早孕期血清PAPP-A检测联合中孕期血清AFP、free β-HCG、uE3检测对DS、ES的筛查效果
2017-10-10杨岚石锦平吴晓施楠郭彩琴赵丽
杨岚,石锦平,吴晓,施楠,郭彩琴,赵丽
(南京医科大学附属无锡妇幼保健院, 江苏无锡214002)
早孕期血清PAPP-A检测联合中孕期血清AFP、free β-HCG、uE3检测对DS、ES的筛查效果
杨岚,石锦平,吴晓,施楠,郭彩琴,赵丽
(南京医科大学附属无锡妇幼保健院,江苏无锡214002)
目的观察早孕期检测血清PAPP-A联合中孕期检测血清AFP、free β-HCG、uE3对胎儿唐氏综合征(DS)和爱德华氏综合征(ES)的筛查效果。方法回顾性分析在我院行产前筛查且有随访结局的7 055例孕妇的临床资料。所有孕妇早孕期检测血清妊娠相关蛋白A(PAPP-A)和游离人绒毛膜促性腺激素β(Free β-hCG),中孕期检测血清甲胎蛋白(AFP)、free β-HCG、游离雌三醇(μE3)。设计3个DS、ES筛查方案。方案A纳入指标为早孕期PAPP-A、Free β-hCG,方案B案纳入指标为中孕期AFP、free β-HCG、μE3,方案C纳入指标为早孕期PAPP-A和中孕期AFP、free β-HCG、μE3。用LifeCycle 4.0软件综合患者年龄、体质量、孕周等数据计算用三种方案筛查得出的患者DS、ES风险值。DS风险值>1/270为高风险,1/1 000~1/270为临界风险;ES风险值>1/350为高风险,1/1 000~1/350为临界风险。比较三种方案的DS高风险、DS临界风险、ES高风险、ES临界风险及阳性筛查结果检出情况。胎儿出生者以胎儿是否确诊DS、ES为金标准,终止妊娠者以羊水核型分析结果为金标准,计算三种方案筛查DS、ES的检出率、假阳性率。结果方案C的DS高风险、DS临界风险、筛查结果总阳性率均低于方案A、B(P均<0.05)。羊水穿刺染色体核型分析和胎儿出生后随访共发现DS患儿9例,其中方案A筛查结果阳性7例,检出率77.8%,假阳性率3.95%;方案B筛查结果阳性6例,检出率66.7%,DS假阳性率4.24%;方案C筛查结果阳性8例,检出率88.9%,假阳性率1.80%。方案C的检出率、假阳性率均优于方案A、B。羊水穿刺染色体核型分析和胎儿出生后随访共发现ES患儿2例,其中1例在3种筛查方案都得到了检出,另1例ES患者用联合方案得出了高风险值,ES检出率联合筛查方案均高于孕早期、孕中期方案。结论早孕期检测血清PAPP-A联合中孕期检测血清AFP、free β-HCG、uE3对胎儿DS和ES的筛查效果优于早孕期检测血清PAPP-A、free β-HCG或中孕期检测血清AFP、free β-HCG、uE3。
唐氏综合征;爱德华氏综合征;早孕期;中孕期;妊娠相关蛋白A;人绒毛膜促性腺激素β;甲胎蛋白;雌三醇
Abstract:ObjectiveTo explore the screening efficiency about the first trimester screening of pregnancy-associated plasma protein A (PAPP-A) combined with the second trimester screening of alpha fetoprotein (AFP), free β-human chorionic gonadotropin (free β-HCG), uncojugated estriol (uE3) strategies for Down's Syndrome (DS) and Edwards' syndrome (ES).MethodsThe clinical data from 7 055 cases of pregnant women with pregnancy outcome, who underwent prenatal screening test, were retrospectively analyzed. The serum PAPP-A, free β-hCG in the first trimester, and AFP, free β-HCG, and uE3 in the second trimester were detected in all pregnant women. We designed three screening tests as three groups: projects A, B, and C. The risk evaluation of project A was serum free β-hCG and PAPP-A levels in the first trimester screening. Project B included free β-hCG, AFP, and uE3 levels in the second trimester. As for project C-so called the integrated serum test, included the serum PAPP-A levels in the first trimester, and AFP, β-hCG, and uE3 in the second trimester. The risks of DS and ES were evaluated by LifeCycle 4.0 software based on the age, weight and gestational week of gravidas. The cut-off value (high risk) and the intermediate risk value were >1/270 and 1/1 000-1/270 in DS, >1/350 and 1/1000-1/350 in ES, respectively. We compared the high risk rate, intermediate risk rate in both DS and ES as well as the total positive rates among the three screening tests mentioned above. The women who terminated pregnancy were identified by prenatal diagnosis via amniocentesis. Furthermore, those who did not terminate pregnancy were confirmed by the pregnancy outcomes, and were followed up based on karyotype of newborns. We evaluated screening efficiency of DS and ES such as the detection rate (DR) and false positive rate (FPR) among the three screening strategies.ResultsThe high risk and the intermediate risk in DS as well as the screening positive rate of the project C were significant lower than those of projects A and B, respectively (P<0.05) (neural tube defect were excluded). A total of 9 fetus with true DS were diagnosed via amniocentesis and follow-up pregnancy outcomes. In the project A, the number of high risk was 7, DR and FPR for trisomy 21 was 77.8% (7/9) and 3.95% (279/7 055), respectively. In the project B, the number of high risk was 6, DR and FPR for trisomy 21 was 66.7% (6/9) and 4. 24% (299/7 055), respectively. In the project C, DR and FPR for trisomy 21 was 88.9% (8/9) and 1.80% (127/7 055) , respectively. The DR and FPR for DS in the project C were both superior to those in the projects A and B (DR:P>0.05, FPR:P<0.05). We diagnosed two fetus with true ES through amniocentesis and follow-up pregnancy outcomes. Only one case was successfully detected by all three screening projects. The other case was detected as high risk in the combined screening test. The detection rate for ES in the project C was higher than those in both projects A and B.ConclusionThe screening efficiency about the first trimester screening of PAPP-A combined with the second trimester screening of AFP, free β-HCG, and uE3 for DS and ES is superior to both the first trimester double test (PAPP-A and free β-HCG) and the second trimester triple test including AFP, free β-HCG, and uE3.
Keywords: Down Syndrome; Edwards syndrome; the first trimester; the second trimester; pregnancy-associated plasma protein A; human chorionic gonadotropin-β; alpha fetoprotein; estriol
唐氏综合征(DS,又名21三体综合征)和爱德华氏综合征(ES,又名18三体综合征)是临床常见的先天出生缺陷病。针对这类胎儿染色体疾病的产前筛查方案,现今国内应用最普遍的是中孕期血清学指标筛查方案[1~2],该方法经济简便,对胎儿无创,可在一定程度上减少这类缺陷儿出生[3],但其检出率较低[4~5]。国外有研究显示将早、中孕期血清学指标整合为一个方案,可将DS、ES检出率提高到88%~95%[6,7]。但国内尚无同类文献报道。本研究对2015年5月~2016年5月在我院规律产检的7 055例单胎孕妇的临床资料进行了回顾行分析,观察早孕期检测血清PAPP-A联合中孕期检测血清AFP、free β-HCG、uE3(联合方案)对胎儿DS和ES的筛查效果。现报告如下。
1 资料与方法
1.1 临床资料 选择同期于我院产前诊断中心规律产检且有完整妊娠结局随访结果的单胎妊娠孕妇共7 055例,预产年龄8~9岁(28.38±2.95岁)。均于早孕期(孕10~13+6周)和中孕期(15~20+6周)进行了产前血清学指标筛查。均无吸烟史,无胰岛素依赖性糖尿病,无DS、ES儿分娩史。本研究已经我院医学伦理委员会批准。孕妇或其家属均签署知情同意书,并填写早、中孕期产前筛查申请单。
1.2 DS、ES筛查方法 ①方案A:孕10~13+6周抽取孕妇肘静脉血3 mL,不抗凝,室温静置2 h后于3 500 r/min离心6 min后分离血清,-20 ℃储存备检。采用 芬兰PerKin Elmer公司生产的1235全自动多标记仪、DELFIA时间分辨荧光法及配套试剂检测血清妊娠相关蛋白A(PAPP-A)和游离人绒毛膜促性腺激素β(free β-hCG)水平。操作按试剂盒说明书进行。用Lifecycler 4.0软件综合患者年龄、体质量、孕周等数据计算患者用早孕期方案筛查DS、ES风险值。DS风险值>1/270为高风险,风险值1/1 000~1/270为临界风险;ES风险值>1/350为高风险,1/1 000~1/350为临界风险。②方案B:纳入的血清学指标为孕中期(孕15~20+6周)血清甲胎蛋白(AFP)、free β-hCG和游离雌三醇(uE3)。其余同孕早期方案。③方案C:纳入的血清学指标为孕中期血清AFP、free β-hCG、uE3和孕早期PAPP-A。其余同孕早期方案。
1.3 DS、ES筛查的准确性评价方法 对筛查出的高风险孕妇进行遗传咨询,建议其接受羊水穿刺进行胎儿染色体核型分析。孕妇胎儿出生者均进行随访确定胎儿是否患有DS或ES。胎儿出生者以胎儿是否确诊DS、ES为金标准,终止妊娠者以羊水核型分析结果为金标准,计算三种方案筛查DS、ES的检出率、假阳性率。
1.4 统计学方法 采用SPSS17.0统计软件。率的比较采用χ2检验和Fisher确切概率法。P<0.05 为差异有统计学意义。
2 结果
2.1 三种方案筛查结果比较 三种方案筛查结果见表1。方案C的DS高风险、DS临界风险率均低于方案A和B(P均<0.05),而其ES高风险、ES临界风险率无统计学差异(P均>0.05);方案A和B的DS高风险、ES高风险、ES临界风险率相比,P均>0.05。
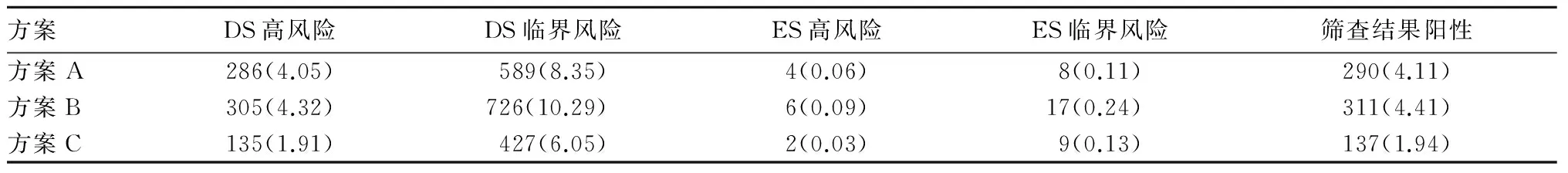
表1 三种方案筛查结果[例(%)]
2.2 三种方案筛查结果准确性比较 羊水穿刺染色体核型分析和胎儿出生后随访共发现DS患儿9例(其中1例为易位型),其中方案A筛查结果阳性7例,灵敏度77.8%,假阳性率3.95%;方案B筛查结果阳性6例,灵敏度66.7%,假阳性率4.24%;方案C筛查结果阳性8例,假阳性率1.80%。方案C的检出率均高于方案A和B(P均<0.05),假阳性率均低于方案A和B(P均<0.05)。见表2。
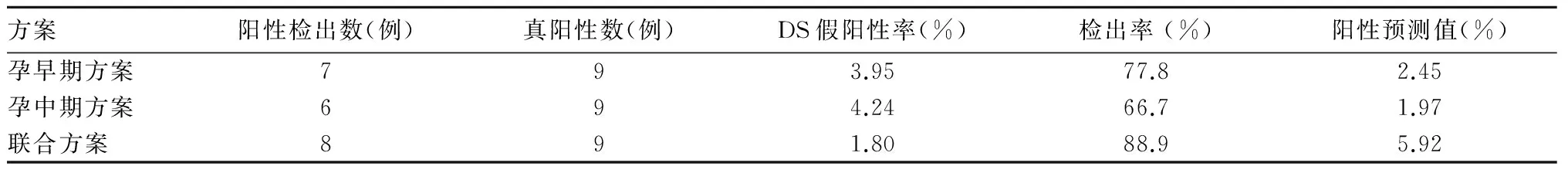
表2 三种方案筛查DS效果比较
2.3 不同筛查方案对11例胎儿非整倍体疾病的筛查风险值比较 详见表1方案C筛查得出的DS、ES儿风险值均比方案A和B高。
3 讨论
前已述及,现今产前筛查的各种筛查方案都有不同的检出率和假阳性率,检出率>80%且假阳性率<5%是比较理想的结果[8]。本研究结果显示三种筛查方案的特异度都>95%,方案C的特异度最高;方案A、B、C筛查DS检出率分别为77.8%、66.7%、88.9%,方案C的检出率高于方案A、B。另外本研究方案B的DS检出率与国内外报道相符[1,2],方案A的检出率略低于文献报道的85%[4,5],分析可能与未纳入超声指标颈项透明层厚度(NT)有关,研究显示,如纳入NT指标则有望将DS的检出率提高到89%以上[9]。早孕筛查最大的优势在于可使高风险孕妇在孕早期即通过绒毛染色体分析得到确诊,以使真阳性孕妇及时终止妊娠,是目前发达国家较常用的筛查方案[10]。本研究方案A、B的DS高风险、DS临界风险及总阳性结果检出虽然比方案C多,但是检出率低于方案C,假阳性率则高于方案C,与相关报道[11,12]相仿。
有研究显示酌情筛查和早中孕全面整合筛查的假阳性率仅2%~3%,DS检出率达到90%以上[7, 13]。但全面整合筛查需符合NT检查要求,酌情筛查需将孕妇合理区分为高风险、临界风险、低风险三类,虽然在筛查成本上较整合筛查更优[14],但需确定该方案的各类风险范围,不同实验室不同地区的标准并不相同。此外,该方案和序贯筛查一样,在早孕期的高风险孕妇需行绒毛穿刺加以确诊,故限制了这两类方案的广泛应用,而整合筛查方案既可获得较高的检出率,又减少了经济压力和有创诊断的社会支出,在早孕期还缓解了孕妇的焦虑情绪,具备较好的成本效益值,在NT检查和绒毛穿刺技术受局限地区,适于临床推广。本研究中确诊的2例ES患儿,用方案A、B筛查仅有1例得到了高风险值,另1例经方案C筛查判定为高风险,可见方案C筛查较方案A、B增加了1例ES的检出。并且方案A、B筛查出的1例ES患儿用方案C测算出的ES风险值高于方案A和B,体现出了方案C对ES良好的筛查价值,该优势在国外学者的研究中也得以证实[15]。
本研究结果显示,方案C可以减少阳性筛查结果数量(假阳性率),从而减少羊水穿刺这种有创性检查的数量。虽然临床上还有一种敏感且无创的高通量测序技术通过对母血中的胎儿游离DNA进行测序对DS、ES进行筛查[16],但目前其高昂的检测成本限制了其普遍开展。本研究结果显示 早孕期检测血清PAPP-A联合中孕期检测血清AFP、free β-HCG、uE3对DS和ES体现出了良好的筛查效率,有助于减低患者经济压力和社会支出,性价比较高,适于临床推广。
[1] Xie Z, Lu S, Li H. Contingent triple-screening for Down syndrome in the second trimester: a feasibility study in Mainland Chinese population[J]. Prenat Diagn, 2010,30(1):74-76.
[2] 苏立,吴玥丽.郑州地区20784例孕妇孕中期产前筛查结果分析[J].中国优生与遗传杂,2012,20(2):69-71.
[3] 杨岚,赵丽,江静颖,等.中孕血清学筛查在产前诊断及指导妊娠结局中的应用[J].南方医科大学学报,2015,35(7):1059-1062, 1072.
[4] Berktold L1, von Kaisenberg CS, Hillemanns P, et al. Analysis of the impact of PAPP-A, free β-hCG and nuchal translucency thickness on the advanced first trimester screening[J]. Arch Gynecol Obstet, 2013,287(3):413-420.
[5] 王胜茂.早孕期联合筛查预测唐氏综合征的效果分析[J]. 吉林医学,2014,35(15):3283-3284.
[6] Malone F, Canick JA, Ball RH, et al. First-trimester or second-trimester screening, or both, for down′s syndrome Firstand Second-Trimester Evaluation of Risk (FASTER) research consortium [J]. N Engl J Med, 2005,353(19):2001-2011.
[7] Benn P, Wright D, Cuckle H. Practical strategies in contingent sequential screening for Down syndrome[J]. Prenat Diagn, 2005,25(8):645-652.
[8] Iles RK, Shahpari ME, Cuckle H, et al. Direct and rapid mass spectral fingerprinting of maternal urine for the detection of Down syndrome pregnancy[J]. Chin Pro, 2015,12(1):9.
[9] Johnson J, Pastuck M, Metcalfe A, et al. First-trimester Down syndrome screening using additional serum markers with and without nuchal translucency and cell-free DNA[J]. Prenat Diagn, 2013,33(11):1044-1049.
[10] Park SY, Jang IA, Lee MA, et al. Screening for chromosomal abnormalities using combined test in the first trimester of pregnancy[J]. Obstet Gynecol Sci, 2016,59(5):357-366.
[11] ACOG Committee on Practice Bulletins. ACOG Practice Bulletin No. 77: screening for fetal chromosomal abnormalities[J]. Obstet Gynecol, 2007,109(1):217-227.
[12] 陈熙, 肖克林, 熊礼宽, 等. 孕早中期血清学检测指标联合筛查唐氏综合征的应用探讨[J]. 中国计划生育学杂志, 2016, 24(7): 476-478.
[13] Cuckle H, Benn P, Wright D. Down syndrome screening in the first and/or second trimester: model predicted performance using meta-analysis parameters[J]. Semin Perinatol, 2005,29(3):252-257.
[14] Gilbert RE, Augood C, Gupta R, et al. Screening for Down′s syndrome: effects, safety, and cost effectiveness of first and second trimester strategies[J]. BMJ, 2001,32(3):1-6.
[15] Palomaki GE, Neveux LM, Knight GJ, et al. Maternal serum-integrated screening for trisomy 18 using both first- and second-trimester markers[J]. Prenat Diagn, 2003,23(3):243-247.
[16] Zhang H, Gao Y, Jiang F, et al. Non-invasive prenatal testing for trisomies 21, 18, and 13: clinical experience from 146, 958 pregnancies[J]. Ultrasound Obstet Gynecol, 2015,45(5):530-538.
Screening efficiency about the first trimester screening of PAPP-A combined with the second trimester screening of AFP, free β-HCG, and uE3 strategies for Down's Syndrome and Edwards' syndrome
YANGLan,SHIJingping,WUXiao,SHINan,GUOCaiqin,ZHAOLi
(WuxiMaternalandChildHealthHospitalAffiliatedtoNanjingMedicalUniversity,Wuxi214002,China)
10.3969/j.issn.1002-266X.2017.35.006
R714.7
A
1002-266X(2017)35-0018-04
2017-03-14)
无锡市医管中心面上项目(YGZXM1510);无锡市科技发展基金项目(CSE31N1511);江苏省妇幼保健重点资助项目(201315)。
杨岚(1979-),女,医学硕士,副主任医师, 主要研究方向为产前筛查、产前诊断。E-mail:lilylan5930@sina.com
赵丽(1978-),女,医学硕士,副主任医师,主要研究方向为产前诊断、遗传咨询。E-mail:our163@163.com
