Highly Responsive and Selective Ethanol Gas Sensor Based on Co3O4-Modi fied SnO2Nano fibers
2017-09-03DongdongChenZhouLiXinJinJianxinYi
Dong-dong Chen,Zhou Li,Xin Jin,Jian-xin Yi
State Key Laboratory of Fire Science,Department of Safety Science and Engineering,University of Science and Technology of China,Hefei 230026,China
Highly Responsive and Selective Ethanol Gas Sensor Based on Co3O4-Modi fied SnO2Nano fibers
Dong-dong Chen,Zhou Li,Xin Jin,Jian-xin Yi∗
State Key Laboratory of Fire Science,Department of Safety Science and Engineering,University of Science and Technology of China,Hefei 230026,China
SnO2nano fibers were synthesized by electrospinning and modi fied with Co3O4via impregnation in this work.Chemical composition and morphology of the nano fibers were systematically characterized,and their gas sensing properties were investigated.Results showed that Co3O4modi fication signi ficantly enhanced the sensing performance of SnO2nano fibers to ethanol gas.For a sample with 1.2 mol%Co3O4,the response to 100 ppm ethanol was 38.0 at 300◦C,about 6.7 times larger than that of SnO2nano fibers.In addition,the response/recovery time was also greatly reduced.A power-law dependence of the sensor response on the ethanol concentration as well as excellent ethanol selectivity was observed for the Co3O4/SnO2sensor.The enhanced ethanol sensing performance may be attributed to the formation of p-n heterojunctions between the two oxides.
SnO2nano fibers,Heterojunction,Electrospinning,Impregnation,Gas sensors
I.INTRODUCTION
Resistance-type gas sensors based on semiconductor metal oxides such as SnO2,ZnO,and TiO2have been widely investigated due to their high sensitivity,fast response,and low cost.Among them,SnO2is widely regarded as one of the most promising sensing materials for detection of various gases.However,SnO2-based gas sensors are still limited by their poor selectivity and relatively long response/recovery time for practical applications.
In order to obtain better selectivity and faster response/recovery speed,much effort has been made on the design of material microstructure and surface modification.Various shapes of SnO2materials on the nanoscale,including flowers[1],spheres[2],rods[3],and fibers[4]have been fabricated. In particular,SnO2nano fibers are of great interest because of their large surface-to-volume ratio.Furthermore,their gas-sensing property can also be effectively enhanced by forming heterojunctions with other semiconductor metal oxides, such as Co3O4[5,6],NiO[7,8],CuO[9],and In2O3[10].Among these oxides,Co3O4has attracted much attention due to its excellent catalytic performance and synergetic effect with SnO2.Jeong et al.synthesized Co3O4-coated SnO2hollow nanospheres via galvanic replacement,and obtained highly improved selectivity to xylene and methylbenzenes[5].Wang et al.modified SnO2nanospheres with Co3O4via a hydrothermal method,and signi ficantly enhanced the response to ammonia gas[11].However,these synthesis methods are difficult for wide-spread use because of the complexity. In contrast,impregnation has been widely adopted as a facile and low cost route for preparation of nano-sized catalysts,which may also be used for synthesis of heterojunction sensing materials.
In this work,SnO2nano fibers were prepared by electrospinning,and modi fied with Co3O4by impregnation.The results showed that gas sensors based on Co3O4/SnO2nano fibers were highly responsive and selective to ethanol gas.Furthermore,signi ficant reduction in both the response and recovery time was also observed relative to that for the SnO2nano fibers.The gas-sensing performance was discussed in relation to the p-n heterojunction.
II.EXPERIMENTS
All reagents were of analytical grade and purchased from Sinopharm Chemical Reagent Co.,Ltd.,China. Pristine SnO2nano fibers were prepared via electrospinning.Typically,0.4 g SnCl2·2H2O,5.6 mL anhydrous ethanol,and 4.7 mL N,N-dimethylformamide(DMF) were mixed and stirred for 30 min under 1000 r/min. 0.8 g polyvinyl pyrrolidone(PVP,Mw=1.3×106)was then added under stirring.The obtained transparent solution was transferred to a plastic syringe.A voltage of 15 kV was applied for electrospinning and the feeding rate was kept constant at 0.4 mL/h using a syringe pump.The as-spun fibers were dried at 80◦C,and then calcined at 600◦C for 3 h to obtain SnO2nano fibers.
Appropriate amounts of SnO2nano fibers were soaked in 0.1 mol/L cobalt nitrate solution followed by filtering. The filtered powders were dried at 80◦C for 2 h,and then heated at 600◦C for 3 h to obtain Co3O4/SnO2nano fibers.These procedures were repeated to obtain another sample with different Co3O4amounts.SnO2nano fibers subjected to this impregnation treatment for 0,1,and 2 times are denoted as SCo-0,SCo-1,and SCo-2,respectively.
Crystal structure was examined by powder X-ray diffraction(XRD,TTR III)with Cu Kα1 radiation.Morphology and microstructure of the nano fibers were studied by scanning electron microscope(JSM-6700F)and transmission electron microscopy(JEM-2011)equipped with an energy-dispersive X-ray spectrometer(EDX).X-ray photoelectron spectroscopy (XPS)was performed on an ESCLAB 250 spectrometer using Al Kα as the exciting source.
To prepare the sensor,nano fibers were dispersed in ethanol under ultrasonic vibration for 10 min.The obtained paste was coated on an alumina tube and then heat-treated at 400◦C for 2 h.The alumina tube has been equipped with a pair of Au electrodes,which was each connected with two platinum wires.A Ni-Cr alloy coil was inserted into the alumina tube as a heater.
The sensing properties of the nano fibers were examined with a WS-30A(Weisheng Electronics Co.Ltd., China)system and an electrometer(Agilent 34461A). The testing method was similar to that described in our previous work[7].Measurements were conducted in an 18 L chamber in a static atmosphere.Appropriate amounts of certi fied analyte gas(Nanjing Specialty Gas Co.,Ltd.) were injected with a syringe,which led to changes in the electrical resistance of the sensor.For ethanol and acetone,vapors were obtained by vaporizing their liquid samples with an evaporator inside the chamber.The sensor response was de fined as S=Rair/Rgas(Rair:resistance in air atmosphere,Rgas: resistance during exposure to the target gas).The time taken by the sensor to reach 90%of the total resistance change was de fined as the response time in the case of response or the recovery time in the case of recovery.
III.RESULTS AND DISCUSSION
FIG.1 shows the XRD patterns of the as-prepared samples.For pristine SnO2,a single-phase tetragonal rutile structure(JCPDS No.41-1445)was obtained.For Co3O4/SnO2composites,all peaks could be indexed to SnO2,and neither presence of Co3O4nor shift of the diffraction peaks was observed.Under the present synthesis conditions,Co3O4would be formed by thermal decomposition of Co(NO3)2·6H2O[12].The absence of Co3O4diffraction peaks is explained as follows.As the concentration of cobalt nitrate solution used was relatively low and the impregnation time was as short as 2 min,only a small amount of Co3O4with small parti-cle size would be formed,which is consistent with the SEM-EDX results discussed below.Similar phenomena have also been observed in other composite materials prepared by impregnation methods[4,13,14].On the other hand,FIG.1 also shows that the diffraction peaks became broadened for the Co3O4/SnO2composites,suggesting larger crystalline size.According to the Debye-Scherrer equation,the crystallite size was estimated to be 9.3 nm for SCo-0,which increased to 33.6 and 48.0 nm for SCo-1 and SCo-2,respectively.The grain growth can be attributed to the repeated calcinations at 600◦C after the impregnation.
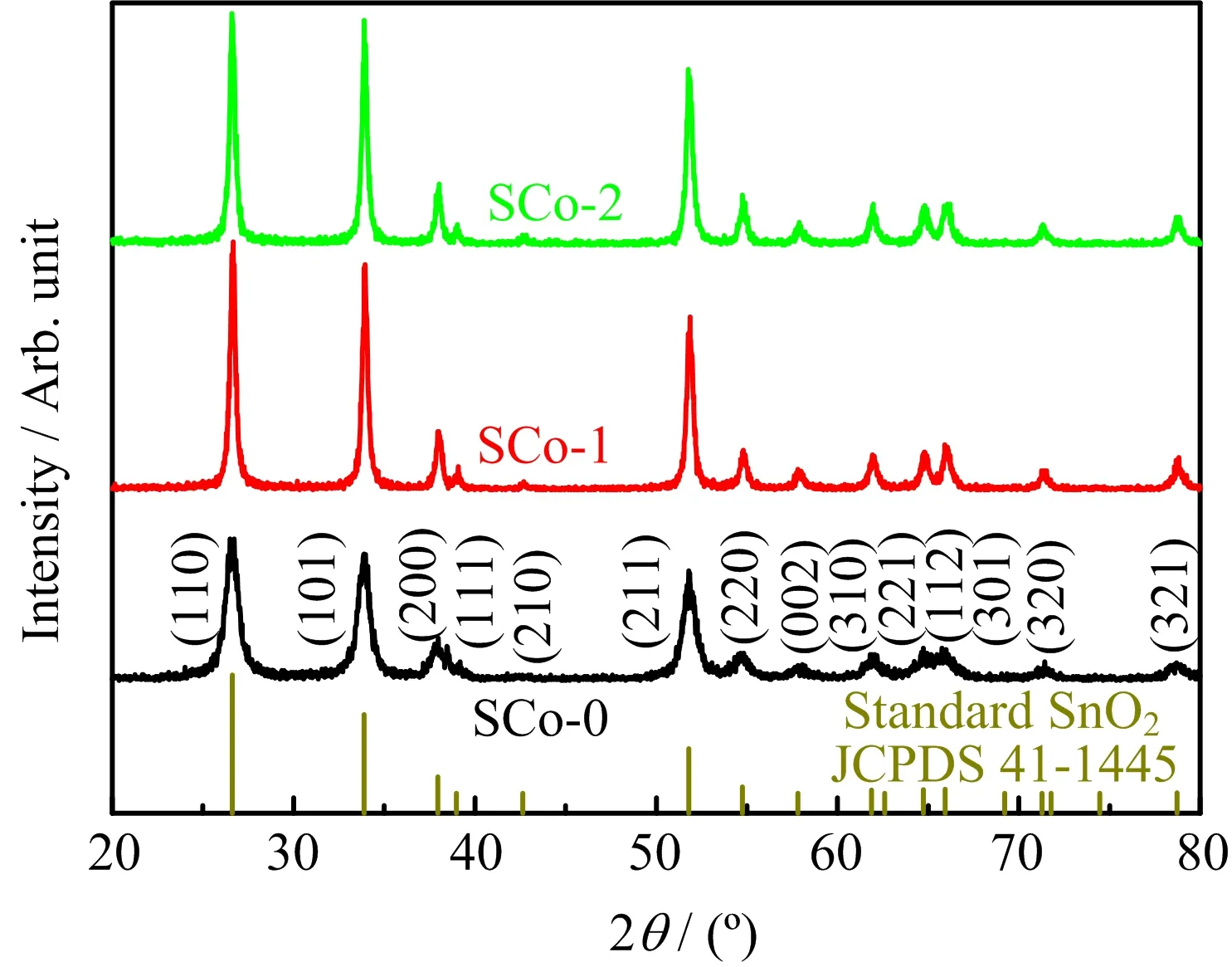
FIG.1 XRD patterns of the as-prepared samples.
XPS was used to analyze the composition and chemical state of the samples,wherein the binding energy for the C 1s peak at 284.8 eV was used as a reference for energy calibration(FIG.2).Sn and O peaks were observed for all the samples,and Co peaks were found for the impregnated samples.The Sn 3d3/2and Sn 3d5/2peaks appeared at 495.0 and 486.6 eV,respectively,which agreed well with other reports for SnO2[15].Co 2p1/2and Co 2p3/2doublets for SCo-1 and SCo-2 were observed at 796.5 and 781.0 eV,respectively,which was consistent with those of Co3O4[16]. The Co/(Sn+Co)ratio was determined to be 6.9 at% for SCo-1 and 9.9 at%for SCo-2(Table I),indicating an increase of Co loading amount with the increase of impregnation times.For the O 1s spectra,each peak was asymmetric and could be deconvoluted into two peaks at~531.4 and~530.3 eV,corresponding to the adsorbed oxygen(Oads)and lattice oxygen(Olat),respectively.As can be seen from Table I,the peak area ratio for adsorbed oxygen to total O 1s decreased monotonically with increasing impregnation time,indicating that oxygen adsorption was depressed by the presence of Co3O4.
SEM images in FIG.3 shows that the length and the diameter of SnO2nano fibers were~1µm and 100−200 nm,respectively.Similar morphology was observed for the SCo-1 and SCo-2 nano fibers.TEM analysis further indicated that the particle size was around 10−50 nm(FIG.4).Some small pores were present in the nano fibers,which would be favorable for achiev-ing high gas accessibility of the materials.EDX analysis revealed a Co/(Sn+Co)ratio of 3.7 at%for SCo-2 nano fibers,corresponding to~1.2 mol%Co3O4.The Co content is much lower than that measured by XPS, which is also consistent with the fact that Co3O4was formed on the surface of SnO2.Direct observation of Co3O4particles was not successful,owing to the low content of Co3O4as well as its small particle size prepared by impregnation.
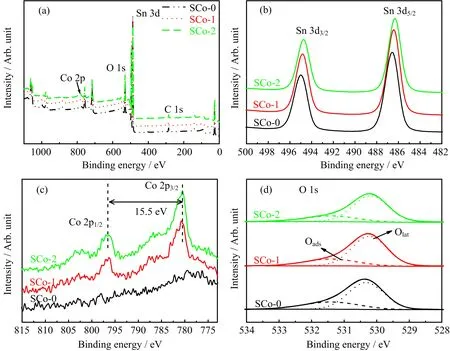
FIG.2(a)Survey XPS spectrum and high resolution spectra for(b)Sn 3d,(c)Co 2p,and(d)O 1s of pristine and Co3O4modi fied SnO2nano fibers.
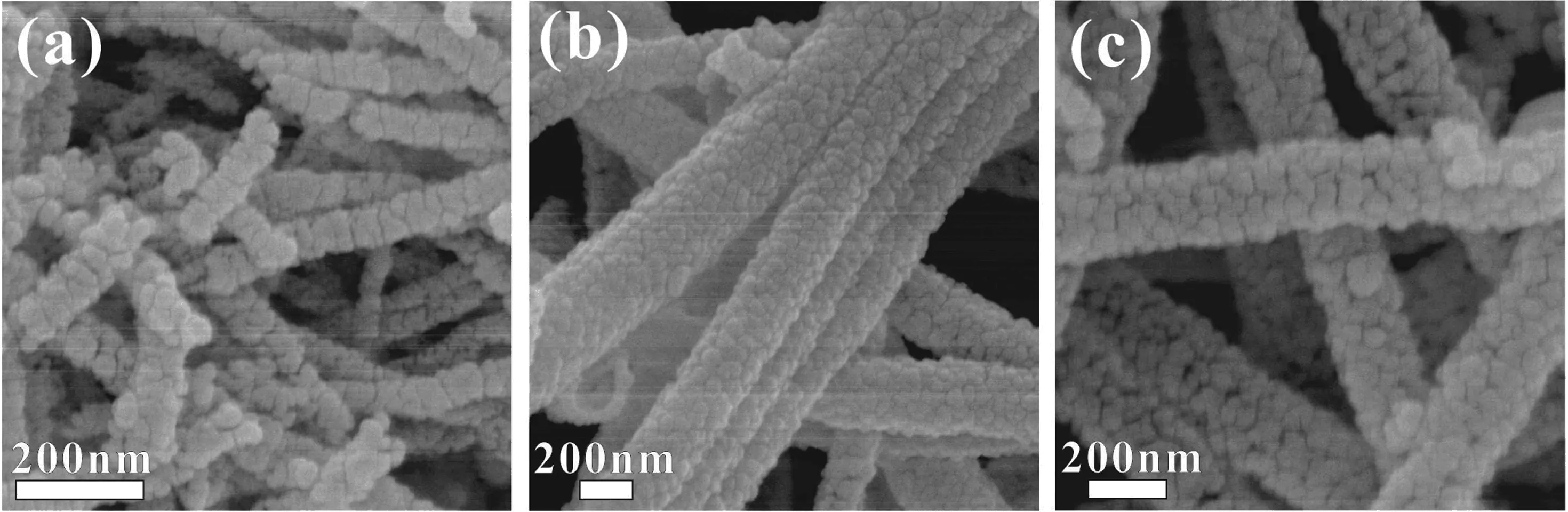
FIG.3 SEM photographs of the as-prepared samples of(a)SCo-0,(b)SCo-1,(c)SCo-2.
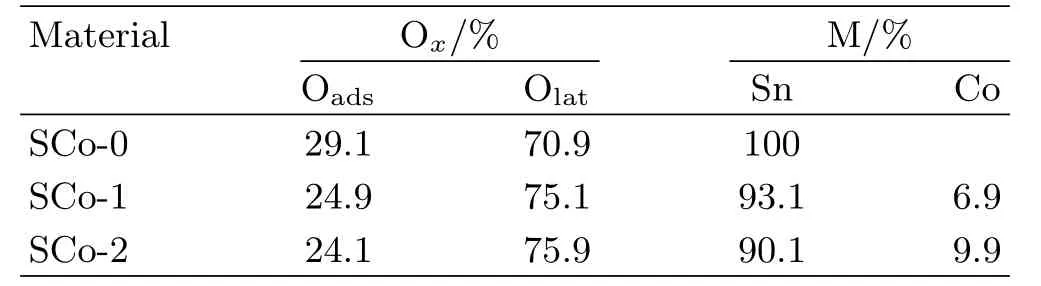
TABLE I Compositions determined from XPS for pristine and Co3O4modi fied SnO2.
FIG.5(a)presents the response of the as-prepared samples to 100 ppm ethanol at different temperatures. For all the samples,the response first increased with temperature,reached a maximum at 300◦C,and then decreased.The response increased signi ficantly with the Co3O4loading.A response of 38.0 was obtained at 300◦C for SCo-2,6.7 times higher than that of SCo-0. Table II shows that the ethanol response of SCo-2 nano fibers is higher than that of some other SnO2-based sensors[17−19].As shown in FIG.5(b),the gas response of the sensors varied linearly with the ethanol concentration on a log-log scale,indicating a power-law type relationship.The distinctly larger slope observed for the SCo-2 sensor suggests a more pronounced enhancement of the response at higher ethanol concentra-tions.Assuming a value of 1.2 as the lowest response [20],the detection limit was estimated by extrapolating the regressed linear line in FIG.5(b)to be 2.3 and 1.5 ppm for SCo-0 and SCo-2,respectively.Further investigation of gas-sensing performance was focused on SCo-2 due to its higher response and lower detection limit.

FIG.4 TEM pictures of the as-prepared samples of(a)SCo-0,(b)SCo-1,(c)SCo-2.
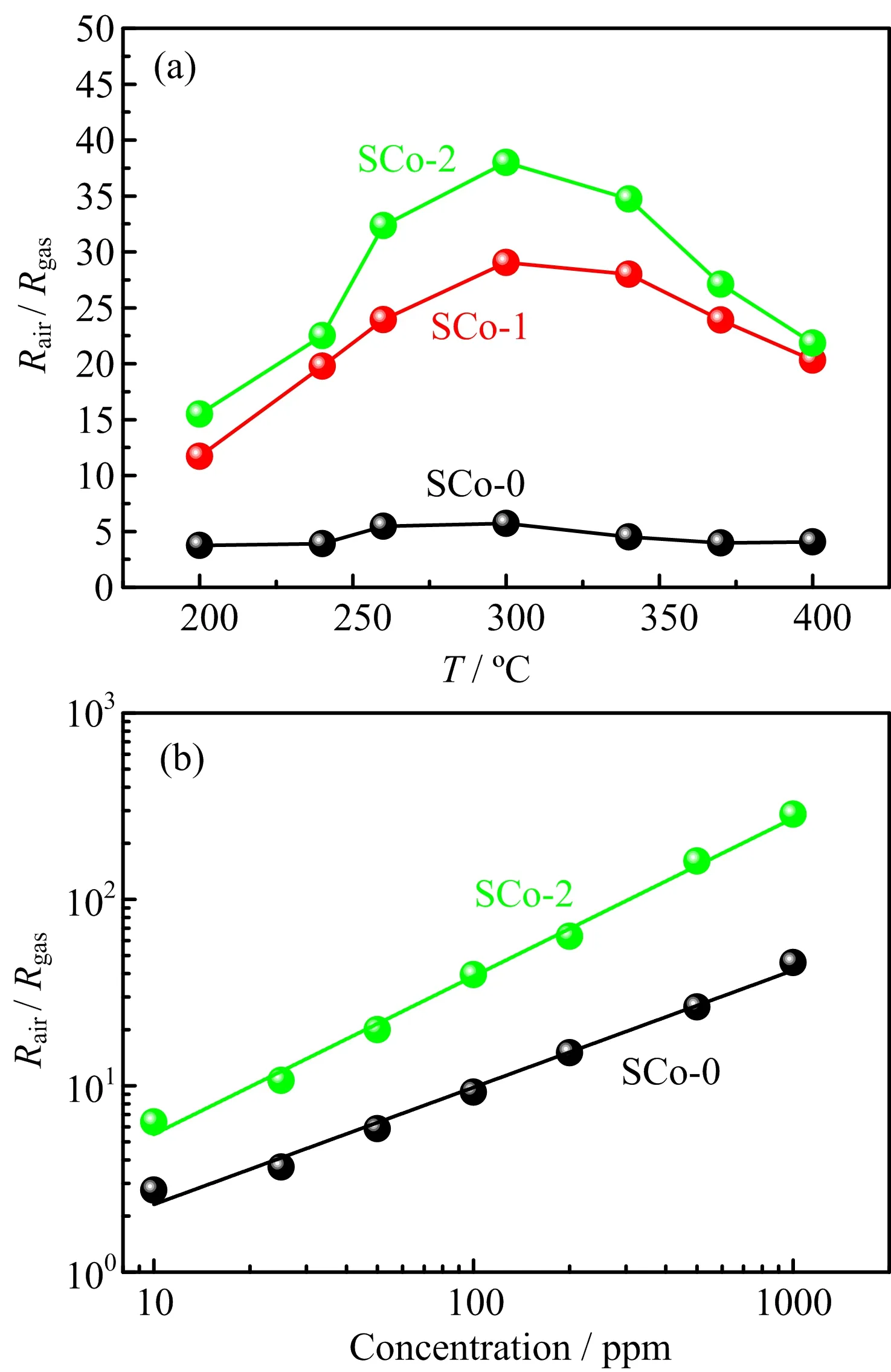
FIG.5(a)Response of as-prepared samples to 100 ppm ethanol gas at different temperatures.(b)Log-log plot for the dependence of response on the ethanol concentration.
FIG.6 presents the continuous response curves of SCo-0 and SCo-2 nano fibers to 100 ppm ethanol gas at 300◦C.It can be seen that the dynamic responserecovery features for both samples were well repeated, and the sensor response could restore to the initial base line after each cycle.The response and recovery time for SCo-2 were found to be 5 and 23 s,respectively,which were remarkably reduced compared with the respectivevalues(10 and 62 s)for SCo-0.
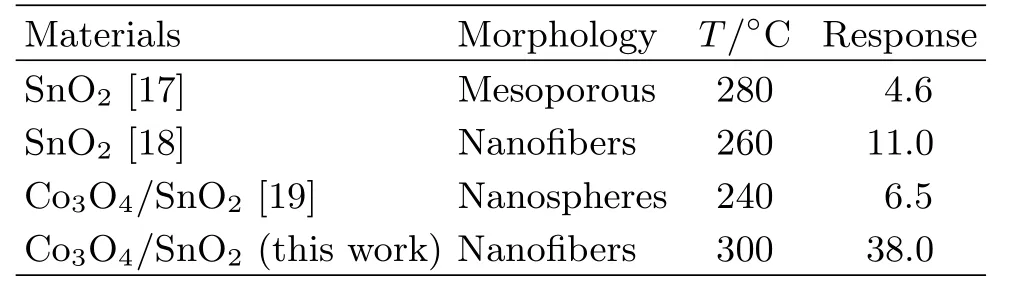
TABLE II The 100 ppm ethanol gas sensing properties of SnO2-based ethanol sensors.
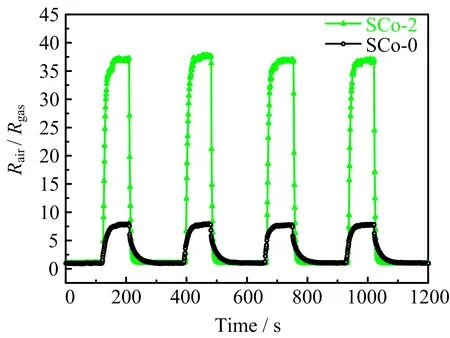
FIG.6 Dynamic response curves of SCo-0 and SCo-2 to 100 ppm ethanol at 300◦C.
The cross sensitivity of SCo-2 nano fibers was examined in the temperature range of 200−400◦C(FIG.7). The sensor exhibited negligible response to methane, propane,and carbon monoxide,and minor response to hydrogen at temperatures above 300◦C.The response to ethanol was over 28 times higher than that to the interferent gases,which clearly demonstrated excellent ethanol selectivity for SCo-2.
It is widely accepted that the gas sensing mechanism of metal oxide semiconductor is based on the adsorption and desorption of gases on the surface of materials [21].In air atmosphere,oxygen molecules are adsorbed on the material surface,and form O2−,O−and O2−ions by capturing electrons from the material.Then an electron depletion layer and a potential barrier are formed on the surface,leading to increase of resistance. When ethanol is present in the atmosphere,adsorbed oxygen species will react with ethanol and the capturedelectrons will be released back to the material.As a result,the electron depletion layer becomes thinner and the resistance decreases.
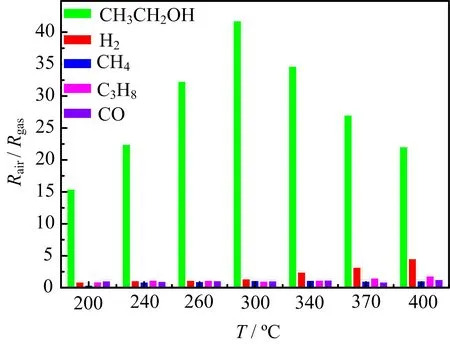
FIG.7 Response of SCo-2 to 100 ppm various gases at different temperatures.
Generally speaking,better surface oxygen adsorption,smaller grain size,and formation of p-n heterojunction are bene ficial to achieving higher gas sensing performance[7,22].The present work showed that Co3O4/SnO2nano fibers were associated with depressed surface oxygen adsorption(Table I)and larger grain size,which may deteriorate the gas sensing properties. Therefore,the remarkable enhancement of ethanol response for SnO2nano fibers by Co3O4decoration may mainly result from formation of p-n heterojunctions between the two oxides,which changes the surface potential barrier and makes the material more sensitive[20].
IV.CONCLUSION
SnO2and Co3O4/SnO2nano fibers were synthesized via electrospinning and impregnation.When compared with pristine SnO2nano fibers,Co3O4/SnO2exhibited greatly enhanced response to ethanol and signi ficantly reduced response/recovery time.A response of 38.0 to 100 ppm ethanol gas and a response/recovery time of 5 and 23 s was observed for SCo-2 at 300◦C.Furthermore,excellent ethanol selectivity against interference of methane,propane,hydrogen,and carbon monoxide was also observed for SCo-2.The improved ethanol sensing performance may be ascribed to formation of p-n heterojunctions between SnO2and Co3O4.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.U1432108)and the Fundamental Research Funds for the Central Universities(No.WK2320000034).
[1]H.L.Zhang,C.G.Hu,X.S.He,L.Hong,G.J.Du, and Y.Zhang,J.Power Sources 196,4499(2011).
[2]Q.R.Zhao,Y.Xie,T.Dong,and Z.G.Zhang,J.Phys. Chem.111,11598(2007).
[3]J.P.Liu,Y.Y.Li,X.T.Huang,R.M.Ding,Y.Y.Hu, J.Jiang,and L.Liao,J.Mater.Chem.19,1859(2009).
[4]Y.G.Zheng,J.Wang,and P.J.Yao,Sens.Actuators B 156,723(2011).
[5]H.M.Jeong,J.H.Kim,S.Y.Jeong,C.H.Kwak,and J.H.Lee,ACS Appl.Mater.Interfaces 8,7877(2016).
[6]R.J.Wu,J.G.Wu,M.R.Yu,T.K.Tsai,and C.T. Yeh,Sens.Actuators B 131,306(2008).
[7]Z.Li and J.X.Yi,Sens.Actuators B 243,96(2017).
[8]L.Liu,Y.Zhang,G.G.Wang,S.C.Li,L.Y.Wang, Y.Han,X.X.Jiang,and A.G.Wei,Sens.Actuators B 160,448(2011).
[9]Y.Zhao,X.L.He,J.P.Li,X.G.Gao,and J.Jia,Sens. Actuators B 165,82(2012).
[10]H.Y.Du,J.Wang,M.Y.Su,P.J.Yao,Y.G.Zheng, and N.S.Yu,Sens.Actuators B 166,746(2012).
[11]L.L.Wang,J.N.Deng,Z.Lou,and T.Zhang,Sens. Actuators B 201,1(2014).
[12]J.W.Yoon,J.K.Choi,and J.H.Lee,Sens.Actuators B 161,570(2012).
[13]H.X.Guo,J.H.Chen,W.Weng,Z.S.Zheng,and D. F.Wang,J.Ind.Eng.Chem.20,3081(2014).
[14]W.Wang,Z.Y.Li,W.Zheng,H.Huang,C.Wang,and J.H.Sun,Sens.Actuators B 143,754(2010).
[15]Y.H.Choi,and S.H.Hong,Sens.Actuators B 125, 504(2007).
[16]S.T.Navale,C.S.T.Liu,P.S.Gaikar,V.B.Patil, R.U.R.Sagar,B.Du,R.S.Mane,and F.J.Stadler, Sens.Actuators B 245,524(2017).
[17]S.Liu,Y.Zhang,B.Yu,Z.Y.Wang,H.R.Zhao, N.Zhou,and T.Zhang,Sens.Actuators B 210,700 (2015).
[18]J.Cao,T.Zhang,F.Li,H.Yang,and S.Liu,New J. Chem.37,2031(2013).
[19]L.L.Wang,Z.Lou,R.Zhang,T.T.Zhou,J.N.Deng, and T.Zhang,ACS Appl.Mater.Interfaces 8,6539 (2016).
[20]C.W.Na,H.S.Woo,I.D.Kim,and J.H.Lee,Chem. Commun.47,5148(2011).
[21]M.Batzil and U.Diebold,Prog.Surf.Sci.79,47(2005).
[22]D.R.Miller,S.A.Akbar,and P.A.Morris,Sens.Actuators B 204,250(2014).
ceived on April 24,2017;Accepted on May 22,2017)
∗Author to whom correspondence should be addressed.E-mail: yjx@ustc.edu.cn,Tel:+86-551-63607817
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- γ-Ray Irradiation-Derived MnO/rGO Composites for High Performance Lithium Ion Batteries
- Intermolecular Interactions in Self-Assembly Process of Sodium Dodecyl Sulfate by Vertically Polarized Raman Spectra
- Identi fication of Superoxide O2−during Thermal Decomposition of Molten KNO3-NaNO2-NaNO3Salt by Electron Paramagnetic Resonance and UV-Vis Absorption Spectroscopy
- Binding Mechanism and Molecular Design of Benzimidazole/Benzothiazole Derivatives as Potent Abl T315I Mutant Inhibitors
- Geometric Design of Anode-Supported Micro-Tubular Solid Oxide Fuel Cells by Multiphysics Simulations
- Laser-Assisted Stark Deceleration of Polar Molecules HC2n+1N(n=2,3,4) in High-Field-Seeking State
