Chemoselective Transfer Hydrogenation of Cinnamaldehyde over Activated Charcoal Supported Pt/Fe3O4Catalyst
2017-09-03YongZhngChunChenWnbingGongJieyoSongYnpingSuHiminZhngGuozhongWngHuijunZho
Yong Zhng,Chun Chen,Wn-bing Gong,Jie-yo Song,Yn-ping Su,Hi-min Zhng, Guo-zhong Wng,Hui-jun Zho,c∗
a.Key Laboratory of Materials Physics,Centre for Environmental and Energy Nanomaterials,Institute of Solid State Physics,Chinese Academy of Sciences,Hefei 230031,China
b.Science Island Branch of Graduate School,University of Science and Technology of China,Hefei 230026,China
c.Centre for Clean Environment and Energy,Griffith University,Gold Coast Campus,Queensland 4222, Australia
Chemoselective Transfer Hydrogenation of Cinnamaldehyde over Activated Charcoal Supported Pt/Fe3O4Catalyst
Yong Zhanga,b,Chun Chena∗,Wan-bing Gonga,b,Jie-yao Songa,b,Yan-ping Sua,b,Hai-min Zhanga, Guo-zhong Wanga,Hui-jun Zhaoa,c∗
a.Key Laboratory of Materials Physics,Centre for Environmental and Energy Nanomaterials,Institute of Solid State Physics,Chinese Academy of Sciences,Hefei 230031,China
b.Science Island Branch of Graduate School,University of Science and Technology of China,Hefei 230026,China
c.Centre for Clean Environment and Energy,Griffith University,Gold Coast Campus,Queensland 4222, Australia
A variety of spherical and structured activated charcoal supported Pt/Fe3O4composites with an average particle size of~100 nm have been synthesized by a self-assembly method using the difference of reduction potential between Pt(IV)and Fe(II)precursors as driving force.The formed Fe3O4nanoparticles(NPs)effectively prevent the aggregation of Pt nanocrystallites and promote the dispersion of Pt NPs on the surface of catalyst,which will be favorable for the exposure of Pt active sites for high-efficient adsorption and contact of substrate and hydrogen donor.The electron-enrichment state of Pt NPs donated by Fe3O4nanocrystallites is corroborated by XPS measurement,which is responsible for promoting and activating the terminal C=O bond of adsorbed substrate via a vertical con figuration.The experimental results show that the activated charcoal supported Pt/Fe3O4catalyst exhibits 94.8%selectivity towards cinnamyl alcohol by the transfer hydrogenation of cinnamaldehyde with Pt loading of 2.46%under the optimum conditions of 120◦C for 6 h,and 2-propanol as a hydrogen donor.Additionally,the present study demonstrates that a high-efficient and recyclable catalyst can be rapidly separated from the mixture due to its natural magnetism upon the application of magnetic field.
Activated charcoal supported Pt/Fe3O4catalysts,Redox method,Transfer hydrogenation Cinnamaldehyde,Cinnamyl alcohol
I.INTRODUCTION
The chemoselective transformation of biomass-based unsaturated aldehyde has been recognized as a potentially promising candidate to bridge a gap between biomass resources and bio-chemicals[1,2].The products of cinnamaldehyde hydrogenation(e.g.hydrocinnamaldehyde,cinnamyl alcohol,hydrocinnamyl alcohol)can serve as signi ficant feed stocks in pharmaceuticals,perfumes,cosmetics,and fine chemicals industries[3–5].However,the chemoselective hydrogenation of C=O bond is more challenging because the hydrogenation of the C=C bond is thermodynamically more favorable than that of the C=O group.These byproducts formed by the hydrogenation of C=C bond will require a sophisticated puri fication procedure,which is labor-intensive and cost-expensive[6–8].Consequently, many endeavors have been devoted to improving the selectivity toward cinnamyl alcohol over the past decades. Indeed,the selectivity towards the unsaturated alcohol has been improved by the presence of some external modi fiers[9–11](e.g.,surface organic ligands or stabilizers,ion electron donating,alkali metal compounds)in the homogeneous and/or heterogeneous catalysis.However,the reaction mechanism will become more complex in the above-mentioned case.The activity and selectivity of the catalysts are strongly correlated with their properties such as geometric[12–14](e.g.,particle size,morphology,crystal phase)and electronic effects[15,16](e.g.,electron-de ficient or electron-rich). In addition,some of the additives are environmentally harmful.Therefore,it is imperative to explore facile methods for the synthesis of catalyst with comprehensive application.
In comparison to conventional hydrogenation reactions by the aid of external H2,transfer hydrogenation reaction is advantageous because of its safety,economy, and handy of operation.Moreover,transfer hydrogenation usually demostrates speci fic seletivity to target pro ducts.Thus,it is reasonable to be used in the reductionof various unsaturated compounds because the high activity and selectivity is independent of H2pressure[17]. The most popular hydrogen donor such as 2-propanol and formic acid/formate salt have received much more attentions[18–20]. For formic acid/formate salt,it is mostly utilized upon the homogeneous catalysts, such as Ir,Ru or Fe organometallic pincer complexes [19,21,22].However,the separation and reusability of catalysts are the major concerns which are needed to be addressed.Additionally,the analysis of products is difficult as gas-chromatography is sensitive to minimal salts.In contrast to formic acid/formate salt, 2-propanol is commonly employed in the heterogeneous catalysis system.These hydrogenation reactions have been carried out with various supported catalysts,including active graphite carbon nitride,metal oxides,zeolites,and hydrotalcite[18,23–25].The catalytic activity and selectivity in the transfer hydrogenation of acetophenone can be adjusted by changing calcination temperature of alumnosilicate.The 94%yield toward phenethyl alcohol is achieved over nickel aluminosilicate nanocomposite calcined at 800◦C under the mild conditions of 90◦C for 3 h[26].The Pt/TiO2catalyst, which is prepared by impregnation method and further reduced under flowing H2at 773 K,shows the excellent catalytic performance with 91%yield of phenethyl alcohol under the conditions of 76◦C for 2 h.Nevertheless, the yield is decreased to 3%after five successive recycles because of the formation of CO and carbonaceous[27]. Meanwhile,the employment of KOH is mandatory in these catalytic systems.It has also been reported that the catalytic activity of transfer hydrogenation of acetophenone is relatively poor in the absence of alkaline promoters[27].However,the alkaline medium will lead to irreversible corrosion of the reaction device,and the residual alkaline needs to be neutralized by inorganic acid at the end of reaction.Thus,it is necessary to develop and design a rational catalyst,which can be synthesized by a facile method and be applied in a simple and clean system of transfer hydrogenation without any alkaline additives.
Herein,a variety of charcoal supported Pt/Fe3O4composites are prepared by a flexible redox method using the different reduction potential between Pt and Fe precursors.The electronic structure of this composite is investigated by XPS technique.The activity test shows that a high chemoselectivity towards cinnamyl alcohol with 95%in the transfer hydrogenation of cinnamaldehyde can be obtained.Besides,this composite can be rapidly separated from the reaction mixture by using its inherent magnetism upon the application of magnetic field.
II.EXPERIMENTS
A.Materials
H2PtCl6·6H2O(AR,≥37.5%Pt)and cinnamaldehyde(GC,≥95%)are purchased from Aladdin Chemical Reagent Co. Ltd. Analytical grade methanol, propanol,2-propanol,2-butanol,pentanol,n-octanol, potassium chloride(KCl),ferrous sulfate heptahydrate (FeSO4·7H2O),and sodium hydroxide(NaOH)are purchased from Sinopharm Chemical Reagent Co.Ltd.All the reagents are used directly without further any puri fication treatment.Deionized water(18 MΩ·cm)is produced by a Milli-Q(Millipore,USA).
B.Catalyst preparation
Here,the activated charcoal has been firstly washed by diluted acid and deionized water for several times. Then it is dried in an oven at 60◦C for overnight.Finally,it is crushed into powder with a size of 20 mesh for further treatment.A series of activated charcoal supported Pt/Fe3O4catalysts have been prepared by a flexible redox method using different reduction potential between metal and support precursor.In a typical procedure,the calculated NaOH aqueous solution is added into FeSO4·7H2O aqueous solution under N2protection, followed by the addition of H2PtCl6·6H2O aqueous solution.Then the solution is heated at 70◦C for 1.8 h, and the activated charcoal powder is then added into the mixture.After treated at 70◦C for another 2 h, the sample is rapidly separated from the slurry by a rectangle magnet.It is washed with deionization water and ethanol for several times,respectively,and then dried at 60◦C overnight in a vacuum oven.The asobtained catalyst are denoted as xPt/Fe3O4-AC,where x represents Pt mass loading(0.83−4.81 wt%).
C.Catalyst characterization
Transmission electron microscopy(TEM)and high resolution transmission electron microscopy(HRTEM) images are recorded on a JEOL-2010 EX instrument operating at 200 kV.The scanning transmission electron microscopy(STEM)images and energy dispersive X-ray spectra(EDS)data are collected on a FEI Titan STEM instrument with a high-angle annular darkfield(HAADF)detector and an EDAX SiLi detector operated at 200 kV,respectively.The crystal phase structure of sample is analyzed by powder X-ray diffraction(PXRD)equipped with Cu Kα(λ=0.154 nm)radiation operating at 40 kV and 40 mA for 2θ angles ranging from 10◦to 75◦.X-ray photoelectron spectra(XPS)measurement is recorded on an ESCALAB 250 photoelectron spectrometer(Thermo-VG Scienti fic Co.,LTD)with Al Kα X-ray radiation as the X-ray source for excitation.The bonding energy of C 1s peak (284.8 eV)is referenced as a calibration.
D.Catalytic activity test
Typically,60 mg of cinnamaldehyde(CAL),30 mg of catalyst,and 8 mL of solvent(hydrogen transferdonor)have been mixed and sealed in a 25-mL Te flonlined steel autoclave vessel.The mixture is heated to a targeted temperature under a flow of N2for several hours.After reaction,the liquid products have been identi fied by gas chromatography mass spectrometry (GC-MS,Thermo Fisher Scienti fic-TXQ),and quantitatively analyzed by GC(Shimadzu,GC-2010 Plus) using n-octanol as an internal standard.As outlined in Scheme 1,the transferred hydrogen atoms can attack the C=C or C=O bond of CAL to form hydrocinnamaldehyde(HCAL)or cinnamyl alcohol(COL),respectively. Hydrocinnamyl alcohol(HCOL)can be formed by the deep hydrogenation of both C=O and C=C bonds.In some case,1-cinnamyl-2-propyl ether (CPE)can also be obtained from etheri fication of formed COL with 2-propanol. The other possible byproducts(e.g.,methylstyrene,phenylpropane)are not detected in this work.
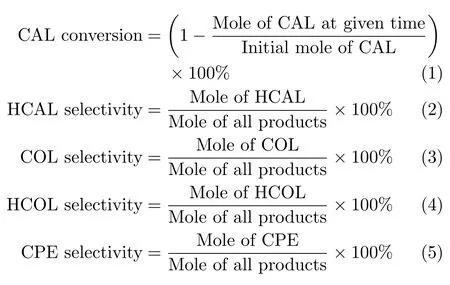
The carbon balance has been checked in every run and it is found to be higher than 90%.Conversion of CAL and selectivities of products are calculated using the following equation:
III.RESULTS AND DISCUSSION
A.Characterization of catalyst
FIG.1 displays the TEM images of 2.46 wt%Pt/ Fe3O4-AC sample.As can be seen,it presents welldispersed sphere-shape hybrids with an average diameter of~100 nm.The composite is formed by the selfassembly between Pt and Fe3O4nanoparticles(NPs) via the driving force of different reduction potential, where the Pt NPs may be entrapped into the matrix of Fe3O4NPs.To more clearly recognize the distribution of element component between Pt and Fe3O4NPs, the HADDF-STEM and corresponding EDX mapping have also been investigated.The interplanar spacing of 0.22 nm is matched well with the(111)crystal plane of Pt NPs on the surface of Fe3O4NPs,as indicated in inserted HRTEM image of FIG.1(c)and marked by black arrows in FIG.2(a).The uniform distribution of Pt NPs is further con firmed by the corresponding EDX mapping in FIG.2(b)−(d).
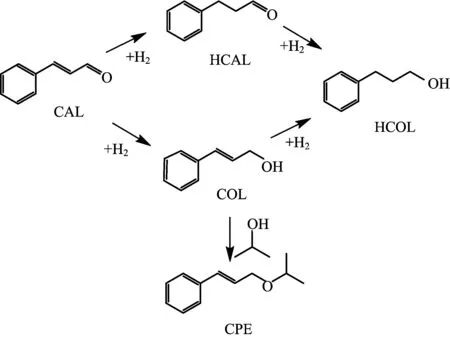
Scheme 1 Hydrogen transfer reaction network of CAL.
The PXRD patterns of Pt/Fe3O4-AC catalysts are shown in FIG.3. For all the samples,main shape diffraction patterns positioned at 2θ of~30.1◦,35.5◦, and 62.6◦can be indexed to the characteristic peaks of cubic Fe3O4phase(JCPDS 88-0866).A board scattering pattern located at 2θ of~23◦can be attributed to the peak of amorphous activated charcoal.At low Pt content,like the samples of 0.83 wt%Pt/Fe3O4-AC and 1.26 wt%Pt/Fe3O4-AC,no obvious diffraction peak of Pt can be observed due to the low Pt loading.As Pt loading increasing to 2.46 wt%and 4.81 wt%(2.46 wt%Pt/Fe3O4-AC and 4.81 wt%Pt/Fe3O4-AC),a very faint broad peak of cubic Pt(111)located at 2θ of~40◦has been detected[28].It is indicated that the complete redox reaction between Pt(IV)and Fe(II)precursors to accelerate the formation of Pt nanocrystallites.Moreover,the aggregation of Pt nanocrystallites can be effectively prevented by the separation and dispersion of Fe3O4NPs.This result is in agreement with the result of HADDF-STEM image,as displayed in FIG.2.
The surface chemical environment of Pt/Fe3O4-AC sample is also investigated by XPS measurement.As shown in FIG.4(a),two obvious satellite peaks positioned at 71.5 and 74.8 eV are attributed to the characteristic peaks of Pt 4f7/2and Pt 4f5/2,respectively. It indicates the existence of metallic Pt in the sample, which is derived from the redox reaction of Pt(IV)and Fe(II)precursors during synthesis process.In comparison with previous supported Pt catalyst,the value is slightly lower by~0.6 eV[29].The peak of metallic Pt shifting to lower level may be caused by electron donating effect from Fe3O4NPs due to its variable valence between Fe2+and Fe3+.This phenomenon can also be affirmed by the shift of Fe3O4peaks.In the Fe 2p XPS spectrum exhibited in FIG.4(b),a couple peaks of Fe 2p3/2and Fe 2p1/2with binding energy of 711.7 and 725.2 eV,are higher by~0.4 eV than that of pure of Fe3O4NPs prepared by solvothermalmethod[30].This intimate electron interaction between Pt and Fe3O4would induce the activation of terminal C=O bond of CAL,leading to high selectivity toward COL[3].

FIG.1 TEM with different magni fication and inserted HRTEM images of 2.46 wt%Pt/Fe3O4-AC catalyst.
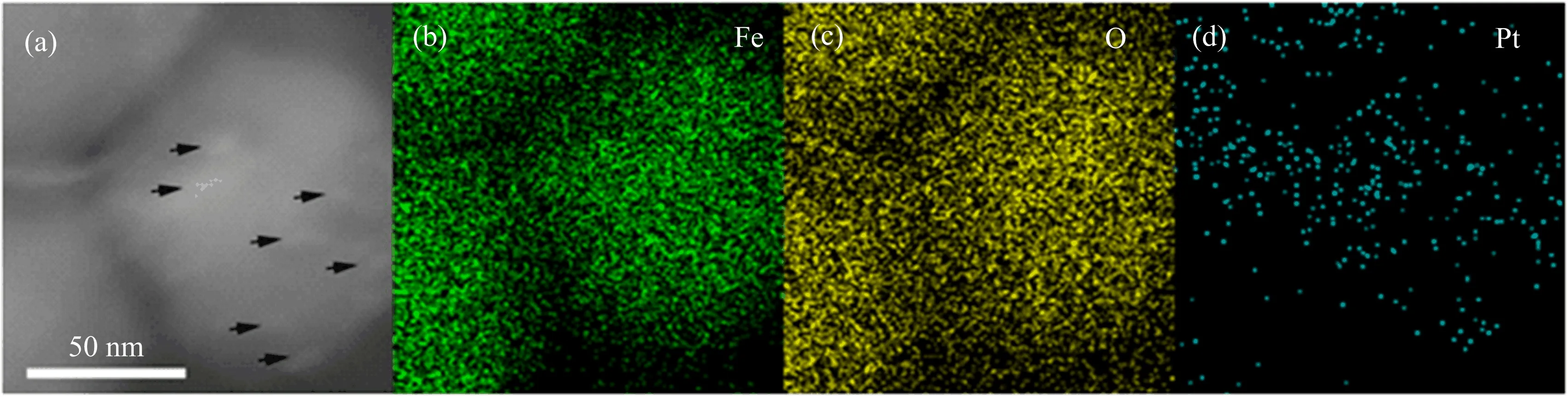
FIG.2(a)HADDF-STEM image and EDX mapping of 2.46 wt%Pt/Fe3O4-AC catalyst((b)Fe,(c)O,(d)Pt).
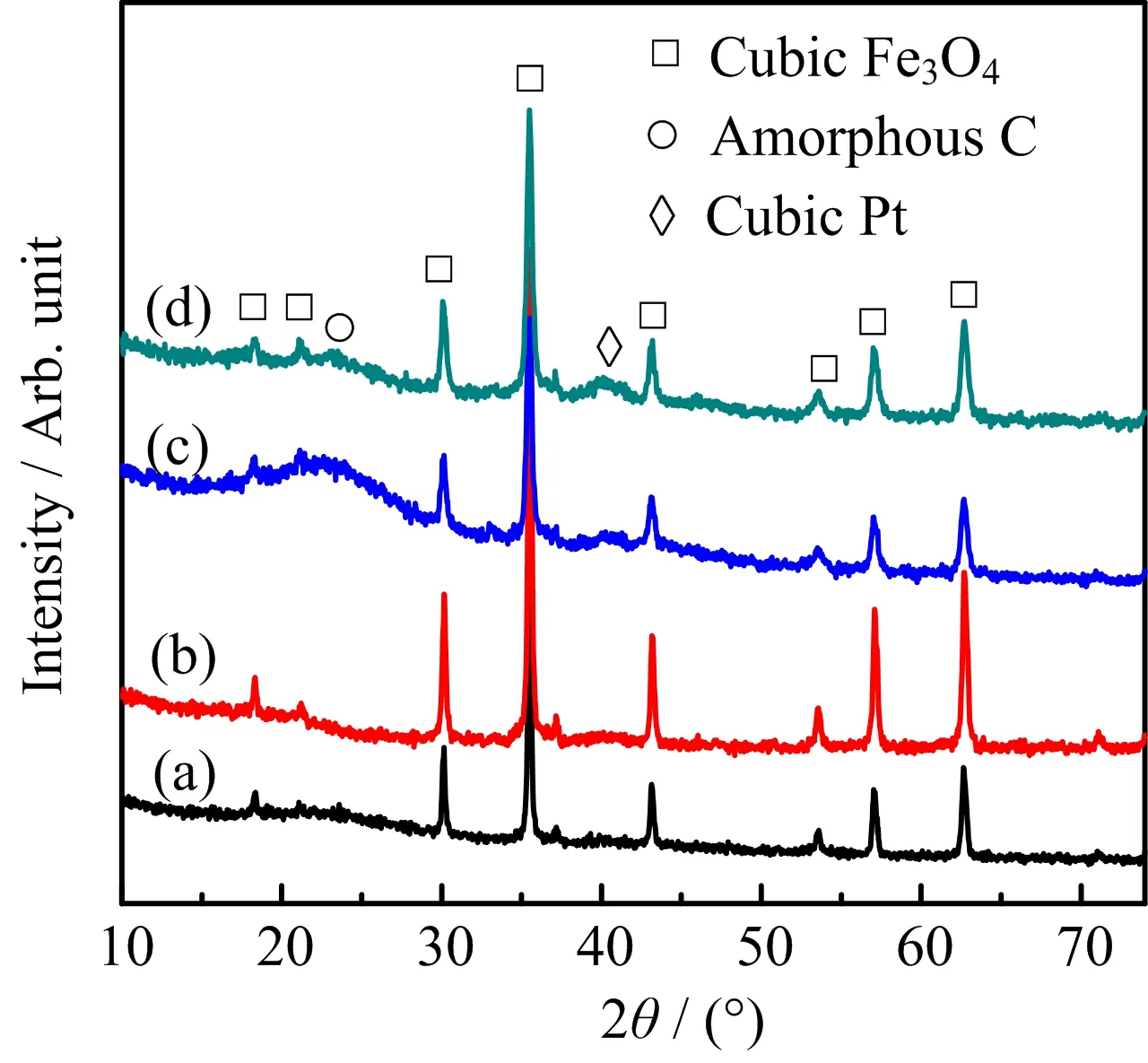
FIG.3 PXRD patternsofPt/Fe3O4-AC catalysts. (a)0.83 wt%Pt/Fe3O4-AC,(b)1.24wt%Pt/Fe3O4-AC, (c)2.46 wt%Pt/Fe3O4-AC,and(d)4.81 wt%Pt/Fe3O4-AC.
B.Catalytic evaluation
The screening of Pt/Fe3O4-AC catalysts has been performed for the transfer hydrogenation of CAL under the conditions of 120◦C for 6 h.The CAL conversion and products selectivity as the function of Pt loading have been investigated and the result is displayed in FIG.5.It is obviously found that the CAL conversion gradually increases from 15.1%to 48.2%with the increase of Pt loading from 0.83 wt%to 2.46 wt%.Theenhanced CAL conversion can be ascribed to more available Pt active sites on the surface of catalyst.Meanwhile,the selectivity for the desired product of COL is maintained higher than 95%for all the samples of 0.83 wt%Pt/Fe3O4-AC,1.24 wt%Pt/Fe3O4-AC,and 2.46 wt%Pt/Fe3O4-AC.Although the CAL conversion is raised up to 61.3%when Pt loading of Pt/Fe3O4-AC catalyst increased to 4.81 wt%(4.81 wt%Pt/Fe3O4-AC),the selectivity of COL reduces to 82.3%following with the increase of HCAL selectivity.It suggests that the hydrogenation of C=C bond is induced andtriggered over the Pt/Fe3O4-AC catalyst with high Pt loading,leading to decrease of COL selectivity.Thus, the 2.46 wt%Pt/Fe3O4-AC sample is selected as the optimal catalyst for the further investigation.
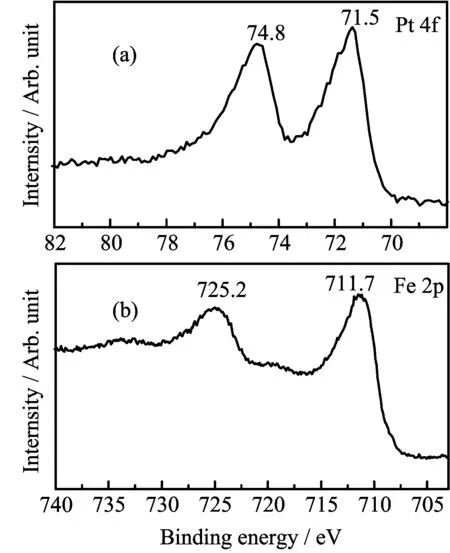
FIG.4XPS spectra of(a)Pt 4f and(b)Fe 2p over 2.46 wt%Pt/Fe3O4-AC catalyst.
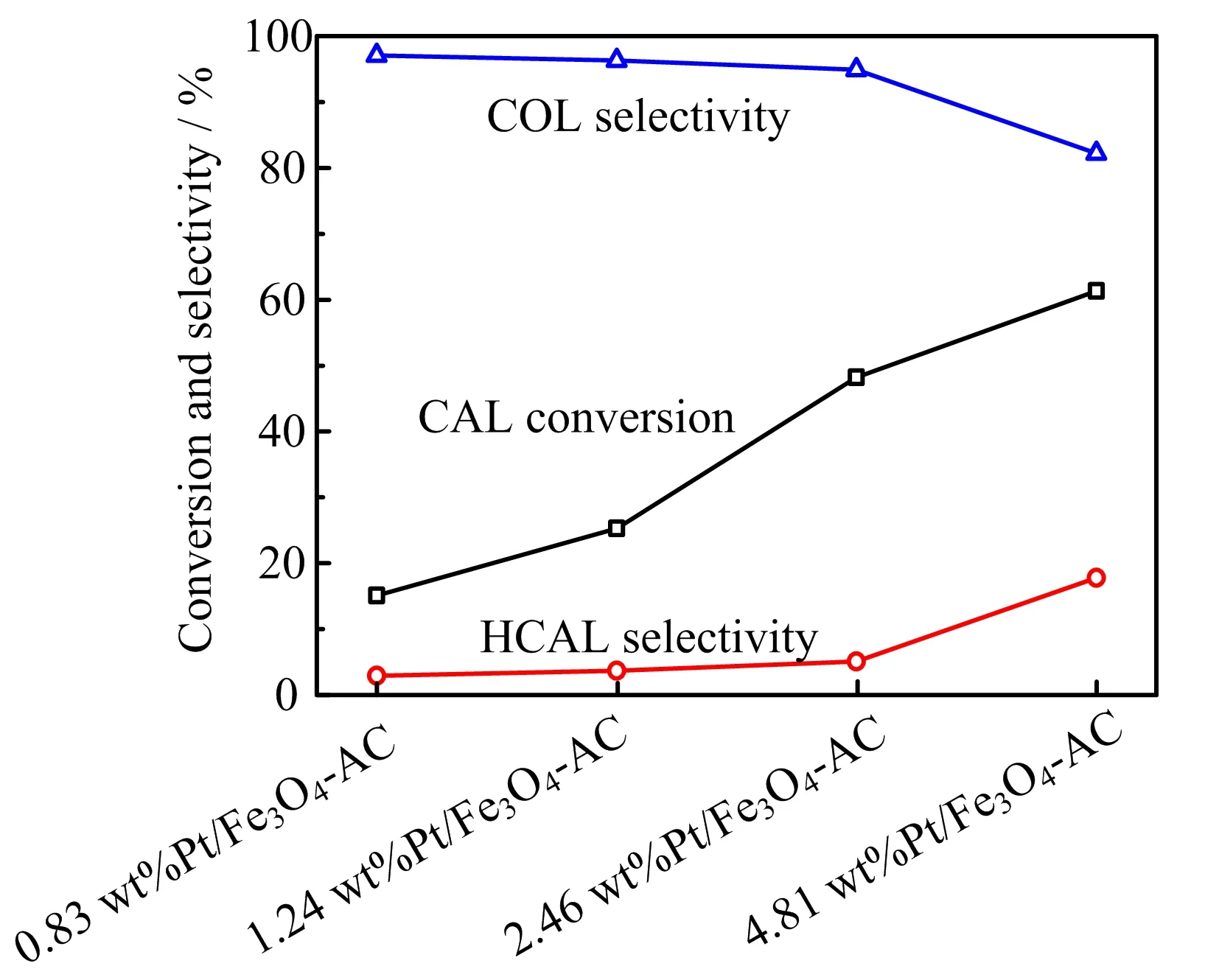
FIG.5 Catalytic performance of transfer hydrogenation of CAL over Pt/Fe3O4-AC catalysts. Reaction conditions: mass ratio of catalyst to CAL=0.5,120◦C,6 h,8 mL 2-propanol as hydrogen donor.
Generally,the catalytic performance of heterogeneous catalyst highly depends on the experimental reaction conditions,such as reaction time and temperature.Therefore,the in fluence of reaction conditions on CAL conversion and products selectivity over 2.46 wt%Pt/Fe3O4-AC catalyst has been investigated and the results are summarized in Table I.As can be seen,the transfer hydrogenation of CAL does not take place efficiently at low reaction temperature of 90◦C due to a quite low reaction rate.Although the conversion of CAL is only 22.6%after 18 h,CAL can convert into COL with almost 100%selectivity(entry 4,Table I).With the reaction temperature elevating from 90◦C to 180◦C,the reaction rate of transfer hydrogenation increases signi ficantly and 92.1%of CAL conversion can be obtained at 180◦C after 18 h(entry 16,Table I). However,the reaction rate for side reactions,like hydrogenation of C=C bonds,also increases signi ficantly giving rise to the obvious change in the selectivity of products.The selectivity for the desired product of COL reduces to 17.9%at 180◦C after 18 h reaction.It can be deduced that the very high activity of transfer hydrogenation over the Pt/Fe3O4-AC catalyst causes the hydrogenation of C=C and C=O bonds simultaneously.
In addition,the hydrogen donors and mass ratio of catalyst to substrate can also in fluence catalytic activity and selectivity in the transfer hydrogenation of CAL over Pt/Fe3O4-AC catalyst.The electronic properties of the hydrogen donor may play an important role in transfer hydrogenation reactions because the product formation strongly depends on the adsorption and activation of hydrogen donor on the surface of catalyst.Generally,the aliphatic secondary alcohol has agood reducibility because the corresponding alkyl diketone has a high reduction potential[31].For example, Di Cosimo reported that aliphatic 2-propanol is better than propanol for the gas-phase transfer hydrogenation of mesityl oxide on MgO catalyst[32].
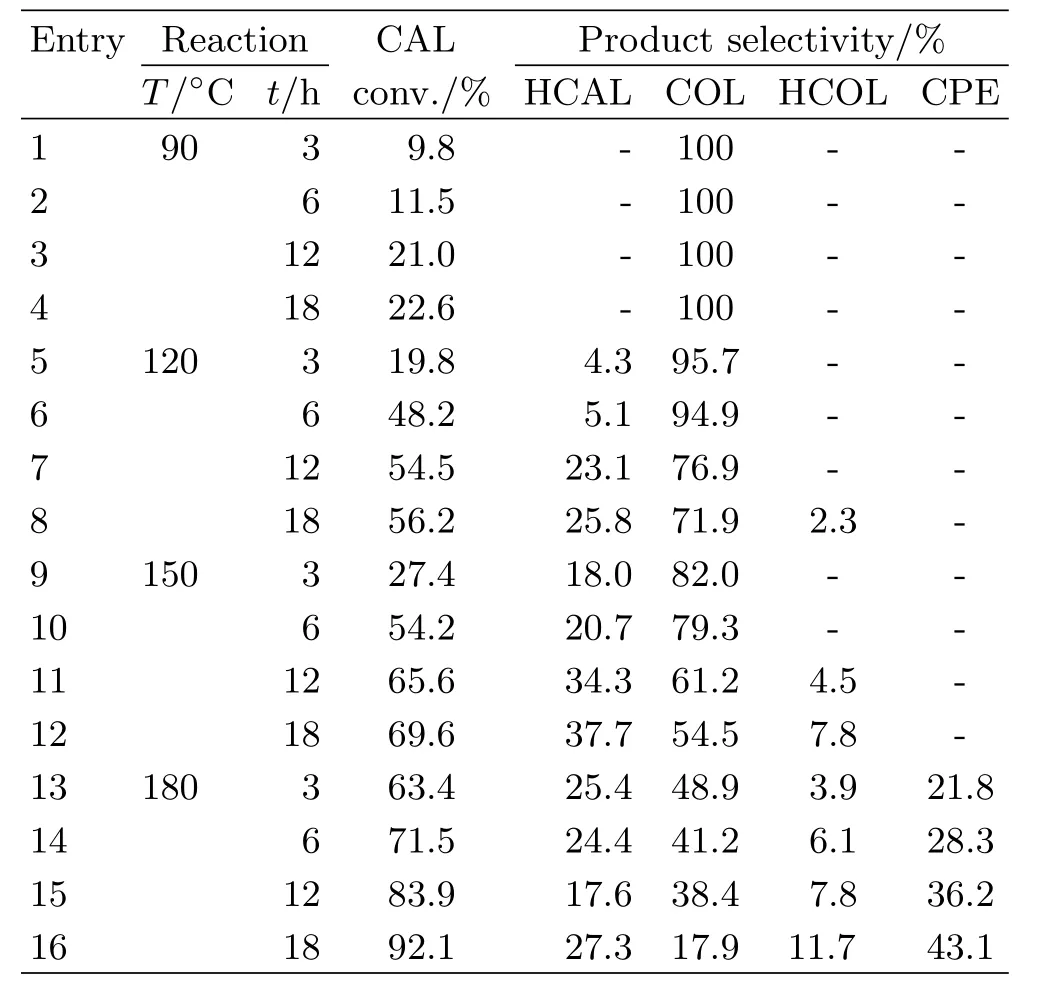
TABLE I Effect of reaction temperature and time on CAL conversion and COL selectivity over 2.46 wt%Pt/Fe3O4-AC catalyst. Reaction conditions:mass ratio of catalyst to CAL=0.5,8 mL 2-propanol as hydrogen donor.
Table II summarizes the catalytic performance of 2.46 wt%Pt/Fe3O4-AC catalyst with different aliphatic alcohols as hydrogen donors.As can be seen,the use of methanol results in a poor CAL conversion(0.9%) and HCAL is the sole product(entry 1,Table II).This may be put down to the feeble capability of transferring hydrogen due to the extremely strong binding effect of hydroxyl group.With the growth of carbon chain, the binding effect of hydroxyl group is weakened,giving rise to an improved CAL conversion(entry 2 and entry 3,Table II).However,the byproduct of ether becomes main product resulting in a sharp decrease of COL selectivity when using pentanol as hydrogen donor (entry 3,Table II).For the secondary alcohol,it shows an obvious improvement on CAL conversion and COL selectivity comparing with primary alcohols.Interestingly,2-propanol shows a comparable catalytic activity and selectivity in comparison with 2-butanol(entry 4 and entry 5,Table II).It may be attributed to similar reduction potential between the methyl ethyl ketone (productof 2-butanol oxidation)and acetone(product of 2-propanoloxidation)[31].However,2-propanol is safer,cheaper and more environment-benign than 2-butanol.Therefore,2-propanol is selected as hydrogen donor for studying the effect of mass ratio of catalyst to substrate on catalytic performance.
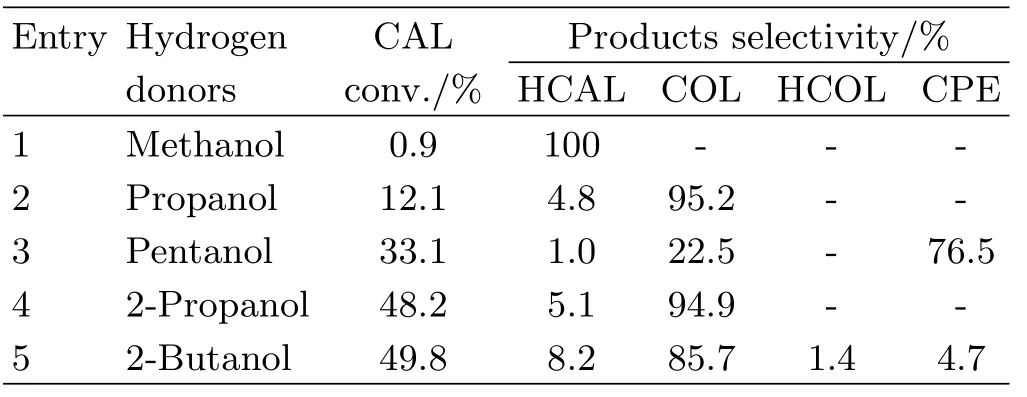
TABLE II Effect of different hydrogen donors on CAL conversion and COL selectivity over 2.46 wt%Pt/Fe3O4-AC catalyst. Reaction conditions:mass ratio of catalyst to CAL=0.5,120◦C,6 h.
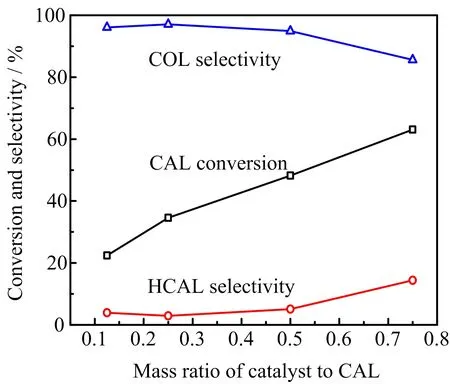
FIG.6 Effect of mass ratio of catalyst to CAL on CAL conversion and COL selectivity over 2.46 wt%Pt/Fe3O4-AC catalyst.Reaction conditions:120◦C,6 h,8 mL 2-propanol as hydrogen donor.
As shown in FIG.6,the CAL conversion is linearly related to the mass ratio of catalyst to substrate.It gradually increases from 22.4%to 63.1%as the mass ratio ranges from 0.125 to 0.75.However,the selectivity towards COL declines from 96.1%to 85.6%.It is worth noting that the selectivity towards COL is beyond 95% when the mass ratio is lower than 0.5.From the experimental results we can infer that the chemoselective transfer hydrogen of C=O bond of CAL is superior than C=C bond over the 2.46 wt%Pt/Fe3O4-AC catalyst. The side reactions for the hydrogenation of C=O bond and/or C=C bond are induced by the redundant available active sites when the mass ratio increases to 0.75, resulting in a decrease of selectivity towards COL.Thus, the optimal mass ratio of catalyst to substrate is fixed at 0.5 for further investigation.
We further explore the reusability of 2.46 wt%Pt/ Fe3O4-AC catalyst(FIG.7).The used catalyst is collected by an external magnet,washed with deionized water and ethanol,and then dried overnight at 60◦C in a vacuum oven.Although the catalyst deactivates in the 5th run,the selectivity towards COL is still reasonably high,suggesting a good reusability.
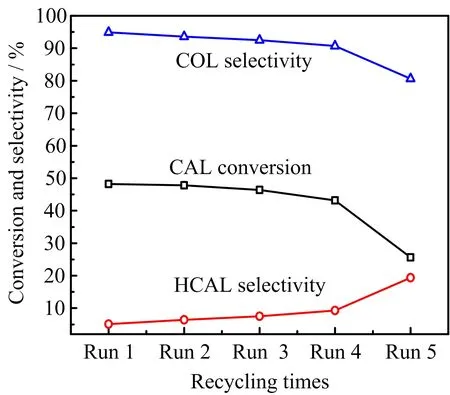
FIG.7 Reusability test of 2.46 wt%Pt/Fe3O4-AC catalyst. Reaction conditions:120◦C,6 h,8 mL 2-propanol as hydrogen donor.
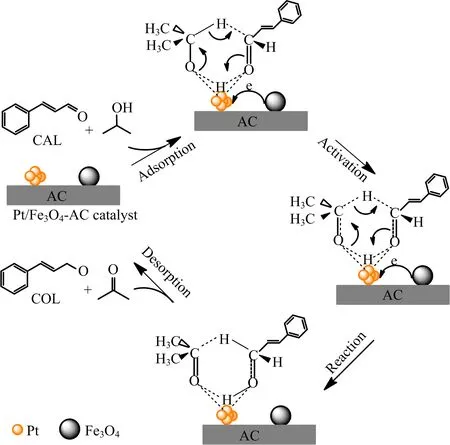
Scheme 2 Probable mechanism of hydrogen transfer reaction of CAL over Pt/Fe3O4-AC catalyst.
C.Mechanism of hydrogenation transfer reduction
The Meerwein-Ponndorf-Verley(MPV)reaction provides a convenient and feasible route which can selectively reduce unsaturated aldehydes into the corresponding alcohols by hydrogen transfer donor,such as isopropanol[33,34].The hydrogen transfer reaction includes an intermediate state where hydrogen atom derived from isopropanol is transferred to the C=O bond of CAL.It probably involves cyclic six-membered transition stats,which has been proposed upon potassium phosphate,magnesium oxide,and so forth[33,35].In view of the previous literature and XPS measurement, we propose a plausible mechanism of hydrogen transfer.As depicted in Scheme 2,the electron-enriched Ptis responsible for CAL adsorption via terminal C=O bond and 2-propanol adsorption by-OH group.Then it triggers hydrogen transfer reaction,where the hydrogen atom of OH and CH group is activated to attack aldehyde group of CAL,leading to formation of transition intermediate compound.Finally,the targeted product of COL can be obtained after the removal of acetone.
IV.CONCLUSION
In summary,a variety of sphere-shape Pt/Fe3O4-AC catalysts with regular morphology and uniform element distribution have been prepared by a redox between Pt(IV)and Fe(II)precursors.The PXRD analysis shows that both main cubic Fe3O4phase and faint cubic Pt phase are observed,inferring the occurrence of redox reaction.The highly dispersed Pt NPs on the surface or entrance into Fe3O4matrix is bene ficial to the adsorption of substrate and hydrogen donor.It is worth noting that the electron-enriched Pt NPs donating from variable Fe3O4NPs promote and activate the terminal C=O bond of CAL.This composite Pt/Fe3O4-AC catalysts show a good selectivity towards COL in the transfer hydrogenation reaction of CAL.The best selectivity towards COL(94.8%)can be obtained over Pt/Fe3O4-AC with 2.46 wt%Pt loading under the optimal conditions of 120◦C,6 h,and using 2-propanol as a hydrogen donor.Additionally,it shows a good reusability pro fiting from rapid separation from the mixture via a magnet due to its natural magnetism.
V.ACKNOWLEDGMENTS
This work is supported by the National Natural Science Foundation of China(No.51372248,No.51432009 and No.51502297),Instrument Developing Project of the Chinese Academy of Sciences(No.yz201421),the CAS/SAFEA International Partnership Program for Creative Research Teams of Chinese Academy of Sciences,China.
[1]S.Bzhogeswararao and D.Srinivas,J.Catal.285,31 (2012).
[2]C.H.Hao,X.N.Guo,Y.T.Pan,S.Chen,Z.F.Jiao, H.Yang,and X.Y.Guo,J.Am.Chem.Soc.138,9361 (2016).
[3]T.N.Ye,J.Li,M.Kitano,M.Sasase,and H.Hosono, Chem.Sci.7,5969(2016).
[4]E.Plessers,D.E.De Vos,and M.B.J.Roeffaers,J. Catal.340,136(2016).
[5]Y.Gu,Y.Zhao,P.Wu,B.Yang,N.yang,and Y.Zhu, Nanoscale 8,10896(2016).
[6]T.Szumelda,A.Drelinkiewicz,R.Kosydar,and J.Gurgul,Appl.Catal.A 487,1(2014).
[7]M.Kolodziej,A.Drelinkiewicz,E.Lalik,J.Gurgul,D. Duraczy´nska,and R.Kosydar,Appl.Catal.A 515,60 (2016).
[8]A.Yepez,J.M.Hidalgo,A.Pineda,R.ˇCern´y,P.J´ıˇsa, A.Garcia,A.A.Romero,and R.Luque,Green Chem. 17,565(2015).
[9]L.X.Dai,W.Zhu,M.Lin,Z.P.Zhang,J.Gu,Y.H. Wang,and Y.W.Zhang,Inorg.Chem.Front.2,949 (2015).
[10]I.Cano,A.M.Chapman,A.Urakawa,and P.W.N. M.van Leeuwen,J.Am.Chem.Soc.136,2520(2014).
[11]M.G.Prakash,R.Mahalakshmy,K.R.Krishnamurthy, and B.Viswanathan,Catal.Today 263,105(2016).
[12]Y.Wang,Z.Rong,Y.Wang,P.Zhang,Y.Wang,and J.Qu,J.Catal.329,95(2015).
[13]E.Bus,R.Prins,and J.A.van Bokhoven,Catal.Commun.8,1397(2007).
[14]C.Milone,R.Ingoglia,L.Schipilliti,C.Crisafulli,G. Neri,and S.Galvagno,J.Catal.236,80(2005).
[15]H.Liu,L.Chang,L.Chen,and Y.Li,ChemCatChem. 8,946(2016).
[16]Q.Wu,C.Zhang,B.Zhang,X.Li,Z.Ying,T.Liu,W. Lin,Y.Yu,H.Cheng,and F.Zhao,J.Colloid.Interface Sci.463,75(2016).
[17]M.J.Gilkey and B.Xu,ACS Catal.6,1420(2016).
[18]J.F.Mi˜nambres,A.Marinas,J.M.Marinas,and F.J. Urbano,J.Catal.295,242(2012).
[19]J.Li,Y.Zhang,D.Han,G.Jia,J.Gao,L.Zhong,and C.Li,Green Chem.10,608(2008).
[20]X.Wu,J.Liu,X.Li,A.Zanotti-Gerosa,F.Hancock, D.Vinci,J.Ruan,and J.Xiao,Angew.Chem.Int.Ed. Engl.45,6718(2006).
[21]I.Szatm´ari,G.Papp,F.Jo´o,and´A.Kath´o,Catal. Today 247,14(2015).
[22]S.Mazza,R.Scopelliti,and X.Hu,Organometallics 34, 1538(2015).
[23]P.Sharma and Y.Sasson,Green Chem.19,844(2017).
[24]Y.Zhu,G.Chuah,and S.Jaenicke,J.Catal.241,25 (2006).
[25]M.a.A.Aramend´ıa,V.Borau,C.Jim´enez,J.M.Marinas,J.R.Ruiz,and F.Urbano,Appl.Catal.A 249,1 (2003).
[26]N.Neelakandeswari,G.Sangami,P.Emayavaramban, S.Ganesh Babu,R.Karvembu,and N.Dharmaraj,J. Mol.Catal.A 356,90(2012).
[27]F.Alonso,P.Riente,F.Rodr´ıguez-Reinoso,J.Ruiz-Mart´ınez,A.Sep´ulveda-Escribano,and M.Yus,J. Catal.260,113(2008).
[28]Z.Rong,J.Lv,Z.Sun,Y.Wang,and Y.Wang,Catal. Lett.144,980(2014).
[29]Z.Tian,Q.Li,Y.Li,and S.Ai,Catal.Commun.61, 97(2015).
[30]J.Lu,X.Jiao,D.Chen,and W.Li,J.Phys.Chem.C. 113,4012(2009).
[31]C.F.d.Graauw,J.A.Peters,H.v.Bekkum,and J. Huskens,Synthesis 10,1007(1994).
[32]J.I.Di Cosimo,A.Acosta,and C.R.Apestegu´ıa,J. Mol.Catal.A 234,111(2005).
[33]R.Radhakrishan,D.M.Do,S.Jaenicke,Y.Sasson,and G.K.Chuah,ACS Catal.1,1631(2011).
[34]S.H.Liu,S.Jaenicke,and G.K.Chuah,J.Catal.206, 321(2002).
[35]T.Pasini,A.Lolli,S.Albonetti,F.Cavani,and M. Mella,J.Catal.317,206(2014).
ceived on March 29,2017;Accepted on May 29,2017)
∗Authors to whom correspondence should be addressed.E-mail: h.zhao@griffith.edu.au,chenchun2013@issp.ac.cn
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- γ-Ray Irradiation-Derived MnO/rGO Composites for High Performance Lithium Ion Batteries
- Identi fication of Superoxide O2−during Thermal Decomposition of Molten KNO3-NaNO2-NaNO3Salt by Electron Paramagnetic Resonance and UV-Vis Absorption Spectroscopy
- Binding Mechanism and Molecular Design of Benzimidazole/Benzothiazole Derivatives as Potent Abl T315I Mutant Inhibitors
- Highly Responsive and Selective Ethanol Gas Sensor Based on Co3O4-Modi fied SnO2Nano fibers
- Geometric Design of Anode-Supported Micro-Tubular Solid Oxide Fuel Cells by Multiphysics Simulations
- Laser-Assisted Stark Deceleration of Polar Molecules HC2n+1N(n=2,3,4) in High-Field-Seeking State
