外源褪黑素对干旱胁迫下番茄叶片光合作用的影响
2017-10-13杨小龙须晖李天来王蕊
杨小龙,须晖,李天来,王蕊
外源褪黑素对干旱胁迫下番茄叶片光合作用的影响
杨小龙,须晖,李天来,王蕊
(沈阳农业大学园艺学院/设施园艺省部共建教育部重点实验室/辽宁省设施园艺重点实验室,沈阳 110866)
【目的】褪黑素是一种广泛存在于高等植物体内的小分子物质,被认为是一种新的植物生长调节剂和生物刺激剂,对于提高植物抗逆性具有重要作用。探索外源褪黑素对干旱胁迫下番茄叶片光合作用的影响,为揭示褪黑素调节植物抗逆性的机制打下基础。【方法】以番茄‘辽园多丽’为试材,首先采用叶片喷施和根施不同浓度褪黑素进行预处理:CK:叶片喷施清水、根施50 mL清水;R5、R50、R100、R150、R250:叶片喷清水,分别根施50mL 5、50、100、150和250 µmol∙L-1褪黑素;L5、L50、L100、L150、L250:根施50 mL清水,叶片分别喷施5、50、100、150和250 µmol∙L-1褪黑素;连续处理3 d后将植株移至温室中,以不浇水作为干旱处理(其中CK0:叶片喷施清水、根施50 mL清水预处理后正常浇水,CK1:叶片喷施清水、根施50 mL清水预处理后干旱处理)。干旱胁迫5 d后,通过比较暗适应下PSII最大光化学量子产量Fv/Fm和PSI最大氧化状态Pm,确定根施和叶片喷施的最佳浓度处理。然后利用光合荧光同步测量系统分析根施和叶片喷施褪黑素对干旱胁迫下番茄叶片气体交换参数,PSII和PSI的光能分配和电子传递速率,类囊体膜的完整性和ATP酶活性的调节。【结果】根施和叶片喷施不同浓度褪黑素均提高了干旱胁迫下番茄叶片的Fv/Fm和Pm,并且随浓度增加表现出先升高后降低的趋势,L100和R100处理下的Fv/Fm和Pm最大,显著高于对照。L100和R100显著缓解了干旱胁迫对气体交换参数的抑制,其中叶片净光合速率(Pn)分别为2.04和1.71 µmol∙m-2∙s-1,显著高于对照(CK1)(0.52 µmol∙m-2∙s-1);蒸腾速率(E)分别为0.66和0.54 mmol∙m-2∙s-1,显著高于CK1(0.25 mmol∙m-2∙s-1),并且显著提高了番茄叶片气孔导度(GH2O)和最大水分利用效率(WUE),降低了气孔限制值(Ls),而L100优于R100处理。快速光响应曲线结果表明L100和R100处理提高了干旱胁迫下番茄幼苗PSII的光化学反应效率Fv'/Fm'及PSII光化学淬灭系数qP,表明褪黑素处理更利于干旱胁迫下番茄叶片PSII光化学反应的高效进行;干旱胁迫下番茄幼苗环式电子传递速率得到显著加强,而L100和R100处理降低了环式电子传递速率,但加强了线性电子传递速率,且L100处理下番茄叶片ETRI和ETRII均高于R100处理;L100、R100处理提高了干旱胁迫下番茄叶片的Y(I)、Y(II),表明褪黑素处理有利于干旱胁迫下番茄叶片吸收光能向光化学反应的方向分配;暗适应后,L100和R100处理番茄叶片P515诱导曲线均高于CK1,照光后,CK0处理番茄幼苗P515信号快速下降,其次是L100和R100处理,而CK1处理降低较慢,表明褪黑素具有保护叶绿体类囊体膜和ATP合成酶免受干旱胁迫伤害的作用。【结论】根施和叶片喷施外源褪黑素能缓解干旱胁迫对番茄幼苗光合性能的抑制,加强光合运转效率,而叶片喷施是一种更简单高效的处理方式;褪黑素能加强作物光合作用对环境胁迫的适应性,对于农作物的生长发育具有调节作用。
褪黑素;干旱胁迫;番茄;光合作用;叶绿素荧光
0 引言
【研究意义】随着全球气温的升高,干旱胁迫对植物的影响越来越严重,设施栽培下的蔬菜作物不可避免的遭受干旱胁迫,干旱胁迫能够间接导致叶片光抑制的发生,降低光合运转效率并对作物造成伤害。近年来研究表明褪黑素能够提高植物抗逆性[1-4],但对逆境下植物光合作用的调节研究还很少,开展应用外源褪黑素调节干旱胁迫下番茄叶片光合作用的研究具有重要的理论和实践意义。【前人研究进展】植物在生长发育过程中不断面临环境胁迫的挑战,如强光、干旱、低温及重金属毒害。干旱胁迫是影响植物光合作用和生长发育的重要逆境因子,干旱胁迫极易引起吸收光能过剩,加强净光抑制的程度,造成光合损伤[5-7]。一些研究表明干旱胁迫显著降低植物叶片光合色素含量和叶片相对含水量,加强活性氧(ROS)的积累和膜脂质过氧化程度,导致叶片叶绿素荧光参数Fv/Fm、光合电子传递速率ETR的下降,非光化学淬灭NPQ的上升,叶绿体结构明显破坏,D1蛋白的含量显著降低[8-10]。褪黑素是生物进化过程中一种保守的小分子物质,具有很强的抗氧化作用,广泛存在于植物体中[11]。植物褪黑素的积累具有一定的组织特异性且与光信号密切相关,在一些品种中呈现昼夜节律,并且许多研究表明逆境能上调褪黑素的含量, 而这与植物体内褪黑素合成与代谢之间的平衡关系密切。近年来的一些研究揭示了褪黑素的合成与分解途径,证实了多个相关酶基因,由色氨酸生成褪黑素需要经过4个连续的酶促反应,而褪黑素也能被催化为2-羟基褪黑素和3-羟基褪黑素[12-14]。褪黑素在植物中具有广泛的生理功能,目前已经开展了许多外源褪黑素调节植物生长发育和抗逆性的研究,Wang等[15]研究表明长期在土壤中添加100 µmol∙L-1褪黑素延迟了干旱诱导的苹果叶片衰老。而外源100 µmol∙L-1褪黑素处理提高了低温胁迫下狼尾草的光合作用,尤其是耐低温基因型[16]。SzafraNska等[17]发现褪黑素处理缓解了百草枯介导的胁迫对豌豆光合机构的损伤。Liu等[18]研究表明褪黑素处理加强了干旱胁迫下番茄幼苗的光合性能并提高了抗氧化能力,褪黑素可能通过抑制叶绿体中ROS的产生以及调节光合电子传递和D1蛋白的合成提高植物耐逆性[19-20]。【本研究切入点】叶绿体是光合作用进行的场所,也是氧自由基产生的主要场所,在植物中褪黑素主要在叶绿体和细胞质中合成[13-14,21],褪黑素能高效的清除自由基,这对缓解光抑制对植物造成的损伤具有重要意义,因此,褪黑素可能对叶片光合作用尤其是光化学反应的进行起着重要的作用,干旱胁迫极易导致活性氧的产生进而加重抑制光合效率。根施和叶片喷施褪黑素如何调节干旱胁迫下番茄幼苗的光合作用有待研究。【拟解决的关键问题】利用光合荧光同步测量技术,通过分析气体交换参数、PSII和PSI的光能分配和电子传递速率以及类囊体膜的完整性和ATP酶活性,筛选出适宜调节干旱胁迫的最佳施用浓度,明确根施和叶片喷施褪黑素对干旱胁迫下番茄幼苗光合机构的调节作用,为深入探索褪黑素调节光合作用的机制提供参考,为设施蔬菜栽培管理提供理论依据。
1 材料与方法
试验于2015年6—10月在沈阳农业大学园艺学院设施蔬菜科研基地的日光温室内进行。
1.1 材料及处理
供试番茄(L.)品种为‘辽园多丽’。采用番茄专用育苗基质育苗,挑选均匀一致的种子播种于50孔的穴盘中,待长至2叶1心时分苗至塑料钵中(13 cm×13 cm),长至6叶1心时进行试验。首先采用叶片喷施和根施不同浓度褪黑素(都莱生物,南京,分析纯,纯度为99%)进行预处理:叶片喷施清水、根施50 mL清水(CK);R5、R50、R100、R150、R250:叶片喷清水,分别根施50 mL 5、50、100、150、250 µmol∙L-1褪黑素;L5、L50、L100、L150、L250:根施50 mL清水,叶片分别喷施5、50、100、150、250 µmol∙L-1褪黑素。喷施以叶片滴水为准,早晚各处理1次,连续处理3 d后将植株移至温室中,以不浇水作为干旱处理,在干旱处理的第5天通过比较暗适应下PSII最大光化学量子产量Fv/Fm和PSI最大氧化状态Pm,确定根施和叶片喷施的最佳浓度处理。之后进行干旱处理:CK0:叶片喷施清水、根施50 mL清水预处理后正常浇水;CK1:叶片喷施清水、根施50 mL清水后干旱处理;R100:叶片喷施清水、根施50 mL 100 µmol∙L-1褪黑素后干旱处理;L100:叶片喷施100 µmol∙L-1褪黑素、根施50 mL清水后干旱处理。干旱处理的第5天进行指标测定。
1.2 气体交换参数、慢速叶绿素荧光诱导动力学和P700活性测定
利用GFS-3000和Dual-pam-100(Heinz Walz,Effeltrich,Germany)光合荧光同步测量系统对活体番茄幼苗叶片的气体交换参数、叶绿素荧光和P700氧化还原状态进行测定[22-23]。每棵植株均以第4片叶为测量对象,测量前进行30 min的暗适应,测量叶室的面积为1.3 cm2,以大气CO2浓度为参比,饱和脉冲光光强为10 000 µmol∙m-2∙s-1,光化光的光强为630 µmol∙m-2∙s-1,流速为400 µmol∙s-1,在室温(25±2)℃条件下测定。气体交换参数包括:净光合速率Pn、气孔导度GH2O、大气CO2浓度Ca、胞间CO2浓度Ci、蒸腾速率E,叶片气孔限制值Ls(Ls=1-Ci/Ca)与水分利用率WUE(WUE=Pn/E)。PSII荧光参数主要包括:暗适应下PSII最大光化学量子产量Fv/Fm[Fv/Fm=(Fm-Fo)/Fm]、PSII量子产量Y(II)、非调节性能量耗散的量子产量Y(NO)、调节性能量耗散的量子产量Y(NPQ)。PSI荧光参数包括:P700最大氧化状态Pm、PSI量子产量Y(I)、由于供体侧限制引起的PSI处非光化学能量耗散的量子产量Y(ND)、由于受体侧限制引起的PSI处非光化学能量耗散的量子产量Y(NA)。
1.3 快速光响应曲线测定
慢速叶绿素荧光诱导动力学测定完后即在快速光响应曲线测定模式下测定光响应曲线(光响应曲线的光强梯度分别设置为29、37、55、113、191、233、349、520、778、1 197、1 474 µmol∙m-2∙s-1),每个光强持续时间为30 s,饱和脉冲为10 000 µmol∙m-2∙s-1,照射300 ms[22,24]。以下参数用于光响应曲线分析:光化学淬灭系数qP、光适应下PSII最大光化学量子产量Fv'/Fm'=(Fm'-Fo)/Fm'、光系统Ⅰ电子传递速率ETR(I)、光系统II电子传递速率ETR(II),并用以下参数来评估环式电子传递:Y(CEF)=Y(I)-Y(II)、Y(CEF)/Y(II)=[Y(I)-Y(II)]/Y(II)。
1.4 P515信号测定
将P515/535模块与Dual-pam-100相连接,利用Dual-PAM v1.19软件控制操作,调平衡后通过检测550—515 nm吸收信号的变化来测量P515活性[22,25]。暗适应1 h后测定单周转饱和闪光诱导的P515信号变化,之后光照6 min(光强为630 µmol∙m-2∙s-1),再暗适应4 min测定单周转饱和闪光诱导的P515信号变化,分别能反映类囊体膜的完整性和ATP酶活性。样品暗适应后,P515诱导曲线表现为慢速下降,这说明膜的完整性很高;经过照光之后,P515诱导曲线表现为信号快速下降,说明ATP酶活性很高。
1.5 数据分析
采用SPSS 22.0软件对数据进行统计分析,取4次重复进行LSD方差分析,用SigmaPlot 12.0软件作图。
2 结果
2.1 褪黑素处理浓度筛选
Fv/Fm反映有活性的PSII反应中心最大光化学效率,Fv/Fm下降是PSII光抑制的典型指标,Pm表示PSI反应中心P700的最大氧化状态,是反映PSI光抑制的一个灵敏指标[26-27],本研究利用这两个指标进行浓度筛选试验。干旱处理后,以喷施、根施清水后进行干旱胁迫的CK植株PSII最大光化学效率Fv/Fm最低,为0.762;而与CK相比,根施和叶片喷施不同浓度褪黑素均提高了Fv/Fm,并且随浓度增加均表现出先升高后降低的趋势,其中L100、L150、R100、R150增幅较大,分别为0.779、0.773、0.776、0.772,均显著高于对照(图1-a)。随着不同浓度褪黑素的处理,Pm也表现出与Fv/Fm相似的趋势,CK处理最低,只有1.56,而L100、L150、L250、R100、R150处理Pm分别为1.71、1.67、1.65、1.69、1.67、1.65,均极显著高于对照(图1-b)。PSII对环境胁迫十分敏感,另外,番茄幼苗PSI对环境胁迫也十分敏感,并且褪黑素处理均能加强番茄幼苗PSI和PSII对干旱胁迫下光抑制的缓解作用。在不同浓度褪黑素处理中,L100 和R100处理下的Fv/Fm和Pm最大,因此,初步确定L100、R100分别为叶片喷施褪黑素和根施褪黑素的最佳浓度处理。

CK:叶片喷施清水、根施50 mL清水;L5、L50、L100、L150、L250:根施50 mL清水,叶片分别喷施5、50、100、150、250 µmol∙L-1褪黑素;R5、R50、R100、R150、R250:叶片喷清水,分别根施50 mL 5、50、100、150、250 µmol∙L-1褪黑素。*和**分别表示在P<0.05 和P<0.01水平差异显著。下同
2.2 气体交换参数
如表1所示,与CK0相比,干旱胁迫(CK1)显著降低了番茄幼苗的净光合速率Pn,而L100和R100处理番茄幼苗Pn分别达到2.04 µmol∙m-2∙s-1和1.71 µmol∙m-2∙s-1,均显著高于CK1(0.52 µmol∙m-2∙s-1);干旱胁迫显著降低了蒸腾速率E,而L100和R100处理下E(分别为0.66和0.54 mmol∙m-2∙s-1)均显著高于CK1(0.25 mmol∙m-2∙s-1);干旱胁迫显著降低了Ci,CK1、L100和R100之间差异不显著;干旱胁迫显著降低了气孔导度GH2O,与CK1相比,L100和R100显著提高了气孔导度;CK1气孔限制值Ls显著高于CK0,而L100和R100处理显著低于CK1;CK0处理的番茄幼苗最大水分利用效率WUE最高,而CK1最低,L100和R100处理均显著高于CK1,表明L100和R100处理缓解了干旱胁迫对番茄幼苗叶片光合气体交换参数的影响,而L100处理对干旱胁迫下番茄叶片光合性能的加强作用表现为优于R100处理。
2.3 PSII光化学效率
从快速光响应曲线可以看出,在光下PSII最大光化学效率Fv'/Fm'以及PSII光化学淬灭系数qP均随着光强的变化先升高后降低,在任何光强下,干旱胁迫处理Fv'/Fm'和qP均显著低于对照CK0,而褪黑素处理L100和R100下的番茄幼苗Fv'/Fm'和qP均高于CK1(图2-a、b),表明褪黑素处理更利于干旱胁迫下番茄叶片PSII光化学反应的高效进行,具有缓解干旱胁迫对PSII光抑制的作用。
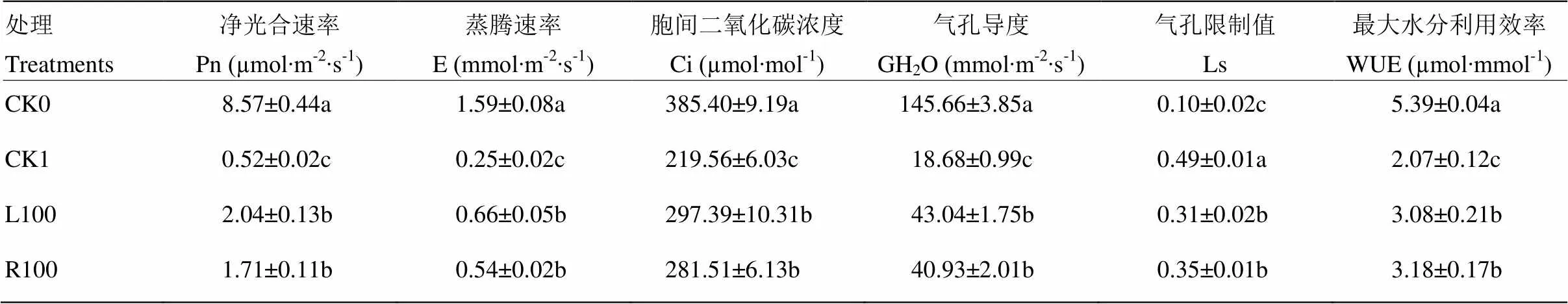
表1 外源褪黑素对干旱胁迫下番茄幼苗光合气体交换参数的影响
CK0:早、晚分别喷施清水、根施50 mL清水预处理后正常浇水;CK1:喷施清水、根施50 mL清水,预处理后进行干旱处理;L100:叶片喷施100 µmol∙L-1褪黑素、根施50 mL清水,预处理后进行干旱处理;R100:叶片喷施清水、根施50 mL 100 µmol∙L-1褪黑素,预处理后进行干旱处理。同列数字后不同字母代表处理间差异在<0.05水平显著。下同
CK0: normal water management after pretreatment by spraying water on leaves and applying 50 mL water on roots; CK1: plants were grown at drought stress after pretreatment by spraying water on leaves and applying 50 mL water on roots; R100: plants were grown at drought stress after pretreatment by spraying water on leaves and applying 50 mL 100 µmol∙L-1melatonin on roots; L100: plants were grown at drought stress after pretreatment by leaf spraying 100 µmol∙L-1melatonin on leaves and applying 50mL water on roots. Different letters (a, b, c) in the same column indicate significant difference between treatments at<0.05. The same as below

图2 外源褪黑素对干旱胁迫下番茄幼苗Fv'/Fm'和qP快速光响应曲线的影响
2.4 PSI和 PSII的光能分配
由于植株缺少光合作用的底物水,干旱胁迫对光能分配的影响很大。由图3-a和b可以看出,与CK0相比,干旱胁迫显著降低了Y(I)和Y(II),并且Y(II)降幅较大,表明干旱胁迫严重抑制了PSII的光化学反应过程,Y(NO)、Y(NPQ)及Y(ND)均显著提高,这表明干旱胁迫降低PSI和PSII吸收光能中分配至光化学反应的能量,而加强了吸收光能向非光化学反应分配的比例;与CK1相比,L100、R100处理降低了番茄叶片Y(ND)、Y(NO)和Y(NPQ),但提高了Y(I)、Y(II),这表明褪黑素对PSI和PSII吸收光能的分配具有一定的影响,在胁迫下褪黑素处理有利于吸收光能向光化学反应的方向分配。
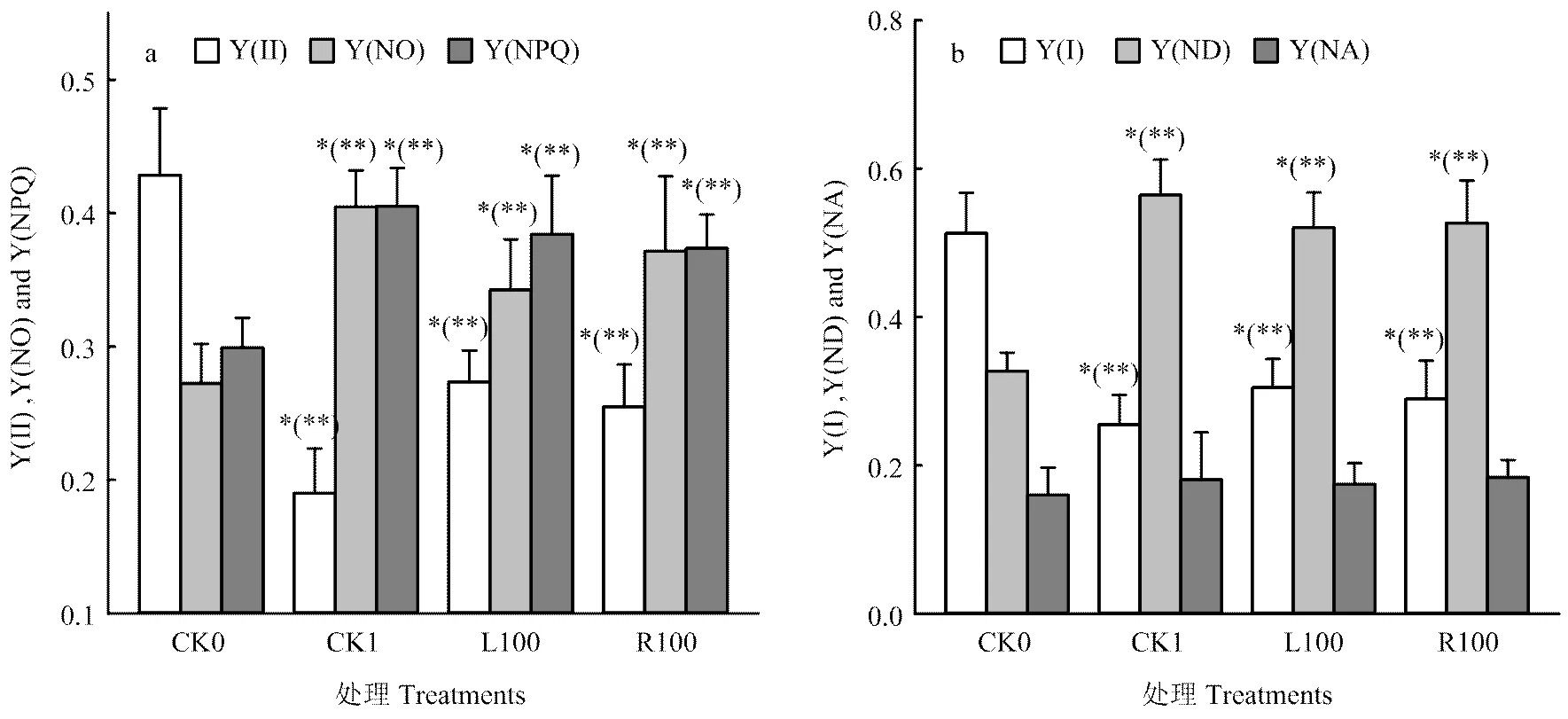
图3 外源褪黑素对干旱胁迫下番茄幼苗PS I和PS II光能分配的影响
2.5 线性和环式电子传递
光合电子传递速率与光照和环境因素密切相关。ETRI和ETRII以及Y(CEF)/Y(II)均随光强的增加而不断增加,在较强光下ETRII增速减慢并趋于稳定,而ETRI在较高光强下才趋于稳定。与CK0相比,干旱处理显著降低了ETRII和ETRI,而L100和R100处理显著缓解了这种降低,表明褪黑素处理具有提高ETRII和ETRI的作用,从图中可以看出,L100处理下番茄叶片ETRII和ETRI均高于R100处理(图4-a、b)。干旱胁迫下番茄幼苗的Y(CEF)/Y(II)均显著高于对照,这说明干旱胁迫加强了环式电子传递速率,而R100、L100处理的幼苗环式电子传递均低于CK1(图4-c)。这些结果表明根施和叶片喷施外源褪黑素能够加强干旱胁迫下番茄幼苗的光合电子传递速率。
2.6 类囊体膜的完整性和ATP酶活性
位于类囊体膜上的光合电子传递链包含多种复合物,因此,类囊体膜的完整性对于光合作用十分重要。充分暗适应后,CK0处理番茄叶片P515诱导曲线表现为慢速下降,均高于胁迫处理,这说明干旱胁迫降低了类囊体膜的完整性,而L100和R100处理均高于CK1,这表明褪黑素具有缓解干旱胁迫对类囊体膜伤害的作用(图5-a)。经过照光后,CK0处理番茄幼苗P515信号快速下降,其次是L100和R100处理,而CK1处理降低较慢,这说明干旱胁迫降低了ATP酶的活性,褪黑素缓解了这种降低(图5-b)。这些结果表明外源施用褪黑素具有保护类囊体膜和ATP酶免受干旱胁迫伤害的作用。
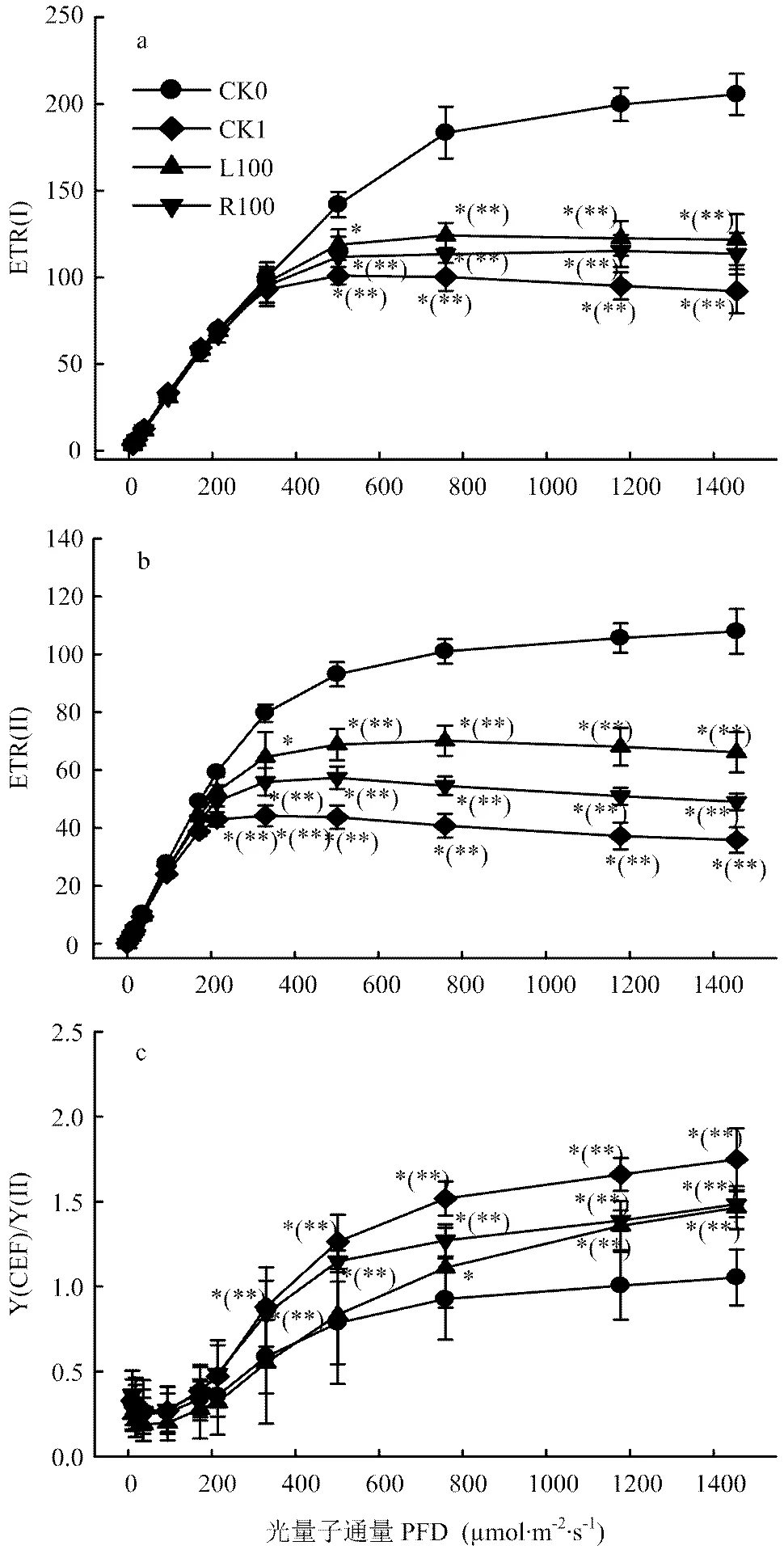
图4 外源褪黑素对干旱胁迫下番茄幼苗电子传递的影响
3 讨论
在植物中发现褪黑素后,大多数研究集中于褪黑素在植物组织中含量的检测和积累规律的分析,近些年关于其在植物中的合成与分解、生理功能以及作用机理的研究已成为热点。褪黑素的抗氧化能力高于抗坏血酸、生育酚、谷胱甘肽等抗氧化剂,通过与自由基的级联互作,一个褪黑素可以清除10个ROS[28-29]。褪黑素能够调节植物多种生育过程,还能直接或间接调控抗氧化酶系统和其他抗氧化剂的水平,缓解环境胁迫及生物胁迫对高等植物造成的损伤,加强植物抗逆性[30-31]。在水稻中分别沉默褪黑素合成的最后两个关键酶基因和均降低了内源褪黑素的含量,突变体延迟了水稻幼苗的生长,而喷施外源褪黑素后这种延迟得到恢复,突变体加速水稻叶片衰老并降低了产量[32]。催化褪黑素分解产生2-羟基褪黑素和环3-羟基褪黑素的酶M2H和的克隆和分析将进一步加快对植物褪黑素生理功能的研究[33-34]。目前,关于褪黑素在植物中作用的信号传导途径研究的还不是很多,研究表明褪黑素能够通过MAPK途径影响植物免疫,MAPK的上游信号MAPKKK3和OXI1激酶也响应褪黑素诱导的防御信号途径,并且这种诱导依赖于ROS和NO信号物质[35-36]。
研究表明外源褪黑素处理显著提高了苹果幼苗抗坏血酸和谷胱甘肽水平[37]。褪黑素处理显著延迟了水稻叶片衰老并加强了抗盐性[38],延迟了干旱胁迫下苹果叶片的衰老[15],加强了番茄抗碱性[31]。在本试验中,不同浓度褪黑素根施和叶片喷施均不同程度提高了干旱胁迫下番茄叶片的Fv/Fm和Pm,其中100 µmol∙L-1褪黑素处理下番茄叶片的Fv/Fm和Pm最高。低温胁迫诱导黄瓜幼苗中积累过多的ROS,严重损伤叶绿体结构,外源200 µmol∙L-1褪黑素预处理加强了ASA-GSH循环和抗氧化酶活性,提高了清除ROS的能力,并且有利于平衡光合电子流的分布[19]。150 µmol∙L-1褪黑素预处理有利于维持盐胁迫下番茄的生长和光合作用,这与加快盐胁迫下D1蛋白的合成和降低ROS的含量有关[20]。100 µmol∙L-1褪黑素处理提高了低温胁迫下狗牙根的叶绿素荧光,提高了一些糖和酸的含量,表明褪黑素具有缓解低温对PSII及物质代谢的损伤[39],Liu等[18]发现100 µmol∙L-1褪黑素预处理显著缓解了干旱胁迫对番茄幼苗PSII的影响,本试验用100 µmol∙L-1褪黑素叶片喷施和根施处理提高了番茄叶片光合电子传递速率,加强了光能向光化学反应方向的分配,保护了类囊体膜免受干旱胁迫的伤害并加强了ATP酶的活性,从而缓解了干旱胁迫下番茄幼苗叶片PSI和PSII光抑制的发生,加强了光合性能。
在生产中,合理选择一些外源物质(激素、渗透调节物质和抗氧化剂类等)对栽培作物进行浸种、叶面喷施、根际施用等可以调节植物代谢过程、缓解或减轻逆境对园艺作物造成的胁迫、提高植株抗逆性进而提高产量、改善品质。这些物质普遍具有用量小、见效快、效果显著等特点[40-41]。本研究表明100 µmol∙L-1褪黑素根施和叶片喷施两种处理方式均能显著缓解干旱胁迫对番茄幼苗造成的伤害,加强叶片的光合性能,而由于处理简单且有更多的褪黑素直接到达靶位点。适量的褪黑素对人体和动物都是安全的,较强的生物学活性、在植物中多样的生理功能以及环境友好型的作用特点,使褪黑素在农作物生产中具有重要的应用潜力[42]。利用褪黑素缓解光抑制的发生来提高作物抗逆性是一种重要的农业调节措施,而褪黑素处理的作用机理和模式仍需要进行广泛而深入的研究。

a:暗适应1 h后单周转饱和闪光诱导的P515信号变化;b:光照6 min(光强为630 µmol∙m-2∙s-1)再暗适应4 min后单周转饱和闪光诱导的P515信号变化
4 结论
利用Fv/Fm和Pm两个指标对褪黑素施用浓度进行了筛选,确定100 µmol∙L-1外源褪黑素为叶片喷施和根施的最佳浓度。干旱胁迫严重降低了光合作用的高效运行,叶片喷施和根施100 µmol∙L-1褪黑素处理均缓解了这种降低,并提高了干旱胁迫下番茄叶片净光合速率和蒸腾速率,提高了PSII和PSI的电子传递速率,加强了光能向光化学反应方向的分配,保护了类囊体膜免受干旱胁迫的伤害并加强了ATP酶的活性,从而增强了干旱胁迫下番茄幼苗的光合性能。与根施相比,叶片喷施是一种更简单高效的调节方式。
References
[1] Wei W, Li Q T, Chu Y N, Reiter R J, Yu X M, Zhu D H, Zhang W K, Ma B, Lin Q, Zhang J S, Chen S Y. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants., 2015, 66(3): 695-707.
[2] Nawaz M A, Huang Y, Bie Z, Ahmed W, Reiter R J, Niu M, Hameed S. Melatonin: current status and future perspectives in plant science., 2015, 6: 1230. DOI: 10.3389/fpls.2015.01230.
[3] ZHANG N, SUN Q, ZHANG H, CAO Y, WEEDA S, REN S, GUO Y D. Roles of melatonin in abiotic stress resistance in plants., 2015, 66(3): 647-656.
[4] Reiter R J, Tan D X, Zhou Z, Cruz M H C, Fuentes- Broto L, Galano A. Phytomelatonin: assisting plants to survive and thrive., 2015, 20(4): 7396-7437.
[5] ZHANG Z S, YANG C, GAO H Y, ZHANG L T, FAN X L, LIU M J. The higher sensitivity of PSI to ROS results in lower chilling–light tolerance of photosystems in young leaves of cucumber., 2014, 137: 127-134. DOI: 10.1016/j.jphotobiol.2013.12.012.
[6] JOHNSON G N, LAWSON T, MURCHIE E H, RAINES C. Photosynthesis in variable environments., 2015, 66(9): 2371-2372.
[7] GURURANI M A, VENKATESH J, TRAN L S P. Regulation of photosynthesis during abiotic stress-induced photoinhibition., 2015, 8(9): 1304-1320.
[8] 须晖, 高洁, 王蕊, 李天来, 马健, 刘满昌. 番茄幼苗叶绿素荧光参数对水分胁迫的响应. 中国农学通报, 2011, 27(10): 189-193.
XU H, GAO J, WANG R, LI T L, MA J, LIU M C. Response of water stress on chlorophyll fluorescence parameters of tomato seedlings., 2011, 27(10): 189-193. (in Chinese)
[9] 曹逼力, 李炜蔷, 徐坤. 干旱胁迫下硅对番茄叶片光合荧光特性的影响. 植物营养与肥料学报, 2016, 22(2): 495-501.
CAO B L, LI W Q, XU K. Effects of silicon on photosynthetic and fluorescence characteristics of tomato leaves under drought stress., 2016, 22(2): 495-501. (in Chinese)
[10] 邵瑞鑫, 李蕾蕾, 郑会芳, 张寄阳, 杨慎娇, 马野, 信龙飞, 苏小雨, 冉午玲, 毛俊, 郑博元, 杨青华. 外源一氧化氮对干旱胁迫下玉米幼苗光合作用的影响. 中国农业科学, 2016, 49(2): 251-259.
SHAO R X, LI L L, ZHENG H F, ZHANG J Y, YANG S J, MA Y, XIN L F, SU X Y, RAN W L, MAO J, ZHENG B Y, YANG Q H. Effects of exogenous nitric oxide on photosynthesis of maize seedlings under drought stress., 2016, 49(2): 251-259. (in Chinese)
[11] HARDELAND R. Melatonin in plants-diversity of levels and multiplicity of functions., 2015, 7: 198. DOI: 10.3389/fpls.2016.00198.
[12] ARNAO M B, HERNANDEZ-RUIZ J. Functions of melatonin in plants: A review., 2015, 59(2): 133-150.DOI: 10.1111/jpi.12253.
[13] BACK K, TAN D X, REITER R J. Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts., 2016, 61(4): 426-437.
[14] 王蕊, 杨小龙, 须晖, 李天来. 高等植物褪黑素的合成和代谢研究进展. 植物生理学报, 2016, 52(5): 615-627.
WANG R, YANG X L, XU H, LI T L. Research progress of melatonin biosynthesis and metabolism in higher plants., 2016, 52(5): 615-627. (in Chinese)
[15] WANG P, SUN X, LI C, WEI Z W, LIANG D, MA F W. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple., 2013, 54(3): 292-302.
[16] HU Z, FAN J, XIE Y, AMOMBO E, LIU A, GITAU M M, KHALDUN A B M, CHEN L, FU J. Comparative photosynthetic and metabolic analyses reveal mechanism of improved cold stress tolerance in bermudagrass by exogenous melatonin., 2016, 100: 94-104. DOI: 10.1016/j.plaphy.2016.01. 008.
[17] SZAFRANSKA K, REITER R J, POSMYK M M. Melatonin Application toL. Seeds positively influences the function of the photosynthetic apparatus in growing seedlings during paraquat-induced oxidative stress., 2016, 7: 1663. DOI: 10.3389/fpls.2016.01663.
[18] LIU J, WANG W, WANG L, SUN Y. Exogenous melatonin improves seedling health index and drought tolerance in tomato., 2015, 77(3): 317-326.
[19] ZHAO H, YE L, WANG Y, WANG Y, ZHOU X, YANG J, WANG J, CAO K, ZOU Z. Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle., 2016, 7: 1814. DOI: 10.3389/fpls.2016.01814.
[20] ZHOU X, ZHAO H, CAO K, HU L, DU T, BALUSKA F, ZOU Z. Beneficial roles of melatonin on redox regulation of photosynthetic electron transport and synthesis of D1 protein in tomato seedlings under salt stress., 2016, 7: 1823.DOI: 10.3389/fpls.2016.01823.
[21] ARNAO M B, HERNANDEZ-RUIZ J, D'MELLO J P F. Melatonin: synthesis from tryptophan and its role in higher plant., 2015: 390-435.
[22] ZHANG G X, LIU YF, NI Y, MENG Z J, LU T, LI T L. Exogenous calcium alleviates low night temperature stress on the photosynthetic apparatus of tomato leaves., 2014, 9(5): e97322. DOI: 10.1371/journal.pone.0097322.
[23] PFUNDEL E, KLUGHAMMER C, Schreiber U. Monitoring the effects of reduced PS II antenna size on quantum yields of photosystems I and II using the Dual-PAM-100 measuring system., 2008, 1: 21-24.
[24] SCHREIBER U, KLUGHAMMER C. Non-photochemical fluorescence quenching and quantum yields in PS I and PS II: analysis of heat-induced limitations using Maxi-Imaging-PAM and Dual-PAM- 100., 2008, 1: 15-18.
[25] SCHREIBER U, KLUGHAMMER C. New accessory for the Dual-PAM-100: The P515/535 module and examples of its application., 2008, 1: 1-10.
[26] 张守仁. 叶绿素荧光动力学参数的意义及讨论. 植物学通报, 1999, 16(4): 444-448.
ZHANG S R. A discussion on chlorophyll fluorescence kinetics parameters and their significance., 1999, 16(4): 444-448. (in Chinese)
[27] KONO M, TERASHIMA I. Elucidation of photoprotective mechanisms of PSI against fluctuating light photoinhibition., 2016, 57(7): 1405-1414.
[28] TAN D X, REITER R J, MANCHESTER L C, YAN M T, EL-SAWI M, SAINZ R M, MAYO J C, KOHEN R, ALLEGRA M C, HARDELAND R. Chemical and physical properties and potential mechanisms: melatonin as a broadspectrum antioxidant and free radical scavenger., 2002, 2(2): 181-197.
[29] TAN D X, MANCHESTER L C, ESTEBAN-ZUBERO E, ZHOU Z, REITER R J. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism., 2015, 20(10): 18886-18906.
[30] PELAGIO-FLORES R, MUNOZ-PARRA E, ORTIZ-CASTRO R, LOPEZ-BUCIO J. Melatonin regulatesroot system architecture likely acting independently of auxin signaling., 2012, 53(3): 279-288.
[31] LIU N, JIN Z Y, WANG S S, GONG B, WEN D, WANG X F, WEI M, SHI Q H. Sodic alkaline stress mitigation with exogenous melatonin involves reactive oxygen metabolism and ion homeostasis in tomato., 2015, 181: 18-25. DOI: 10.1016/j.scienta.2014. 10.049.
[32] BYEON Y, BACK K. Low melatonin production by suppression of either serotonin N-acetyltransferase or N-acetylserotonin methyltransferase in rice causes seedling growth retardation with yield penalty, abiotic stress susceptibility, and enhanced coleoptile growth under anoxic conditions., 2016, 60(3): 348-359.
[33] LEE K, ZAWADZKA A, CZARNOCKI Z, RUSSEL J R, BACK K. Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice ()., 2016, 61(4): 470-478.
[34] BYEON Y, BACK K. Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice ()., 2015, 58(3): 343-351.
[35] LEE H Y, BACK K. Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants., 2016, 60(3): 327-335.
[36] LEE H Y, BACK K. Melatonin is required for H2O2-and NO-mediated defense signaling through MAPKKK3 and OXI1 in., 2017: e12379. DOI: 10.1111/jpi. 12379.
[37] WANG P, YIN L H, LIANG D, LI C, MA F W, YUE Z Y. Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle., 2012, 53(1): 11-20.
[38] LIANG C, ZHENG G, LI W, WANG Y, HU B, WANG H, WU H, QIAN Y, ZHU X G, TAN D X, CHEN S Y, CHU C. Melatonin delays leaf senescence and enhances salt stress tolerance in rice., 2015, 59(1): 91-101.
[39] FAN J, HU Z, XIE Y, CHAN Z, CHEN K, AMOMBO E, CHEN L, FU J. Alleviation of cold damage to photosystem II and metabolisms by melatonin in bermudagrass., 2015, 6: 925. DOI: 10.3389/fpls.2015.00925.
[40] ZIOGAS V, TANOU G, BELGHAZI M, FILIPPOU P, FOTOPOULOS V, GRIGORIOS D, MOLASSIOTIS A. Roles of sodium hydrosulfide and sodium nitroprusside as priming molecules during drought acclimation in citrus plants., 2015, 89(4/5): 433-450.
[41] BORGES A A, JIMENEZ-ARIAS D, EXPOSITO-RODRIGUEZ M, SANDALIO L M, PEREZ J A. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms., 2014, 5: 642. DOI: 10.3389/fpls.2014.00642.
[42] JANAS K M, POSMYK M M. Melatonin, an underestimated natural substance with great potential for agricultural application., 2013, 35(12): 3285-3292.
(责任编辑 赵伶俐)
Effects of Exogenous Melatonin on Photosynthesis of Tomato Leaves Under Drought Stress
YANG XiaoLong, XU Hui, LI TianLai, WANG Rui
(College of Horticulture, Shenyang Agricultural University / Key Laboratory of Protected Horticulture of Education Ministry and Liaoning Province/Key Laboratory of Protected Horticulture of Liaoning Province, Shenyang 110866)
【Objective】Melatonin is a small molecules widely exist in higher plants which is regarded as a new plant growth regulator and biological stimulant. Melatonin plays an important role in improving plant resistance, but research about melatonin involved in regulation of plant photosynthesis under adversity stress is rarely reported. The objective of this study is to explore the influence of exogenous melatonin on photosynthesis of tomato leaves under drought stress. 【Method】Tomato cultivar ‘LiaoYuanDuoLi’ was used as the experimental materials, concentration screening tests were firstly carried out: CK: Leaf spray with water and root application with 50 mL water. R5, R50, R100, R150, R250: leaf spray with water and root application with 50 mL 5, 50, 100, 150, 250 µmol∙L-1melatonin. L5, L50, L100, L150, L250: root application with 50 mL water and leaf spray with 5, 50, 100, 150, 250 µmol∙L-1melatonin. This process was repeated in the morning and afternoon for three consecutive days and the drought stress treatment was conducted for subsequent three days (CK0: normal water after leaf spray with water and root application with 50 mL water, CK1: drought treatment after leaf spray with water and root application with 50 mL water). The optimal melatonin concentration was screened comparing the maximum photochemical quantum yield of PSII (Fv/Fm) and a parameter representing the quantity of efficient PSI complex (Pm). Then the influence of root application and leaf spray with exogenous melatonin on gas exchange parameters, light energy distribution and electron transfer rate of PSI and PSII and the integrity of the thylakoid membrane and ATP enzyme activity of tomato leaves under drought stress were analyzed by using photosynthetic fluorescence synchronous measurement technology. 【Result】Root application and leaf spray with different concentrations of melatonin both increased the Fv/Fm and Pm of tomato leaves under drought stress, and all showed a trend of increase at first and then decreased with the increase of concentration, the values of Fv/Fm and Pm were the highest under L100 and R100, both significantly higher than that of control, thus it was determined that L100 and R100 were the optimal concentration treatments for leaf spray and root application, respectively. L100 and R100 significantly alleviated the inhibition of drought stress on the gas exchange parameters, and the leaf net photosynthetic rate (Pn) was 2.04 µmol∙m-2∙s-1and 1.71 µmol∙m-2∙s-1, respectively, which both significantly higher than the control (CK1) (0.52 µmol∙m-2∙s-1); transpiration rate (E) was 0.66 mmol∙m-2∙s-1and 0.54 mmol∙m-2∙s-1, respectively, and both significantly higher than that of CK1 (0.25 mmol∙m-2∙s-1). L100 and R100 treatments significantly increased the stomatal conductance (GH2O) and the maximum water use efficiency (WUE) and significantly reduced stomatal limitation (Ls) of tomato leaves under drought stress, it was also found that L100 treatment was superior to R100. The results of rapid light response curve showed that L100 and R100 enhanced Fv'/Fm' and qP of tomato leaves, indicated melatonin is beneficial to improve photochemical reaction efficiency of PSII of tomatoes under drought stress. Cyclic electron flow of tomato seedlings under drought stress was significantly enhanced, while melatonin treatment reduced on cyclic electron flow, but strengthened ETRI and ETRII, and they were both higher under L100 compared with R100. Y (I) and Y (II) both improved under L100 and R100 compared with CK1, indicate melatonin treatment strengthened light energy distribution to the direction of photochemical reaction of PSI and PSII under drought stress. P515 induction curves of L100 and R100 were higher than that of CK1 after dark adaptation, and after illumination, P515 signal of CK0 fell fast, followed by L100 and R100, CK1 treatment decreased slowest, showed that exogenous melatonin protected the thylakoid membrane from damage caused by drought stress and strengthened the ATP-synthase activity. 【Conclusion】Root application and leaf spraying with exogenous melatonin can relieve the inhibition of drought stress on photosynthetic performance of tomato leaves, strengthen the photosynthetic efficiency, leaf spraying is a more simple and efficient measure compared with root application. Melatonin can enhance crop photosynthesis adaptability to the environment stresses and has an regulatory role in crop growth and development.
melatonin; drought stress; tomato; photosynthesis; chlorophyll fluorescence
2017-01-04;接受日期:2017-06-15
“十三五”国家重点研发计划(2016YFD0201004)
杨小龙,E-mail:xiaolongyang0214@163.com。通信作者王蕊,E-mail:ruiwangsyau@126.com
