Production of Benzene from Lignin through Current Enhanced Catalytic Conversion
2017-09-03XiopingWuMinghuiFnQunxinLi
Xio-ping Wu,Ming-hui Fn,Qun-xin Li∗
a.Department of Chemical Physics,Anhui Key Laboratory of Biomass Clean Energy,Key Laboratory of Urban Pollutant Conversion,Chinese Academy of Sciences,University of Science and Technology of China,Hefei 230026,China
b.Anhui Key Laboratory of Tobacco Chemistry,China Tobacco Anhui Industrial Co.,Ltd.,Hefei 230088, China
Production of Benzene from Lignin through Current Enhanced Catalytic Conversion
Xiao-ping Wua,Ming-hui Fanb,Quan-xin Lia∗
a.Department of Chemical Physics,Anhui Key Laboratory of Biomass Clean Energy,Key Laboratory of Urban Pollutant Conversion,Chinese Academy of Sciences,University of Science and Technology of China,Hefei 230026,China
b.Anhui Key Laboratory of Tobacco Chemistry,China Tobacco Anhui Industrial Co.,Ltd.,Hefei 230088, China
The directional production of benzene is achieved by the current-enhanced catalytic conversion of lignin.The synergistic effect between catalyst and current promotes the depolymerization of lignin and the selective recombinant of the functional groups in the aromatic monomers.A high benzene yield of 175 gbenzene/kgligninwas obtained with an excellent selectivity of 92.9 C-mol%.The process potentially provides a promising route for the production of basic petrochemical materials or high value-added chemicals using renewable biomass.
Benzene,Current enhanced catalytic conversion,Lignin,Zeolite
I.INTRODUCTION
Lignin,a main constituent of lignocellulosic biomass, is a rich and natural amorphous polymer that can provide bulk renewable aromatic compounds[1].Much efforts have been made to produce value-added chemicals and bio-fuels from lignin by various strategies[1, 2],such as hydrogenation reduction[3],oxidation[4], pyrolysis[5,6],catalytic pyrolysis[7],hydrolysis[8], aqueous phase reforming[9],and enzymatic conversion/degradation of lignin[10].For hydrogenation reduction of lignin,typical reactions involve lignin depolymerization followed by the removal of the extensive functionality of the lignin subunits to form simpler monomeric compounds such as phenols,benzene, toluene,and xylenes. These simple aromatic compounds can then be hydrogenated to alkanes or used as platform chemicals in the synthesis of fine chemicals using technology developed in the petroleum industry[1,3]. For example,Xu et al. successfully hydrodeoxygenated the lignin with the Pt/C catalyst, to phenolic monomers with conversion of 21 wt%[11]. Yan et al. explored a two-step processes combining NaOH-catalyzed lignin depolymerization with hydrodeoxygenation,and achieved a high yield of C8−C18alkanes[12].The products from lignin hydrogenation reduction,depending on catalysts and reaction conditions,generally include a wide range of compounds such as phenols,aromatic hydrocarbons,alkanes and low oligomers[12].The challenges for lignin hydrogenation such as improving selectivity of target products and suppressing catalyst deactivation remain[1,12].Lignin catalytic oxidation generally produces a mixture such as aromatic aldehydes,acids,alcohols,and quinines compounds[13−15].Noble metals,transition metals and metal oxide catalysts have been explored for the catalytic oxidation of lignin or its model compounds. For example,Stark et al.developed a method for production of 2,6-dimethoxy-1,4-benzoquinone(11.5 wt% yield)through the oxidative depolymerization of lignin in ionic liquid[EMIM][CF3SO3]using the Mn(NO3)2catalyst[15].So far,both the selectivity and yield of speci fic product(for example,vanillaldehyde[4])by catalytic oxidation of lignin are low and need to be further improved.Pyrolysis of lignin primarily produces various phenolic compounds and tar in liquid products [16−18].Catalytic pyrolysis of lignin with zeolite catalysts has been widely investigated,mainly resulting in aromatic hydrocarbons mixture(such as benzene, toluene,xylenes,alkylbenzenes,naphthalenes,and indenes)[7,19,20].Zakzeski et al.reported the solubilization and aqueous phase reforming of lignin using H2SO4and Pt/Al2O3for the first time,and revealed that the alkyl chains and methoxy groups in the aromatic rings were readily reformed to produce hydrogen and simple aromatic platform chemicals,particularly guaiacol and syringol[21].
Lignin conversion into benzene,toluene and xylenes can provide the basic feedstocks for the petrochemical industry[7,22].These aromatics,also,can serve as the most important aromatic platform molecules for the development of high-end chemicals[23−25].However, directional and efficient synthesis of these platform m-olecules using lignin is still an unsolved problem[1]. Herein,we show a new route to directionally produce one of the aromatic platform molecules,benzene, by the current enhanced catalytic conversion of lignin (Scheme 1).Important feature of this strategy is using the synergistic effect between catalyst and current to promote the lignin depolymerization(Scheme 1,step 1) and the selective recombinant of the functional groups in the aromatic monomers(Scheme 1,step 2).
II.EXPERIMENTS
A.Biomass feedstocks
The lignin material,supplied from Lanxu Biotechnology Co.Ltd.(Hefei,China),was a brown and sulfurfree lignin powder manufactured from wheat straw (lignin>91 wt%,ash<5 wt%,moisture content<2 wt%, molecular weight of 2500−3500 Da).The lignin contained carbon of 62.8 wt%,hydrogen of 5.5 wt%,oxygen of 29.8 wt%and nitrogen of 1.9 wt%(in dry and ash free),which was carried out by the elemental analysis with a elemental analyzer(Vario EL-III,Elementar, Germany).
B.Catalysts
In previous studies,on the catalytic cracking of biomass and bio-oil[26,27],the catalysts of Ni/HZSM-5 and Re/Y were selected for the catalytic pyrolysis of lignin and for removing the functional groups of aromatics in the lignin-derived oil,respectively.HZSM-5(25) zeolite with a Si/Al ratio of 25 obtained from Nankai University catalyst Co.,Ltd.(Tianjin,China)was calcined in air atmosphere at 550◦C for 4 h prior to use. For the preparation of the Ni/HZSM-5 catalyst,20 g HZSM-5 was impregnated in 100 mL Ni(NO3)2solution (0.18 mol/L,Sinopharm,99.9%)at room temperature overnight,followed by rotary-evaporation at 60◦C,drying at 80◦C for 6 h,and calcinating at 550◦C for 5 h. The Ni content(4.8 wt%)in the catalyst was measured by inductively coupled plasma and atomic emission spectroscopy(ICP-AES,Atomscan Advantage,Thermo Jarrell Ash Co.,USA).The Re/Y-zeolite catalyst(containing 2.0 wt%Re)was obtained from Nankai University catalyst Co.(Tianjin,China),and calcined in nitrogen atmosphere at 550◦C for 4 h prior to use.
C.Experimental setups and procedures
The depolymerization of lignin was performed in the fixed bed catalytic pyrolysis system[22].The system was mainly composed of a quartz tube reactor(inner diameter of 33 mm,length of 400 mm),a feeder for solid reactants,two condensers and a gas analyzer.Prior to the runs,lignin was mixed with 4.8 wt%Ni/HZSM-5(25) catalyst and sieved to a particle size of 60−80 mesh. For a typical case,10 g of lignin was mixed with 30 g of Ni/HZSM-5 catalyst(catalyst/lignin weight ratio of3).The solid mixture(lignin and catalyst)was fed into the reactor by the feeder with a typical feeding rate of about 50 g lignin/h.Before the reactions,the reactor was flushed with argon at a flow rate of 1000 cm3/min for 3 h.Here,we performed and compared the depolymerization of lignin into the lignin-derived oil with following three modes:the current-enhanced catalytic pyrolysis,the catalytic pyrolysis and the pyrolysis.For the current-enhanced catalytic pyrolysis mode,an annular Ni-Cr metal wire(length of 2.9 m and diameter of 1.2 mm)was installed in the center of the reactor, and the metal wire was passed through a given electronic current by adjusting the voltage of the AC(alternating current)power supply.To obtain a speci fied reaction temperature,the reactor was simultaneously heated by an outside furnace.The temperature in the center of the reactor was controlled by adjusting the power of the outside furnace,meanwhile,the current of the metal wire installed in the catalyst bed was fixed. The center temperature in the reactor,which was close to the averaged temperature for our selected temperature range(450−550◦C),was approximately used as the reaction temperature.For the depolymerization of lignin by the conventional catalytic pyrolysis,the catalytic bed was homogeneously heated by the outside furnace.To directly investigate the impact of current, the metal wire was sometimes retained in the reactor but the power source connected with the wire was turn o ff(without the current).For the pyrolysis of lignin, the catalyst was substituted by quartz sand and the reactor was heated by the outside furnace.The difference between current-enhanced catalytic pyrolysis and catalytic pyrolysis was that a given current was supplied in the catalytic reactor for the current-enhanced catalytic pyrolysis runs.
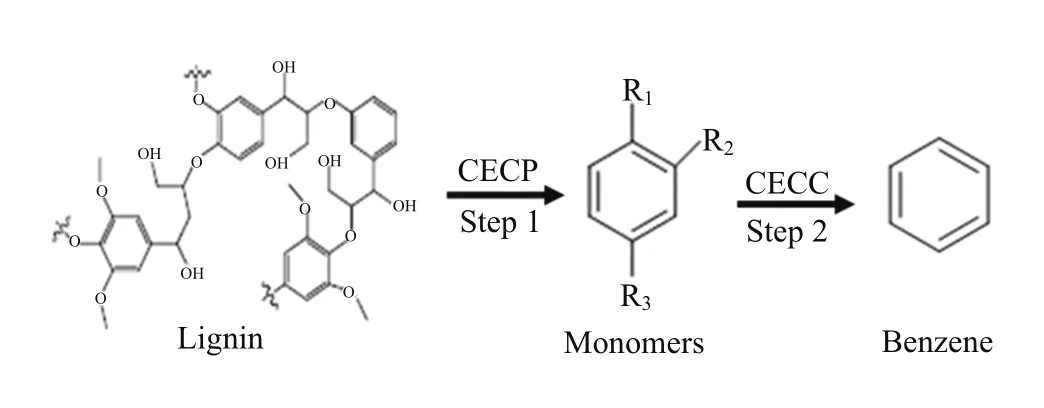
Scheme 1 Directional production of benzene through two consecutive steps that involve current-enhanced catalytic pyrolysis(CECP)of lignin followed by current-enhanced catalytic conversion(CECC)of aromatic monomers.R1, R2and R3represent the groups such as OH,CH3,OCH3or CH3CH2.
The selective conversion of lignin-derived oil or the model compounds(such as toluene and anisole)into benzene was carried out in a continuous flowing reaction system using a quartz fixed-bed reactor(inner diameter of 33 mm,length of 400 mm)under atmospheric pressure.We compared the second catalytic re fining of lignin-derived oil between the current-enhanced catalytic conversion and the conventional catalytic con-version.For the current-enhanced catalytic conversion run,an annular Ni-Cr metal wire(length of 2.1 m and diameter of 1.2 mm)entwined around a quartz column was installed in the center of the catalyst bed, and 10 g of 2.0 wt%Re/Y-zeolite with a particle size of 60−80 mesh was uniformly filled in around the metal wire.The metal wire was passed through a given electronic current for the current-enhanced catalytic conversion run. The liquid reactants(lignin-derived oil or model compounds)were fed into the reactor using the multi-syringe pump(Model:TS2−60,Baoding Longer Precision Pump)with a typical weight hourly space velocity of 0.6 h−1.Argon(99.99%)was used as carrier gas.
The integrated system for one-step conversion of lignin into benzene was mainly composed of a quartz tube reactor(inner diameter of 40 mm,length of 600 mm),a feeder for solid reactants,two condensers, and a gas analyzer.The reactor consists of two sections:the upstream region used for the depolymerization of lignin and downstream region used for the second catalytic re fining of lignin-derived oil vapor.For the current-enhanced catalytic conversion runs,an annular Ni-Cr metal wire(length of 3.2 m and diameter of 1.2 mm)entwined around a quartz column was installed in the reactor,and 10 g of 2.0 wt%Re/Y-zeolite with a particle size of 60−80 mesh was uniformly filled around the metal wire in the second section.Prior to the runs, lignin was mixed with 4.8 wt%Ni/HZSM-5(25)catalyst (catalyst/lignin weight ratio of 3).After the reactor was flushed with argon at a flow rate of 1000 cm3/min for 3 h,the metal wire was passed through a given electronic current by adjusting the voltage of the power supply.The temperature in the reactor can be controlled by adjusting the power of the outside furnace. When the temperatures reached the stable value,the solid mixture(lignin and catalyst)was fed into the pyrolysis reactor by a feeder with a typical feeding rate of about 50 glignin/h for the integrated tests.For the conventional catalytic conversion,the metal wire was removed from the reactor and the catalytic bed was heated by the outside furnaces.
D.Product analysis and data evaluation
For gas product analysis,the gas in each test was collected with air bags,and analyzed using a gas chromatograph(GC-SP6890,Shandong LunanRuihong Chemical Instrument Co.,Ltd.,Tengzhou China)with two detectors,a TCD(thermal conductivity detector)for analysis of H2,CO,CH4,and CO2separated on TDX-01 column,and a FID( flame ionization detector)for gas hydrocarbons separated on Porapak Q column.The moles of a gas product were determined by the normalization method with standard gas.The total gas weight was summed up based on the GC analysis.The liquid products(oil and water)in each test were collected by two liquid nitrogen/ethanol bath condensers.Condensed products from the condensers were weighed to obtain the mass of liquid products.The carbon contents in the liquid products were measured by a Vario EL III elemental analyzer,and the water content was analyzed by a moisture analyzer(Model ZSD-1,Shanghai,China). The main components of the organic liquid products were further analyzed by gas chromatograph(Techcomp GC-7900 equipped with a SE-30 capillary column, Shanghai,China)and GC-MS(gas chromatographymass spectroscopy,Thermo Trace GC/ISQ MS with a TR-5 capillary column,USA).For solid product analysis,the solid residues after each experiment were immediately removed from the heating zone and cooled to room temperature in an argon flow.The solid residues in each experiment were weighed,and measured by the elemental analysis(Vario EL-III,Elementar,Germany) and TGA analysis(Q5000IR thermogravimetric analyzer,USA).Overall carbon yields of the gas,liquid and solid products(Yj(C-mol%)),carbon yield of a speci fic product(Yl(C-mol%)),aromatic selectivity of a speci fic aromatic product(SA(C-mol%))were calculated based on Eqs.(1−3)as previously described[26].All tests were repeated three times and the data reported are the mean values of three trials.
For the carbon balance analysis,the carbon contents in the gas,liquid and solid products were measured by gas chromatograph analysis,elemental analysis and TGA analysis,respectively.The carbon content in each feed was also determined by the elemental analysis.The carbon balance was evaluated by the overall carbon yields obtained from the gas,liquid and solid products. For mass balance analysis,the weights of the gas,liquid and solid products as well as feeds were measured by the weighing method and combined with composition analysis.The carbon balance and mass balance evaluated ranged from 89.7 C-mol%to 107.3 C-mol% in this work.
III.RESULTS AND DISCUSSION
A.The depolymerization of lignin using three different routes
Depolymerization of complex aromatic polymer in lignin into monomers is an initial step for the directional production of benzene.Here,the transformation of lignin to aromatic monomers was explored by the current-enhanced catalytic pyrolysis with the Ni/HZSM-5(25)catalyst,which was compared to pyrolysis and catalytic pyrolysis.The above three val-orization routes were not fully selective toward the speci fied target product(benzene),but can obtain various types of aromatic monomers.As shown in Table I,the liquid products from lignin pyrolysis were main variously phenolic compounds,tar,and water,and the noncatalytic pyrolysis only produced small amounts of aromatic hydrocarbons.For the catalytic pyrolysis of lignin at 550◦C with the Ni/HZSM-5(25)catalyst, however,the yield of aromatic hydrocarbons signi ficantly increased to 28.9 C-mol%carbon yield.Aromatics selectivities of benzenes,toluene,and xylenes were about 9.2 C-mol%,26.0 C-mol%,and 21.7 C-mol%,respectively. The difference between the non-catalytic pyrolysis and catalytic pyrolysis should be attributed that the intermediate oxygenates originated from lignin pyrolysis are further catalytically converted to hydrocarbon products through decarbonylation,decarboxylation,dehydration,and oligomerization with the catalyst.With regard to current-enhanced catalytic pyrolysis with the same catalyst and same temperature (Table I),the weight yield and carbon yield of aromatic hydrocarbons signi ficantly increased to 41.9 C-mol%. Especially,the formation of oxygenates(0.2 C-mol%) and char/coke(16.3 C-mol%)by current-enhanced catalytic pyrolysis was remarkably lower than the levels obtained by pyrolysis or catalytic pyrolysis.
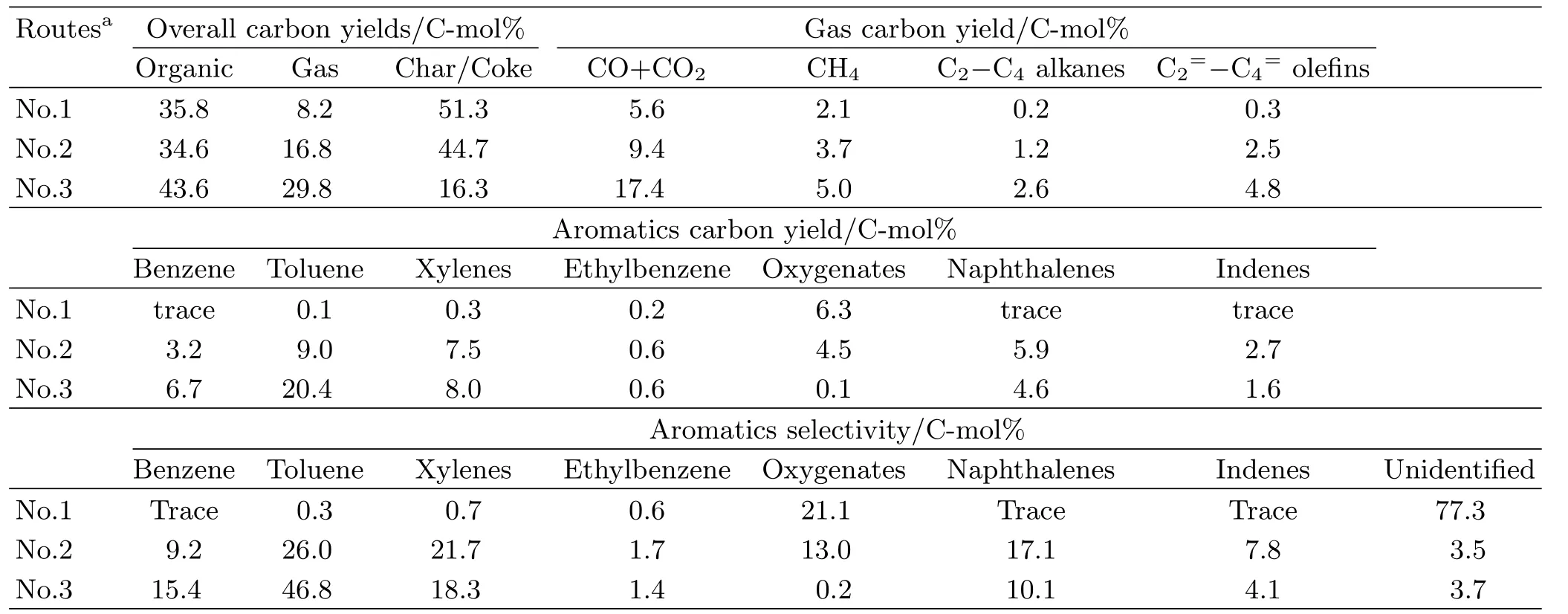
TABLE I Transformation of lignin to aromatic monomers using three different routes for the depolymerization of lignin. Reaction conditions:temperature of 550◦C,argon gas flow rate of 500 cm3/min,lignin feeding speed of 50 glignin/h. Pyrolysis:sand/lignin ratio of 3;catalytic pyrolysis:Ni/HZSM-5 catalyst/lignin ratio of 3;current enhanced catalytic pyrolysis:Ni/HZSM-5 catalyst/lignin ratio of 3,current of 4 A.
B.Comparison between the catalytic conversion and current-enhanced catalytic conversion
It is noticed that the organic liquid products from current-enhanced catalytic pyrolysis or catalytic pyrolysis of lignin still consisted of a variety of aromatic compounds.These mixtures need to be further converted into the target product by the second catalytic re fining.We think that selective recombination of functional groups in the aromatic monomers is one of the key steps for the directional production of the aromatic platform molecules from lignin.With regard to the production of benzene,it is required to completely remove the redundant groups(such as methyl and methoxy) from the aromatic monomers.Here,we compared two different second re fining processes involved in the catalytic conversion and current-enhanced catalytic conversion(Table II).For the catalytic conversion process operated at 550◦C with the Re/Y-zeolite catalyst, 77.1 C-mol%aromatic hydrocarbons(mainly consisting of 55.8 C-mol%benzene and 20.2 C-mol%toluene) with a benzene selectivity of 72.3 C-mol%were yielded. Interestingly,we found that the selective transformation of the monomers into benzene was sensitive to the current passing through the catalyst.As shown in Table II,the production of benzene from currentenhanced catalytic conversion shows much higher efficiency and selectivity than that from catalytic conversion with the same catalyst.For example,the benzene selectivity was effectively improved from 72.3 C-mol% to 92.9 C-mol%,meanwhile the benzene yield increased from 55.8 C-mol%to 70.1 C-mol%with increasing the current from 0 A to 4.0 A at 550◦C.The selectivities towards other aromatics such as toluene,xylenes,ethylbenzene,and phenols were prominently depressed.
C.Mechanism study on current-enhanced catalytic conversion
To gain further insight on the mechanism of the current-enhanced catalytic conversion of lignin,we per-formed and added the following experiments using toluene and anisole as the selected model compounds, which contain methyl and methoxy groups in the aromatic ring,respectively.We investigated the transformation of these model compounds to benzene by the conventional catalytic conversion(without current)and the current-enhanced catalytic conversion(with current),respectively.The results show that the methyl and methoxy groups can be effectively removed from the aromatics with current-enhanced catalytic conversion(Tables III−IV).
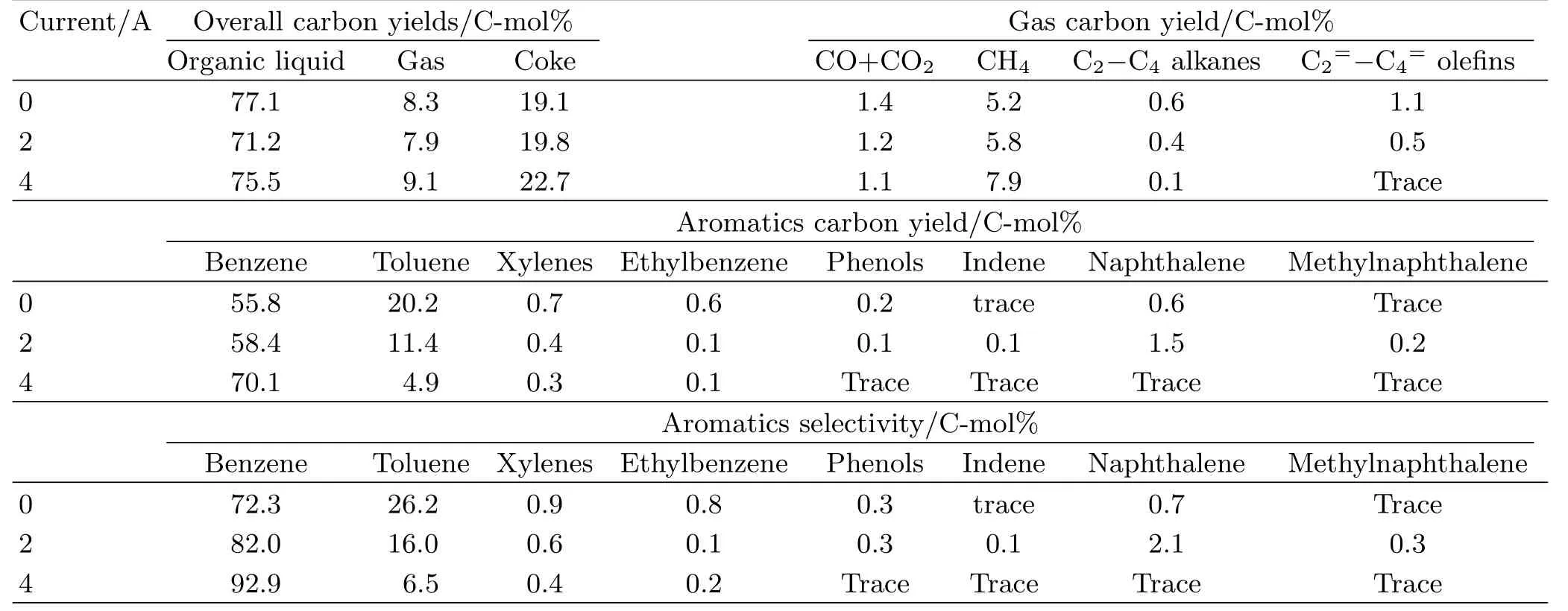
TABLE II Transformation of the aromatic monomers to benzene through the current enhanced catalytic conversion and non-current enhanced catalytic conversion.Reaction conditions:temperature of 550◦C,current of 0−4 A,weight hourly space velocity of 0.6 h−1,argon gas flow rate of 150 cm3/min,and the Re/Y-zeolite catalyst of 10 g.The data evaluations were calculated based on Eqs.(1−3).The values reported are averages of three trials.
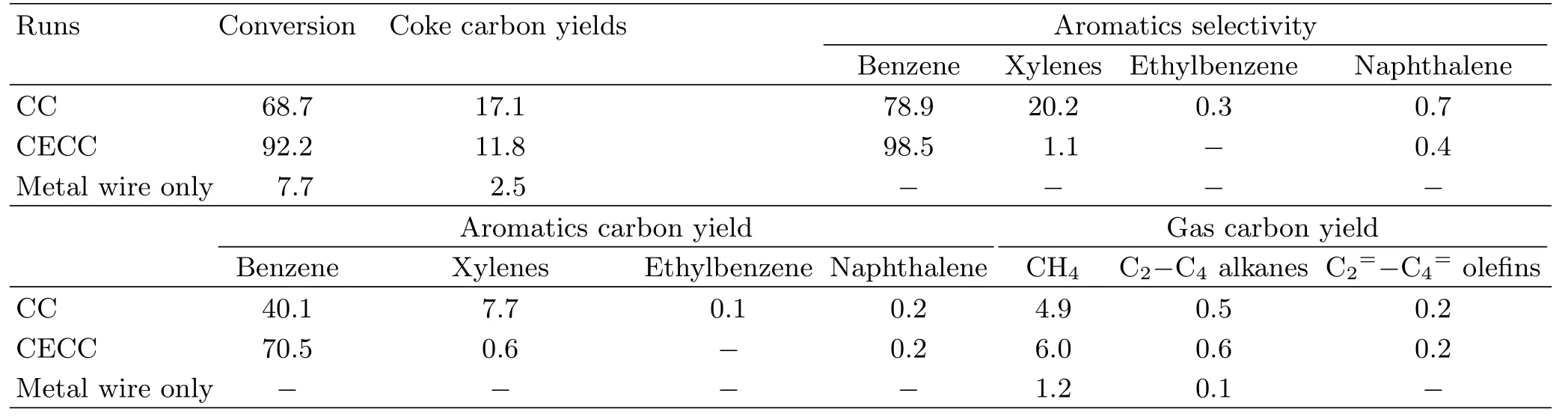
TABLE III Transformation of toluene to benzene through the current enhanced catalytic conversion(CECC)in 4 A and conventional catalytic conversion(CC)in 0 A.Reaction conditions:temperature of 450◦C,weight hourly space velocity of 0.6 h−1,argon gas flow rate of 150 cm3/min,and the Re/Y-zeolite catalyst of 10 g.The data evaluations were calculated based on Eqs.(1−3).The values reported are averages of three trials.Conversion,coke carbon yields,aromatics selectivity, gas carbon yield,and aromatics carbon yield in C-mol%.Metal wire only in 0 A,sand.
1.Comparison between current-enhanced catalytic conversion and catalytic conversion of toluene
To investigate the removal of methyl functional group from the methyl-containing aromatic compounds, the transformation of toluene to benzene was carried out through the catalytic conversion and the currentenhanced catalytic conversion. Almost all of liquid products(98.5 C-mol%)obtained from currentenhanced catalytic conversion of toluene over the Re/Y-Zeolite catalyst were benzene,which was formed through demethylation process(Table III).About 20.2 C-mol%of xylenes was generated by methylation of toluene in the catalytic conversion process,but signi ficantly decreased to 1.1 C-mol%in the currentenhanced catalytic conversion process.The transformation of toluene to benzene by current-enhanced catalytic conversion shows much higher benzene yield and selectivity than that by catalytic conversion with the same catalyst.With increasing the current from 0 A to 4 A at 450◦C,the yield of benzene distinctly increased to from 40.1 C-mol%to 70.5 C-mol%,meanwhile,the aromaticsselectivity of benzene was improved from 78.9 C-mol% to 98.5 C-mol%.In addition,the metal wire itself had very low catalytic activity for the transformation of the model compounds to benzene(Tables III−IV).
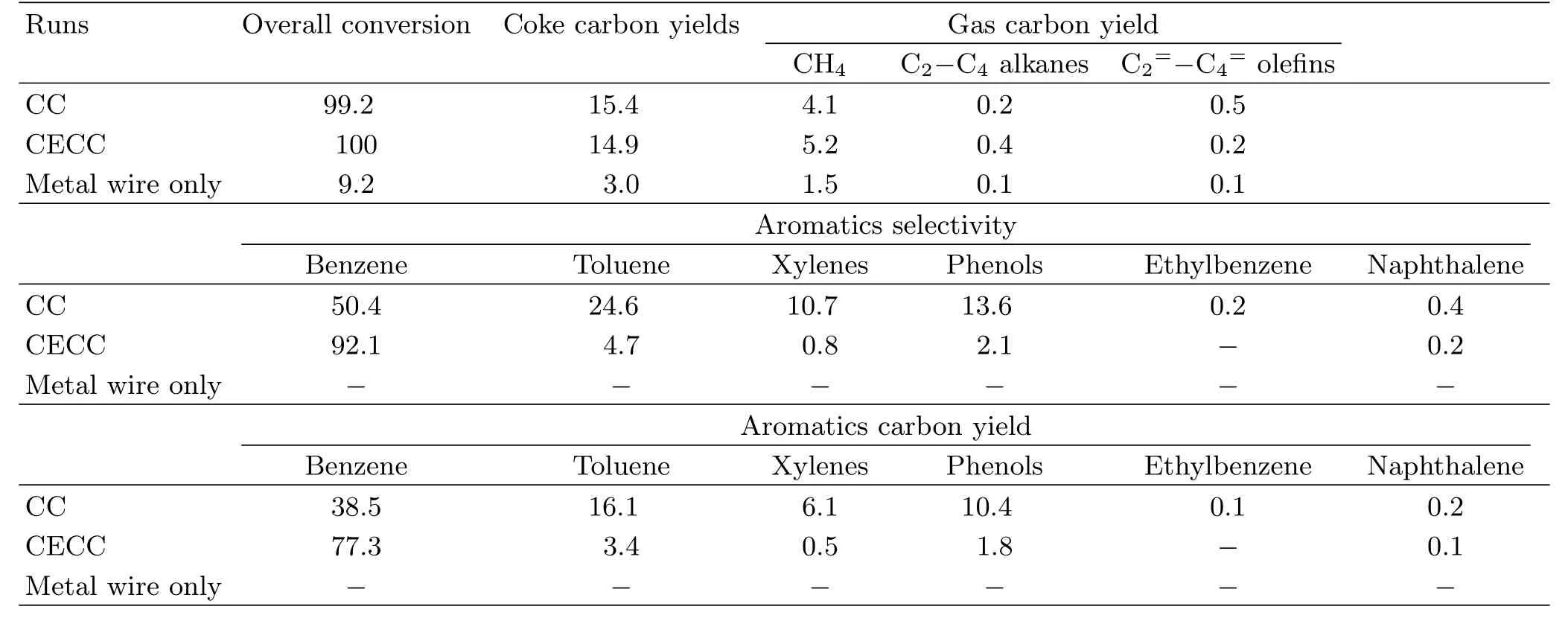
TABLE IV Transformation of anisole to benzene through the CECC in 0 A and CC in 4 A.Reaction conditions:temperature of 450◦C,weight hourly space velocity of 0.6 h−1,argon gas flow rate of 150 cm3/min,and the Re/Y-zeolite catalyst of 10 g. The data evaluations were calculated based on Eqs.(1−3).The values reported are averages of three trials.Conversion,coke carbon yields,aromatics selectivity,gas carbon yield,and aromatics carbon yield in C-mol%.Metal wire only in 0 A,sand.
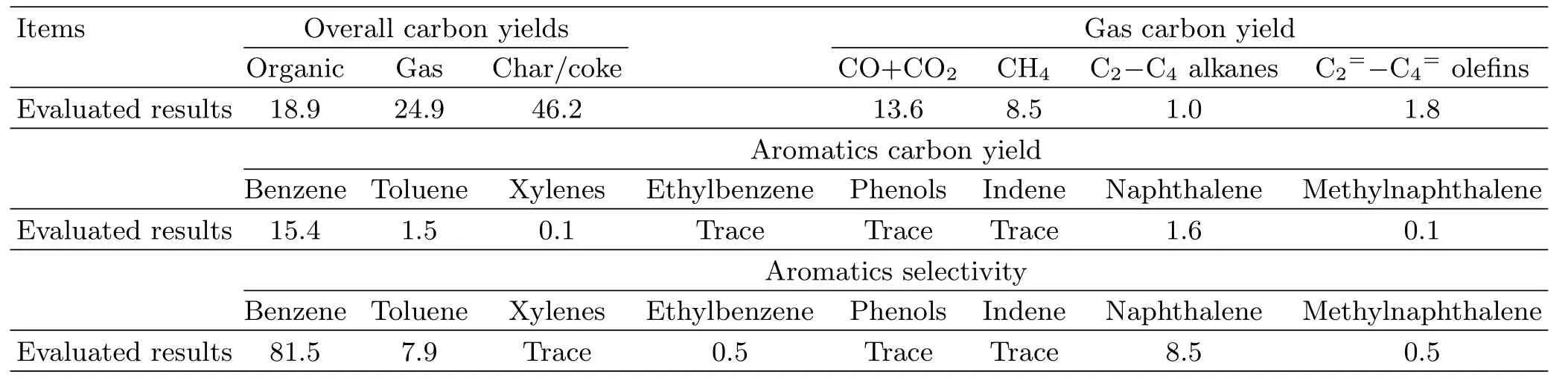
TABLE V One-step conversion of lignin into benzene by the system integration.Reaction conditions:temperature of 550◦C,current of 4 A,argon gas flow rate of 150 cm3/min,catalyst/lignin ratio of 3,weight ratio of Ni/HZSM-5 to Re/Y-zeolite at 2:1 and lignin feeding speed of 50 g lignin/h.Overall carbon yields,aromatics selectivity,gas carbon yield,and aromatics carbon yield in C-mol%.
2.Comparison between current-enhanced catalytic conversion and catalytic conversion of anisole
To investigate the removal of the methoxy group from the aromatics,the transformation of anisole over Re/Y-zeolite was performed by catalytic conversion and current-enhanced catalytic conversion respectively(Table IV).For the catalytic conversion process,the products from the anisole conversion were a wide range of compounds and mainly consisted of benzene(50.4 C-mol%),toluene(24.6 C-mol%), xylenes(10.7 C-mol%)and phenols(13.6 C-mol%), implying that the demethoxylation of anisole,alkylation of benzene/toluene and anisole demethylation reactions occurred over the zeolite. Contrary to the catalytic conversion process,the main product from current-enhanced catalytic conversion of anisole was 92.1 C-mol%benzene together with a small amount of toluene(4.7 C-mol%).The benzene yield from the anisole conversion by current-enhanced catalytic conversion reached 77.3 C-mol%,which was signi ficantly higher than the value of 38.5 C-mol%by catalytic conversion at 450◦C.The directional conversion of anisole to benzene in the current-enhanced catalytic conversion process was attributed to suppressing the side reactions and subsequent decomposing of the by-products to benzene.
To gain insight on the mechanism of the currentenhanced catalytic conversion of lignin,we performed the following experiments using toluene and anisole as the selected model compounds,which contain methyl and methoxy groups in the aromatic ring,respectively. We investigated the transformation of thesemodel compounds to benzene over the Re/Y-zeolite catalyst by catalytic conversion and current-enhanced catalytic conversion,respectively. For the currentenhanced catalytic conversion of toluene(Table III), almost all of liquid product(98.5 C-mol%)was benzene formed through demethylation process. About 20.2 C-mol%of xylenes was generated by methylation of toluene in the catalytic conversion process,but signi ficantly decreased to 1.1 C-mol%in the current-enhanced catalytic conversion process.With increasing the current from 0 A to 4 A at 450◦C,the aromatics selectivity of benzene was improved from 78.9 C-mol% to 98.5 C-mol%,and the benzene yield distinctly increased from 40.1 C-mol%to 70.5 C-mol%.With regard to the anisole conversion(Table IV),the products from CC were a wide range of compounds and mainly consisted of benzene(50.4 C-mol%),toluene (24.6 C-mol%),xylenes(10.7 C-mol%),and phenols (13.6 C-mol%),implying that the demethoxylation of anisole,alkylation of benzene/toluene and anisole demethylation reactions occurred over the zeolite.Contrary to the catalytic conversion process,the main product from current-enhanced catalytic conversion of anisole was 92.1 C-mol%benzene,and its yield reached 77.3 C-mol%which was signi ficantly higher than the value of 38.5 C-mol%by catalytic conversion at 450◦C. The directional conversion of anisole to benzene in the current-enhanced catalytic conversion process was attributed to suppressing the side reactions and subsequent decomposing of the by-products to benzene.
D.The test of one-step conversion of lignin into benzene
The system integration is very important to make practical use of new technical route.Here,one-step conversion of lignin into benzene was tested.The reaction condition was balanced at temperature of 550◦C, argon gas flow rate of 150 cm3/min and current of 4 A, so that the lignin depolymerization and selective conversion of monomers into benzene can occur in the onepot reactor.The one-step conversion showed an absolute yield of 103 gbenzene/kgligninwith a benzene selectivity of 81.5 C-mol%(Table V),which was lower than the level obtained by the separated two-steps processes(yield of 175 gbenzene/kgligninand selectivity of 92.9 C-mol%).Therefore,the setup and condition for the system integration still needs to be further optimized.
E.Lifetime of catalysts and reaction-regeneration cycles
As shown in FIG.1,the catalyst lifetime and reaction-regeneration cycles were tested for conversion of lignin into benzene through the current-enhanced catalytic conversion in the integrated system. For the reaction step,we tested the current-enhanced catalytic conversion of lignin under the following conditions:temperature of 550◦C,current of 4 A,argon gas flow rate of 150 cm3/min,catalyst/lignin ratio of 3,weight ratio of Ni/HZSM-5 to Re/Y-zeolite at 2:1, and lignin feeding speed of about 50 glignin/h.For the regeneration step,the combustion of the coke deposited on the catalyst was carried out in oxygen at 550◦C for 4 h(coke burn-o ffmethod)and ensured almost complete elimination of coke.Operating current-enhanced catalytic conversion of lignin after 90 min,benzene selectivity decreased from 82.1 C-mol%to 38.9 C-mol%, accompanied by a decrease in the benzene yield from 15.8 C-mol%to 4.8 C-mol%.A similar catalyst deactivation was also observed in the conventional catalytic conversion for time on stream of 1.5 h.The decrease in the catalyst activity is mainly caused by the carbon deposition on the catalyst in the lignin conversion process,considering that the regeneration of the deactivated catalyst can be achieved by the coke burno ffmethod(the coke yield under the different conditions was given in Table II and Table V).Therefore,a reaction-regeneration recycle con figuration can be assembled for conversion of lignin into benzene(FIG.1).
IV.CONCLUSION
In conclusion,we presented a route for directional production of benzene through two consecutive steps that involve the current-enhanced catalytic depolymerization of lignin and selective conversion of aromatic monomers to benzene.Present results suggested that the synergistic effect between catalyst and current promoted the lignin depolymerization and the selective recombinant of the groups in the monomers. The mechanism studies revealed that the groups such as methyl and methoxy in the aromatic were able to effectively remove from the aromatics,and the thermal electrons enhanced the dissociation of aromatic molecules in the current-enhanced catalytic conversion process. The proposed route shows an excellent benzene selectivity of 92.9 C-mol%and a high benzene yield of 175 gbenzene/kglignin.The process can be carried out at medium temperature and atmospheric pressure without external hydrogen,which potentially provide a promising route for production of the basic petrochemical materials and development of high-end chemicals using renewable lignin.
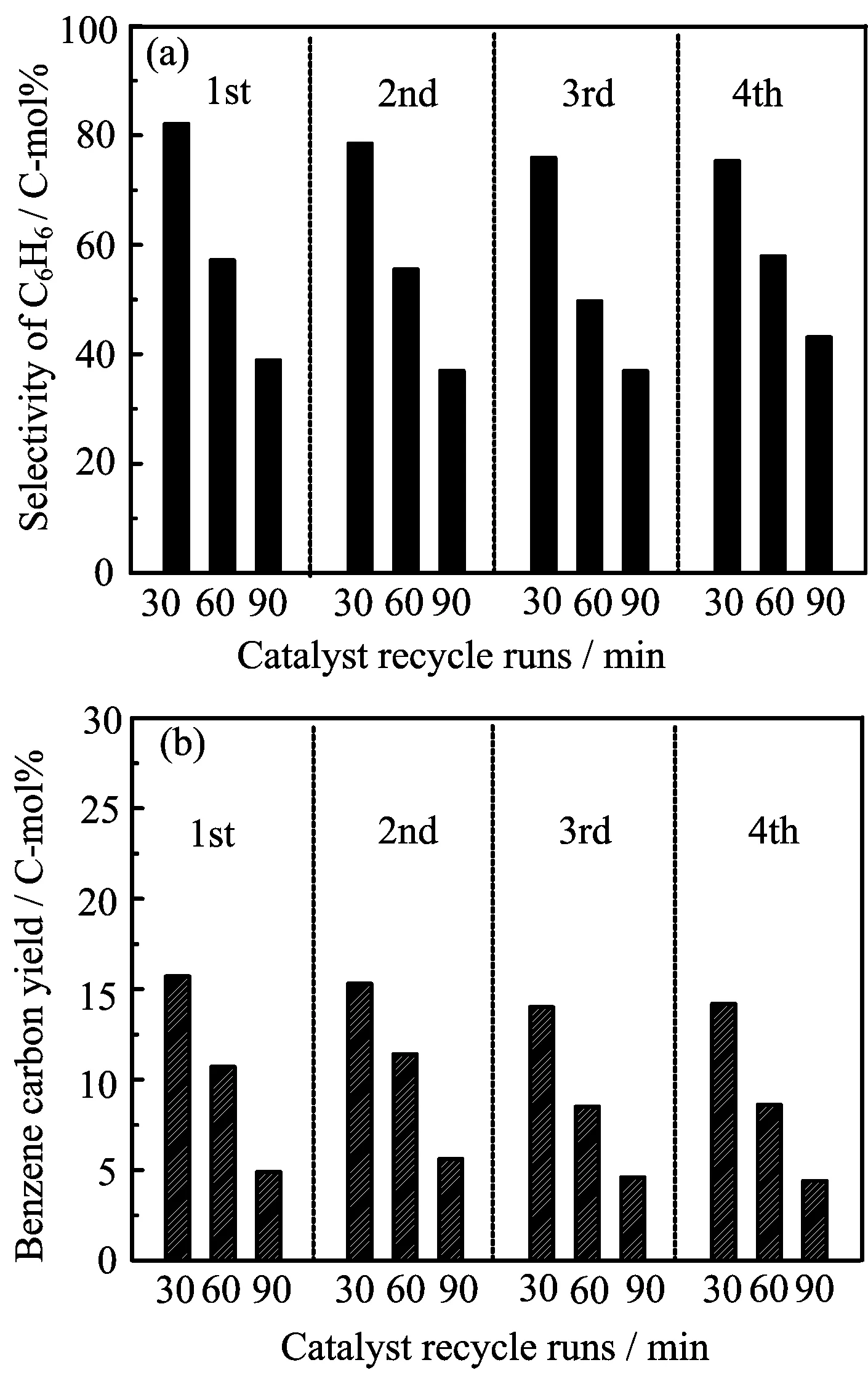
FIG.1 The catalyst reaction-regeneration cycles were tested for conversion of lignin into benzene through the currentenhanced catalytic conversion in the integrated system.Reaction conditions:temperature of 550◦C,current of 4 A, argon gas flow rate of 150 cm3/min,catalyst/lignin ratio of 3,weight ratio of Ni/HZSM-5 to Re/Y-zeolite at 2:1,and lignin feeding speed of about 50 g lignin/h.The regeneration of the deactivated catalyst was carried out by the coke burn-o ffmethod at 550◦C in oxygen for 4 h.
This work was supported by the National Natural Sci-Tech Support Plan(No.2014BAD02B03),the Program for Changjiang Scholars and Innovative Research Team in University and the Fundamental Research Funds for the Central Universities(No.wk2060190040).
[1]J.Zakzeski,P.C.A.Bruijnincx,A.L.Jongerius,and B.M.Weckhuysen,Chem.Rev.110,3552(2010).
[2]G.W.Huber and A.Corma,Angew.Chem.Int.Ed. 46,7184(2007).
[3]M.Kleinert,J.R.Gasson,and T.Barth,J.Anal.Appl. Pyrolysis 85,108(2009).
英国民族文化有着非常悠久的历史,其中占据着主要地位的是正统、传统文化。在英国的传统文化中,民众应保守、自律、保持着绅士态度等等。并且,在英国人看来,应始终如一地对本民族的文化保持着自信心与自豪感,把坚守传统看作与提高自己不被别人超越一样重要。这一点,在许多英国文学作品中也可以看到,如小说中的主人公罗宾汉、贝奥武夫等等都有着传统的绅士风度与骑士精神,并且也会积极的进取。当然,英国始终如一的坚守着传统也造就了文学评论多数属于中规中矩的,个性不足。美国的民族文化比较开放、自由,在这种多元文化思想的碰撞下,美国文学创作与文学评论就有了更多更好的创新空间,从而为美国文学评论体系结构的建立提供有力支撑。
[4]H.Deng,L.Lin,and S.Liu,Energy Fuels 24,4797 (2010).
[5]Q.Yao,Z.Tang,J.Guo,Y.Zhang,and Q.Guo,Chin. J.Chem.Phys.28,209(2015).
[6]S.Wang,H.Lin,B.Ru,W.Sun,Y.Wang,and Z.Luo, J.Anal.Appl.Pyrolysis 108,78(2014).
[7]P.Bi,J.Wang,Y.Zhang,P.Jiang,X.Wu,J.Liu,H. Xue,T.Wang,and Q.Li,Bioresour.Technol.183,10 (2015).
[8]F.Bouxin,S.Baumberger,J.H.Renault,and P.Dole, Bioresour.Technol.102,5567(2011).
[9]J.Zakzeski and B.M.Weckhuysen,ChemSusChem 4, 369(2011).
[10]Q.Wu,L.Ma,J.Long,R.Shu,Q.Zhang,T.Wang, and Y.Xu,Chin.J.Chem.Phys.29,474(2016).
[11]W.Xu,S.J.Miller,P.K.Agrawal,and C.W.Jones, ChemSusChem 5,667(2012).
[12]N.Yan,C.Zhao,P.J.Dyson,C.Wang,L.T.Liu,and Y.Kou,ChemSusChem 1,626(2008).
[13]J.Dai,A.F.Patti,and K.Saito,Tetrahedron Lett.57, 4945(2016).
[14]C.Diaz-Urrutia,B.B.Hurisso,and P.M.P.Gauthier, J.Mol.Catal.A 423,414(2016).
[15]K.Stark,N.Taccardi,A.Bosmann,and P.Wasserscheid,ChemSusChem 3,719(2010).
[16]D.J.Nowakowski,A.V.Bridgwater,D.C.Elliott,D. Meier,and P.Wild,J.Anal.Appl.Pyrolysis 88,53 (2010).
[17]D.K.Shen,S.Gu,K.H.Luo,S.R.Wang,and M.X. Fang,Bioresour.Technol.101,6136(2010).
[18]G.Jiang,D.J.Nowakowski,and A.V.Bridgwater, Energy Fuels 24,4470(2010).
[19]Y.Zhao,L.Deng,B.Liao,Y.Fu,and Q.X.Guo, Energy Fuels 24,5735(2010).
[20]C.A.Mullen,and A.A.Boateng,Fuel Process.Technol.91,1446(2010).
[21]J.Zakzeski and B.M.Weckhuysen,ChemSusChem 4, 369(2011).
[22]M.Fan,S.Deng,T.Wang,and Q.Li,Chin.J.Chem. Phys.27,221(2014).
[23]J.Zhu,J.Wang,and Q.Li,Chin.J.Chem.Phys.26, 477(2013).
[24]Y.Zhang,P.Bi,J.Wang,P.Jiang,X.Wu,H.Xue,J. Liu,X.Zhou,and Q.Li,Appl.Energy 150,128(2015).
[25]J.Wang,P.Bi,Y.Zhang,H.Xue,P.Jiang,X.Wu,J. Liu,T.Wang,and Q.Li,Energy 86,488(2015).
[26]M.Fan,P.Jiang,P.Bi,S.Deng,L.Yan,Q.Zhai,T. Wang,and Q.Li,Bioresour.Technol.143,59(2013).
[27]A.G.Gayubo,A.Alonso,B.Valle,A.T.Aguayo,M. Olazar,and J.Bilbao,Fuel 89,3365(2010).
[28]P.Bi,Y.Yuan,M.Fan,P.Jiang,Q.Zhai,and Q.Li, Bioresour.Technol.136,222(2013).
[29]L.Yuan,T.Ye,F.Gong,Q.Guo,Y.Torimoto,M. Yamamoto,and Q.Li,Energy Fuels 23,3103(2009).
ceived on March 24,2017;Accepted on April 30,2017)
∗Author to whom correspondence should be addressed.E-mail: liqx@ustc.edu.cn,Tel.:+86-551-63601118,FAX:+86-551-63606689
猜你喜欢
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- γ-Ray Irradiation-Derived MnO/rGO Composites for High Performance Lithium Ion Batteries
- Identi fication of Superoxide O2−during Thermal Decomposition of Molten KNO3-NaNO2-NaNO3Salt by Electron Paramagnetic Resonance and UV-Vis Absorption Spectroscopy
- Binding Mechanism and Molecular Design of Benzimidazole/Benzothiazole Derivatives as Potent Abl T315I Mutant Inhibitors
- Highly Responsive and Selective Ethanol Gas Sensor Based on Co3O4-Modi fied SnO2Nano fibers
- Geometric Design of Anode-Supported Micro-Tubular Solid Oxide Fuel Cells by Multiphysics Simulations
- Laser-Assisted Stark Deceleration of Polar Molecules HC2n+1N(n=2,3,4) in High-Field-Seeking State
