最近在水体[41-44]、污泥[42,45-46]、消防基地[47]、城市垃圾填埋场渗滤液[48]等中都发现了6:2 FTSA。在我国,PFOS的替代物F-53B作为雾抑制剂广泛地应用于镀铬工业。Wang等[49]在温州一个镀铬工业的废水处理厂的进水、出水中检测到了高浓度的F-53B。由于污水处理厂不能够有效去除该类物质,在污水处理厂排放的地表水中也可以检测到高浓度的F-53B。最近一些研究发现在污泥中也检测到F-53B。据统计,F-53B年产量高达10多吨,其销售保持逐年稳步增长的态势。由于全球禁止使用PFOS,F-53B在未来可能会有更大的市场份额[50-51]。在欧洲,PFOA替代物GenX作为加工助剂广泛应用于氟聚树脂制造业,年产量高达10~100吨[50]。此外,在中国含氟乳化剂用6:2 FTCA替代了PFOA。目前在水体、污泥等介质中均已检测到PFASs替代物的存在(表2)。

表1 全氟和多氟化合物(PFASs)及其替代品Table 1 Per- and polyfluoroalkyl substances (PFASs) and PFASs alternatives
3 生物及人体暴露水平(The exposure of PFASs alternatives in the human and organisms)
自2002年3M公司终止全氟辛烷磺酰氟(POSF)的生产和PFOS正式进入POPs名单以来,PFASs在环境中的浓度虽然有所下降,但是PFASs替代物在生物体不断检出。一项研究表明,在东格陵兰岛海洋哺乳动物环海豹、北极熊、虎鲸中发现了高浓度F-53B[53]。Shi等[54]在小清河和汤逊湖中鲫鱼血清、肾、性腺、肝脏以及心脏中检出了F-53B。同时Shi等[55]研究发现食鱼偏好者、镀铬工人以及普通人群尿液和血清也存在F-53B。另一项对6:2 FTSA的检测发现,6:2 FTSA也存在于生物以及人体中(表3)。
暴露途径研究是准确认识污染物健康效应的一个重要前提。Shi等[55]研究认为灰尘/空气、饮用水和食物可能是PFASs替代物人体暴露的重要途径。偏好食鱼的人群血清中F-53B浓度中值是普通人的20倍,说明淡水鱼可能是人体暴露的重要途径。镀铬工人血清中浓度也远远超过普通人,可能通过灰尘/空气、皮肤接触等暴露途径。最近一项研究也发现,通过罗纳河的沉积物暴露,钩虾体内发现了6:2 FTSA,因此食物和呼吸可能是钩虾暴露的途径[42]。
高度重视PFASs替代物的人体累积和清除是科学研究的一个热点。与普通人群不同的是,一些特殊人群可能面临比普通人群更高或者不同特征的PFASs替代品暴露,这种情况值得高度关注。一项对食鱼偏好者、镀铬工人、以及普通人体血液和尿液中血清中的F-53B和PFOS浓度清除半衰期的研究表明,人体对F-53B的血液清除较慢,半衰期较长,其半衰期的中值分别为15.3和6.7年,F-53B的半衰期显著长于PFOS[55]。
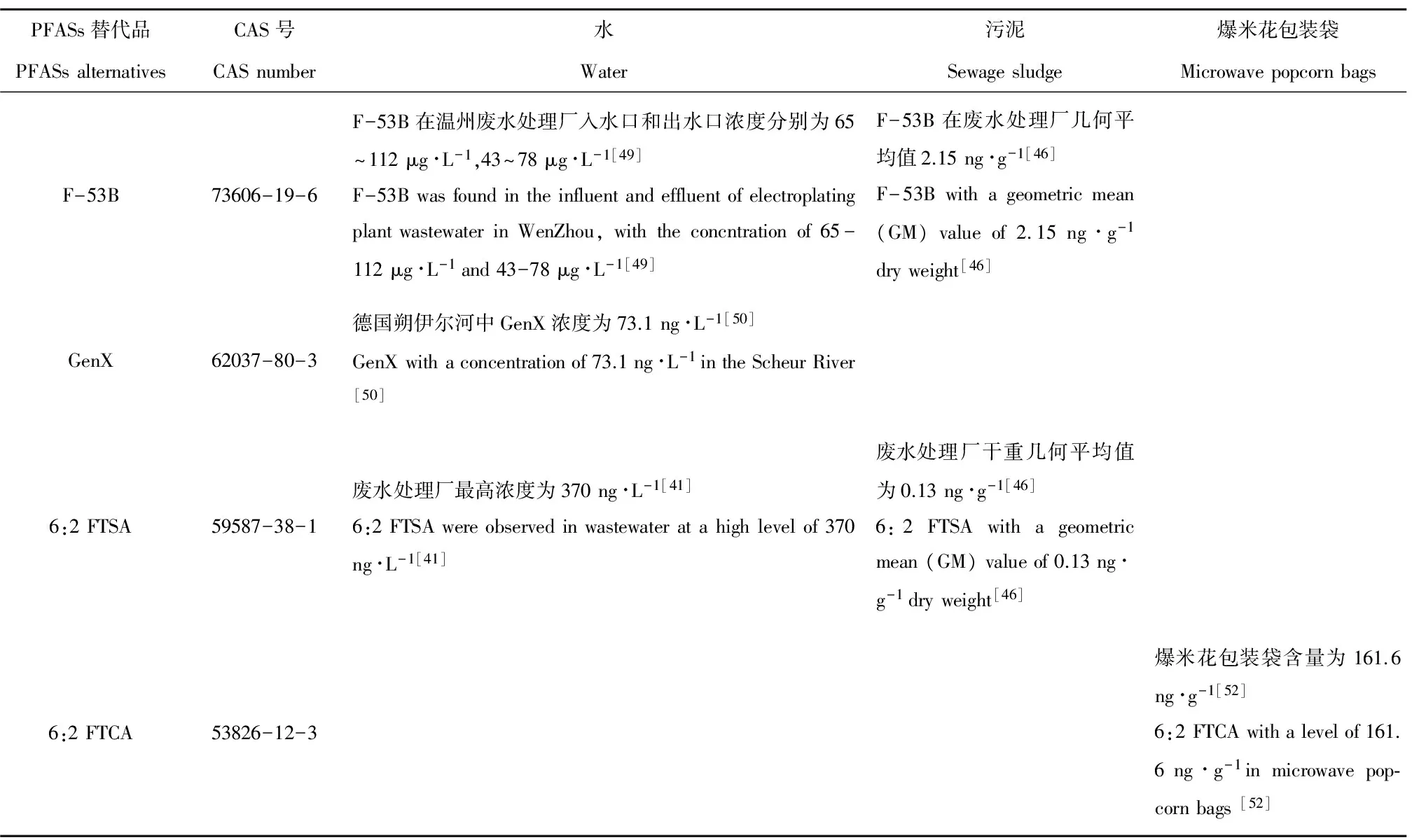
表2 PFASs替代品在环境中的检出量Table 2 The detection of PFASs alternatives in the environment
4 PFASs替代品的潜在影响(The potential impact of PFASs alternatives)
最近一些研究表明短链全氟替代物生物富集因子较低,基本没有生物富集趋势[42,60]。但仍有研究学者提出部分PFASs替代物具有持久性、生物累积性以及毒性等问题。最近研究发现有些替代物的降解产物仍然具有毒性。如全氟丁基磺酰氟(PBSF)和6:2全氟调聚物最终降解为短链PFASs和其他物质,如高毒性的碳酰氟(COF2)[61]。因此,PFASs替代物对环境和生态影响仍然值得关注。关于PFASs替代物的特性见表4。
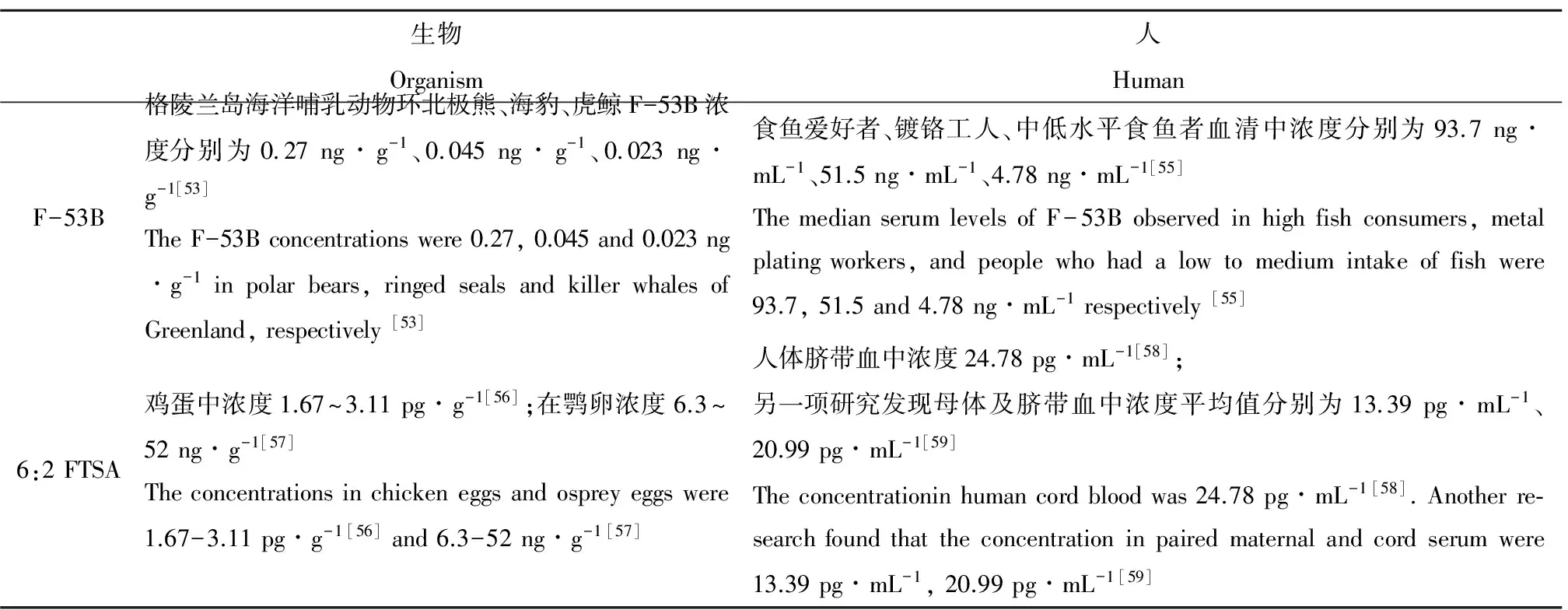
表3 PFASs替代品在生物及人体组织中的检出量Table 3 The detection of PFASs alternatives in the human and organisms
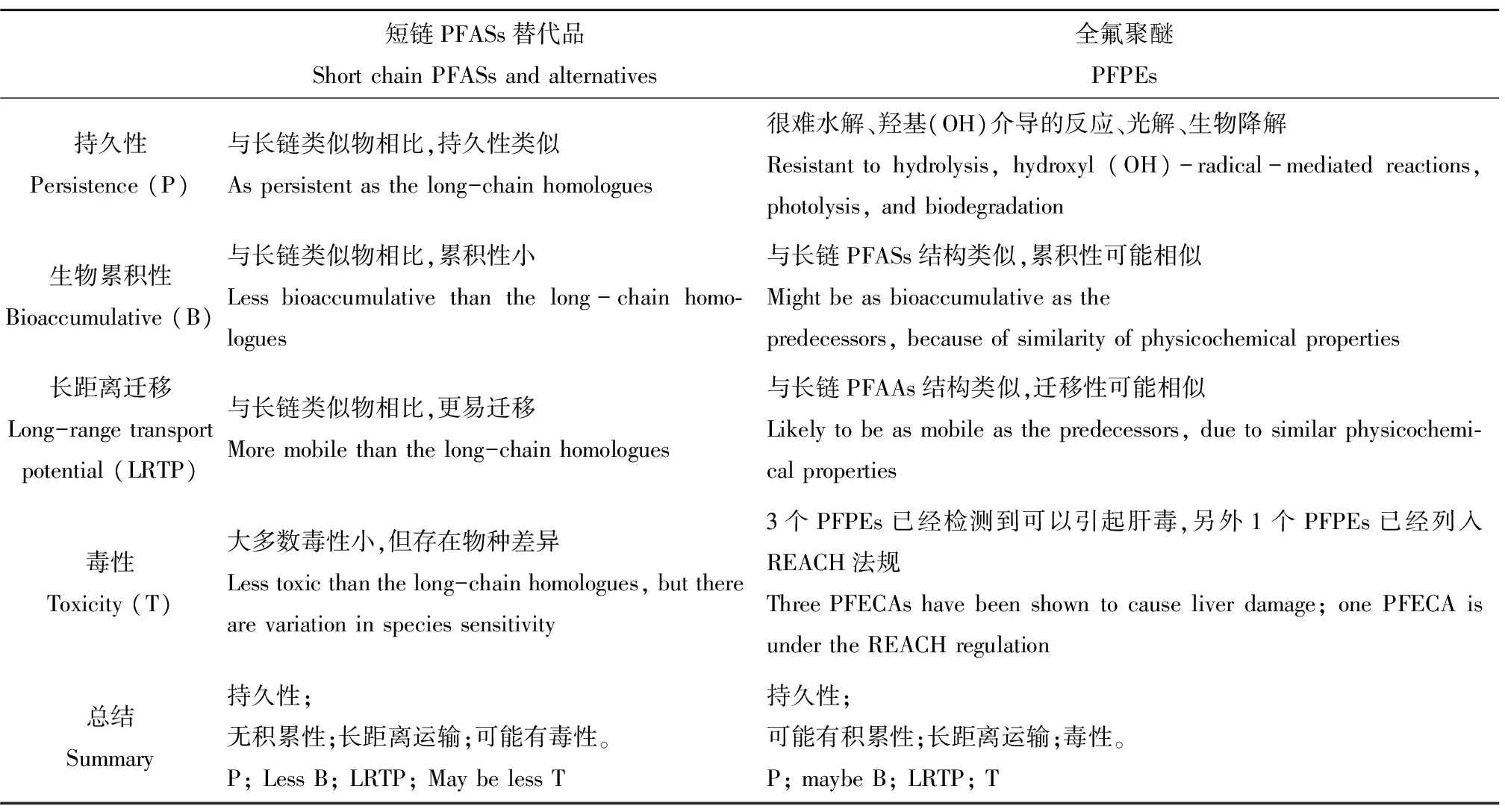
表4 短链PFASs和全氟聚醚(PFPEs)的理化性质Table 4 The physical and chemical properties of short chain PFASs and perfluoropolyether (PFPEs)
4.1 持久性
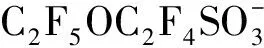
4.2 生物累积性
生物累积性是衡量污染物是否属于POPs的一个极其重要依据,也是近年来PFASs研究的一个重要内容。与长链类似物相比,短链全氟化合物在生物和人体内基本没有生物累积趋势[60],但在植物的叶、茎、果实中很易积累[69-70]。最近研究发现,6:2 FTSA对虹鳟鱼的生物富集系数< 40,同时研究发现虹鳟鱼对6:2 FTSA的食物吸收效率、生长半衰期以及食物放大因子分别为0.435、23.1和0.295,因此虹鳟鱼对6:2 FTSA的积累性很低[71]。Shi等[55]对不同人群血液及尿液中的F-53B的消除动力学进行了分析,F-53B的血液清除半衰期小于PFOS的清除半衰期,但是由于物种的敏感度、灌喂的剂量方法以及实验技术等原因,关于PFECAs和PFESAs的生物累积性还需要更多的验证。
4.3 长距离迁移
许多PFASs具有POPs的远距离迁移特性,与长链类似物相比,具有较高水溶性、低吸附性的短链PFASs更易在环境中迁移[72-73]。PFECAs和PFESAs不仅与PFCAs和PFSAs结构类似,而且具有持久性,因此在水中也可能具有长距离运输的潜力[74]。到目前为止,还未在偏远地区发现PFECAs和PFESAs,可能由于(1)有些PFECAs和PFESAs近期才开始使用,使用量少并且排放量少;(2)PFECAs和PFESAs可能以前体形式存在;(3)排放和迁移到偏远地区形成污染物之间有一定的时间间隔;(4)没有灵敏的检测方法以及检测标准。
4.4 毒性
高度重视人体健康效应研究是PFASs环境研究的一个前沿和重点领域。研究表明,与长链类似物相比,大多数的短链PFASs替代品对人体和环境中生物没有明显的毒性效应[75-76]。在对水生生物的急性和慢性毒性暴露研究发现,6:2 FTSA对水生生物几乎没有毒性。如虹鳟鱼96 h半数效应浓度(96 h-LC50)> 107 mgL-1,大型蚤48 h的半数效应浓度(48 h-EC50) > 112 mgL-1[72]。Hoke等[77]研究发现6:2 FTCA对水蚤的48 h-LC50> 97.5 mgL-1,海藻72 h-EC50= 47.9 mgL-1,摇蚊10 d-LC50= 75.2 mgL-1,浮萍7 d-EC50= 1.3 mgL-1。根据美国环保署毒品管理条例(USEPA TSCA)中水生动物毒性评估条例的规定,急性毒性的参考值为1 mgL-1< EC/LC50< 100 mgL-1,因此,6:2 FTCA对水生无脊椎动物毒性并不大。Mitchell等[78]也研究发现6:2 FTCA对淡水无脊椎动物的毒性不大。如6:2 FTCA抑制小球藻生长的EC50= 26.2 mgL-1,对月牙藻的生长抑制EC50>53 mgL-1,而对端足虫10 d的死亡LC50= 33.1 mgL-1。Phillips等[79]也发现6:2 FTCA抑制摇蚊生长的EC50= 63 mgL-1。Wang等[38]研究发现F-53B对斑马鱼毒性与PFOS类似,其96 h-LC50分别为15.5 mgL-1和17 mgL-1。GenX不仅对皮肤、眼睛造成刺激,在低剂量浓度连续暴露(≤ 10 mgkg-1d-1)还可能引起肝癌[70]。关于PFASs替代品的毒性还需要更多的实验验证。

表5 PFASs替代品的降解性实验Table 5 The degradability of PFASs alternatives
5 PFASs替代品研究存在的问题与展望(The existing problems and perspectives of PFASs alternatives)
PFASs替代品对人和环境是否安全?最近一些研究发现结构类似物的替换并不能真正解决问题,相反会出现“锁定”问题[38]。为了解决这一问题,PFASs替代品的信息如特性、产量、用量、排放量以及毒性需要公开。然而,目前由于新型化合物研发费用高、时间长以及公司为了增加竞争力,PFASs替代品信息并未完全公开。同时对PFASs替代品的环境行为及生态毒理效应方面的研究也尚未系统开展。今后应该开展如下研究:鼓励公司公开替代品的信息,加强各部门之间的交流;注重PFASs替代品在各环境介质以及人体内的监测,加快对PFASs替代品在各种环境介质降解机制的研究;完善新的替代品在环境介质和生物中的分析方法,以使分析数据具有可比性;系统地进行全氟替代品的来源、分布、迁移转化等研究;重视暴露途径、生物有效性的研究,并与风险评估相结合;开展低剂量、长期、慢性毒性和复合毒性研究,整合多种组学从分子、基因等水平研究其毒性机制,这些研究结果为研发新的无潜在生物蓄积性、无毒性的高性能化合物提供技术支撑。
[1] Buck R C, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins [J]. Integrated Environmental Assessment Management, 2011, 7(4): 513-541
[2] Olsen G W, Burris J M, Ehresman D J, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers [J]. Enviromental Health Perspectives, 2007, 115(9): 1298-1305
[3] Moody C A, Martin J W, Kwan W C, et al. Monitoring perfluorinated surfactants in biota and surface water samples following an accidental release of fire-fighting foam into Etobicoke Creek [J]. Environmental Science & Technology, 2002, 36(4): 545-551
[4] Moody C A, Kwan W C, Martin J W, et al. Determination of perfluorinated surfactants in surface water samples by two independent analytical techniques: Liquid chromatography/tandem mass spectrometry and 19F NMR [J]. Analytical Chemistry, 2001, 73(10): 2200-2206
[5] Lu Z B, Song L N, Zhao Z, et al. Occurrence and trends in concentrations of perfluoroalkyl substances (PFASs) in surface waters of eastern China [J]. Chemosphere, 2015, 119: 820-827
[6] Gomez-Canela C, Barth J A, Lacorte S. Occurrence and fate of perfluorinated compounds in sewage sludge from Spain and Germany [J]. Environmental Science and Pollution Research International, 2012, 19(9): 4109-4119
[7] Yan H, Zhang C J, Zhou Q, et al. Short- and long-chain perfluorinated acids in sewage sludge from Shanghai, China [J]. Chemosphere, 2012, 88(11): 1300-1305
[8] Higgins C P, Field J A, Criddle C S, et al. Quantitative determination of perfluorochemicals in sediments and domestic sludge [J]. Environmental Science & Technology, 2005, 39(11): 3946-3956
[9] Shoeib T, Hassan Y, Rauert C, et al. Poly- and perfluoroalkyl substances (PFASs) in indoor dust and food packaging materials in Egypt: Trends in developed and developing countries [J]. Chemosphere, 2016, 144: 1573-1581
[10] Yao Y M, Sun H W, Gan Z W, et al. Nationwidedistribution of per- and polyfluoroalkyl substances in outdoor dust in mainland China from eastern to western areas [J]. Environmental Science & Technology, 2016, 50(7): 3676-3685
[11] Kannan K, Koistinen J, Beckmen K, et al. Accumulation of perfluorooctane sulfonate in marine mammals [J]. Environmental Science & Technology, 2001, 35(8): 1593-1598
[12] Rotander A, Karrman A, van Bavel B, et al. Increasing levels of long-chain perfluorocarboxylic acids (PFCAs) in Arctic and North Atlantic marine mammals, 1984-2009 [J]. Chemosphere, 2012, 86(3): 278-285
[13] Olsen G W, Church T R, Miller J P, et al. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors [J]. Environmental Health Perspectives, 2003, 111(16): 1892-1901
[14] Starling A P, Engel S M, Whitworth K W, et al. Perfluoroalkyl substances and lipid concentrations in plasma during pregnancy among women in the Norwegian Mother and Child Cohort Study [J]. Environmental International, 2014, 62: 104-112
[15] Kato K, Wong L Y, Jia L T, et al. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008 [J]. Environmental Science & Technology, 2011, 45(19): 8037-8045
[16] Barry V, Winquist A, Steenland K. Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant [J]. Environmental Health Perspectives, 2013, 121(11-12): 1313-1318
[17] Steenland K, Zhao L, Winquist A, et al. Ulcerative colitis and perfluorooctanoic acid (PFOA) in a highly exposed population of community residents and workers in the mid-Ohio valley [J]. Environmental Health Perspectives, 2013, 121(8): 900-905
[18] Darrow L A, Stein C R, Steenland K. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005-2010 [J]. Environmental Health Perspectives, 2013, 121(10): 1207-1213
[19] Winquist A, Steenland K. Modeled PFOA exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts [J]. Environmental Health Perspectives, 2014, 122(12): 1299-1305
[20] Nelson J W, Hatch E E, Webster T F. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population [J]. Environmental Health Perspectives, 2010, 118(2): 197-202
[21] Winquist A, Steenland K. Perfluorooctanoic acid exposure and thyroid disease in community and worker cohorts [J]. Epidemiology, 2014, 25(2): 255-264
[22] Lopez-Espinosa M J, Mondal D, Armstrong B, et al. Thyroid function and perfluoroalkyl acids in children living near a chemical plant [J]. Environmental Health Perspectives, 2012, 120(7): 1036-1041
[23] Okada E, Sasaki S, Saijo Y, et al. Prenatal exposure to perfluorinated chemicals and relationship with allergies and infectious diseases in infants [J]. Environmental Research, 2012, 112: 118-125
[24] Grandjean P, Andersen E, Budtz-Jørgensen E, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds [J]. Jama-Journal of the American Medical Association, 2012, 307(4): 391-397
[25] Fei C, McLaughlin J K, Tarone R E, et al. Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort [J]. Environmental Health Perspectives, 2007, 115(11): 1677-1682
[26] Fei C, McLaughlin J K, Tarone R E, et al. Fetal growth indicators and perfluorinated chemicals: A study in the Danish National Birth Cohort [J]. American Journal of Epidemiology, 2008, 168(1): 66-72
[27] Lam J, Koustas E, Sutton P, et al. The Navigation Guide—Evidence-based medicine meets environmental health: Integration of animal and human evidence for PFOA effects on fetal growth [J]. Environmental Health Perspectives, 2014, 122(10): 1040-1051
[28] Zhang C, Sundaram R, Maisog J, et al. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes [J]. Fertil Steril, 2015, 103(1): 184-189
[29] Lau C, Anitole K, Hodes C, et al. Perfluoroalkyl acids: A review of monitoring and toxicological findings [J]. Toxicological Sciences, 2007, 99(2): 366-394
[30] Mariussen E. Neurotoxic effects of perfluoroalkylated compounds: Mechanisms of action and environmental relevance [J]. Archives of Toxicology, 2012, 86(9): 1349-1367
[31] Lau C, Butenhoff J L, Rogers J M. The developmental toxicity of perfluoroalkyl acids and their derivatives [J]. Toxicology and Applied Pharmacology, 2004, 198(2): 231-241
[32] Negri S, Maestri L, Esabon G, et al. Characteristics, use and toxicity of fluorochemicals: Review of the literature [J]. Giornale Italiano di Medicina del Lavoro ed Ergonomia, 2008, 30(1): 61-74
[33] Zhang L, Ren X M, Guo L H. Structure-based investigation on the interaction of perfluorinated compounds with human liver fatty acid binding protein [J]. Environmental Science & Technology, 2013, 47(19): 11293-11301
[34] Knox S S, Jackson T, Javins B, et al. Implications of early menopause in women exposed to perfluorocarbons [J]. Journal of Clinical Endocrinology & Metabolism, 2011, 96(6): 1747-1753
[35] White S S, Fenton E, Hines E P. Endocrine disrupting properties of perfluorooctanoic acid [J]. Journal of Steroid Biochemistry and Molecular Biology, 2011, 127(1-2): 16-26
[36] European Chemical Agency (ECHA). Candidate list of substances of very high concern for authorisation [DB/OL]. (2013-12-21) [2016-06-18]. http://echa.europa.eu/web/guest/candidate-list-table
[37] Wang Z Y, Cousins T I, Scheringer M, et al. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions [J]. Environment International, 2015, 75: 172-179
[38] Wang Z Y, Cousins I T, Scheringer M, et al. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors [J]. Environment International, 2013, 60: 242-248
[39] Zhou Z, Liang Y, Shi Y L, et al. Occurrence and transport of perfluoroalkyl acids (PFAAs), including short-chain PFAAs in Tangxun Lake, China [J]. Environmental Science & Technology, 2013, 47(16): 9249-9257
[40] Yang X L, Huang J, Zhang K L, et al. Stability of 6:2 fluorotelomer sulfonate in advanced oxidation processes: Degradation kinetics and pathway [J]. Environmental Science Pollution Research, 2014, 21(6): 4634-4642
[41] Schultz M M, Barofsky D F, Field J A, et al. Quantitative determination of fluorinated alkyl substances by large-volume-injection liquid chromatographytandem mass spectrometry-characterization of municipal wastewaters [J]. Environmental Science & Technology, 2006, 40(1): 289-295
[42] Bertin D, Labadie P, Ferrari B J, et al. Potential exposure routes and accumulation kinetics for poly- and perfluorinated alkyl compounds for a freshwater amphipod: Gammarus spp. (Crustacea) [J]. Chemosphere, 2016, 155: 380-387
[43] Schiltz M M, Barofsky D F, Field J A. Quantitative determination of fluotelomer sulfonates in groudwater by LC MS/MS [J]. Environmental Science & Technology, 2014, 38: 1828-1835
[44] Houtz E F, Sutton R, Park J S, et al. Poly- and perfluoroalkyl substances in wastewater: Significance of unknown precursors, manufacturing shifts, and likely AFFF impacts [J]. Water Research, 2016, 95: 142-149
[45] Zhang S, Lu X X, Wang N, et al. Biotransformation potential of 6:2 fluorotelomer sulfonate (6:2 FTSA) in aerobic and anaerobic sediment [J]. Chemosphere, 2016, 154: 224-230
[46] Ruan T, Lin Y, Wang T, et al. Identification of novel polyfluorinated ether sulfonates as PFOS alternatives in municipal sewage sludge in China [J]. Environmental Science & Technology, 2015, 49(11): 6519-6527
[47] Baduel C, Paxman C J, Mueller J F. Perfluoroalkyl substances in a firefighting training ground (FTG), distribution and potential future release [J]. Journal of Hazardous Materials, 2015, 296: 46-53
[48] Huset C A, Barlaz M A, Barofsky D F, et al. Quantitative determination of fluorochemicals in municipal landfill leachates [J]. Chemosphere, 2011, 82(10): 1380-1386
[49] Wang S, Huang J, Yang Y, et al. First report of a Chinese PFOS alternative overlooked for 30 years: Its toxicity, persistence, and presence in the environment [J]. Environmental Science & Technology, 2013, 47(18): 10163-10170[50] Heydebreck F, Tang J, Xie Z, et al. Alternative and legacy perfluoroalkyl substances: Differences between European and Chinese river/estuary system [J]. Environmental Science & Technology, 2015, 49(14): 8386-8395
[51] 黄澄华, 李训生, 金广泉, 等. 电解氟化及其下游精细氟化工产品(待续)[J]. 化工生产与技术, 2010, 17(5): 1-9
Huang C H, Li X S, Jin G Q, et al. Electro fluorination and its fine-fluorine production branches [J]. Chemical Production and Technology, 2010, 17(5): 1-9 (in Chinese)
[52] Zabaleta I, Bizkarguenaga E, Bilbao D, et al. Fast and simple determination of perfluorinated compounds and their potential precursors in different packaging materials [J]. Talanta, 2016, 152: 353-363
[53] Gebbink W A, Bossi R, Riget F F, et al. Observation of emerging per- and polyfluoroalkyl substances (PFASs) in Greenland marine mammals [J]. Chemosphere, 2016, 144: 2384-2391
[54] Shi Y, Vestergren R, Zhou Z, et al. Tissue distribution and whole body burden of the chlorinated polyfluoroalkyl ether sulfonic acid F-53B in crucian carp (Carassius carassius): Evidence for a highly bioaccumulative contaminant of emerging [J]. Environmental Science & Technology, 2015, 49(24): 14156-14165
[55] Shi Y, Vestergren R, Xu L, et al. Human exposure and elimination kinetics of chlorinated polyfluoroalkyl ether sulfonic acids (Cl-PFESAs) [J]. Environmental Science & Technology, 2016, 50(5): 2396-2404
[56] Wang M, Wang Y X, Yang L, et al. Determination of perfluorosulfonate and perfluorocarboxylate precursors in eggs by ultra-highperformance liquid chromatography-mass spectrometry [J]. Zhonghua Yu Fang Yi Xue Za Zhi, 2016, 50(5): 439-444
[57] Eriksson U, Roos A, Lind Y, et al. Comparison of PFASs contamination in the freshwater and terrestrial environments by analysis of eggs from osprey (Pandion haliaetus), tawny owl (Strix aluco), and common kestrel (Falco tinnunculus) [J]. Environmental Research, 2016, 149: 40-47
[58] 石瑀. 全氟有机化合物及其前体物质的产前暴露水平及与胎儿生长发育的关系[D]. 北京: 中国疾病预防控制中心, 2014: 1-8
[59] Yang L, Wang Z, Shi Y, et al. Human placental transfer of perfluoroalkyl acid precursors: Levels and profiles in paired maternal and cord serum [J]. Chemosphere, 2016, 144: 1631-1638
[60] Conder J M, Hoke R A, De Wolf W, et al. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds [J]. Environmental Science & Technology, 2008, 42(4): 995-1003
[61] Center for Disease Control and Prevention. NIOSH Pocket Guide to Chemical Hazards [DB/OL]. (2011-04-11) [2016-06-18]. http://www.cdc.gov/niosh/npg/npgd0108.html.
[62] Parsons J R, Sáez M, Dolfing J, et al. Biodegradation of perfluorinated compounds [J]. Reviews of Environmental Contamination and Toxicology, 2008, 196: 53-71
[63] Young C J, Mabury S A. Atmospheric perfluorinated acid precursors: Chemistry, occurrence, and impacts [J]. Reviews of Environmental Contamination and Toxicology, 2010, 208: 1-109
[64] Hori H, Murayama M, Kutsuna S. Oxygen-induced efficient mineralization of perfluoroalkylether sulfonates in subcritical water [J]. Chemosphere, 2009, 77(10): 1400-1405
[65] Zhang K L, Cao Z G, Huang J, et al. Mechanochemical destruction of Chinese PFOS alternative F-53B [J]. Chemical Engineering Journal, 2016, 286: 387-393
[66] Park S, Lee L S, Medina V F. Heat-activated persulfate oxidation of PFOA, 6:2 fluorotelomer sulfonate, and PFOS under conditions suitable for in-situ groundwater remediation [J]. Chemosphere, 2016, 82(6): 376-383
[67] Wang N, Liu J, Buck R C, et al. 6:2 fluorotelomer sulfonate aerobic biotransformation in activated sludge of waste water treatment plants [J]. Chemosphere, 2011, 82(6): 853-858
[68] European Chemical Agency (ECHA). European Chemicals Agency Registered Substances [DB/OL]. (2014-04-03) [2016-06-18]. http://echa.europa.eu/information-on-chemicals/registered-substances
[69] Felizeter S, McLachlan M S, DeVoogt P. Root uptake and translocation of perfluorinated alkyl acids by three hydroponically grown crops [J]. Journal of Agricultural and Food Chemistry, 2014, 62(15): 3334-3342
[70] Krippner J, Brunn H, Falk S, et al. Effects of chain length and pH on the uptake and distribution of perfluoroalkyl substances in maize (Zea mays) [J]. Chemosphere, 2014, 94: 85-90
[71] Hoke R A, Barbra D, Ferrell B D, et al. Aquatic hazard, bioaccumulation and screening risk assessment for 6:2 fluorotelomer sulfonate [J]. Chemosphere, 2015, 128: 258-265
[72] Venkatesan A K, Halden R U. Loss and in situ production of perfluoroalkyl chemicals in outdoor biosolids-soil mesocosms [J]. Environmental Research, 2014, 132: 321-327
[73] Vierke L, Moller A, Klitzke S. Transport of perfluoroalkyl acids in a water-saturated sediment column investigated under near-natural conditions [J]. Environmental Pollution, 2014, 186: 7-13
[74] Gomis M I, Wang Z, Scheringer M, et al. A modeling assessment of the physicochemical properties and environmental fate of emerging and novel per- and polyfluoroalkyl substances [J]. Science of the Total Environment, 2015, 505: 981-991
[75] Borg D, Hakansson H. Environmental and health risk assessment of perfluoroalkylated and polyfluoroalkylated substances (PFASs) in Sweden, Report 6513 [R]. Stockholm: The Swedish Environmental Protection Agency, 2012
[76] Daikin. Measures concerning use of PFOA [DB/OL]. [2016-06-18]. http://www.daikin.com/chm/csr/.
[77] Hoke R A, Bouchelle L D, Ferrell B D, et al. Comparative acute freshwater hazard assessment and preliminary PNEC development for eight fluorinated acids [J]. Chemosphere, 2012, 87(7): 725-733
[78] Mitchell R J, Myers A L, Mabury S A, et al. Toxicity of fluorotelomer carboxylic acids to the algae Pseudokirchneriella subcapitata and Chlorella vulgaris, and the amphipod Hyalella azteca [J]. Ecotoxicology and Environmental Safety, 2011, 74(8): 2260-2267
[79] Phillips M M, Dinglasan-Panlilio M J, Mabury S A, et al. Fluorotelomer acids are more toxic than perfluorinated acids [J]. Environmental Science & Technology, 2007, 41(20): 7159-7163
◆
TheCurrentResearchStatusofSeveralKindsofFluorinatedAlternatives
Zhou Xiujuan, Sheng Nan, Wang Jianshe, Dai Jiayin*
Institute of Zoology, Chinese Academy of Sciences, Chinese Academy of Sciences Key Laboratory of Animal Ecology and Conservation Biology, Beijing 100101, China
10.7524/AJE.1673-5897.20160618001
2016-06-18录用日期2016-08-22
1673-5897(2017)3-003-10
X171.5
A
戴家银(1965—),男,博士,研究员,主要从事持久性污染物的生态毒理学研究工作,发表学术论文90余篇。
国家自然科学基金(31320103915,21477126)
周秀鹃(1990-),女,硕士研究生,研究方向为生态毒理学,E-mail: zhouxiujuan@ioz.ac.cn;
*通讯作者(Corresponding author), E-mail: daijy@ioz.ac.cn
周秀鹃, 盛南, 王建设, 等. 全氟和多氟化合物替代品的研究进展[J]. 生态毒理学报,2017, 12(3): 3-12
Zhou X J, Sheng N, Wang J S, et al. The current research status of several kinds of fluorinated alternatives [J]. Asian Journal of Ecotoxicology, 2017, 12(3): 3-12 (in Chinese)
