Tailoring the Self-assembly of Melamine on Au(111)via Doping with Cu Atoms
2017-09-03HexiaShiWenyuanWangZheLiLiWangXiangShao
He-xia Shi,Wen-yuan Wang,Zhe Li,Li Wang,Xiang Shao
Department of Chemical Physics,CAS Key Laboratory of Urban Pollutant Conversion,Synergetic, Innovation Center of Quantum Information and Quantum Physics,University of Science and Technology of China,Hefei 230026,China
Tailoring the Self-assembly of Melamine on Au(111)via Doping with Cu Atoms
He-xia Shi,Wen-yuan Wang,Zhe Li,Li Wang,Xiang Shao∗
Department of Chemical Physics,CAS Key Laboratory of Urban Pollutant Conversion,Synergetic, Innovation Center of Quantum Information and Quantum Physics,University of Science and Technology of China,Hefei 230026,China
The doping effect of Cu on the self-assembly film of melamine on an Au(111)surface has been investigated with scanning tunneling microscopy(STM).The evaporated Cu adatoms occupy the positions underneath the amino groups and change the hydrogen bonding pattern between the melamine molecules.Accordingly,the self-assembly structure has changed stepwise from a well-de fined honeycomb into a track-like and then a triangular structure depending on the amount of Cu adatoms.The interaction between Cu adatom and melamine is moderate thus the Cu adatoms can be released upon mild heating to around 100◦C.These findings are different from previous observations of either the coordination assembly or the physically trapped metal adatoms.
Melamine,Self-assembly,Scanning tunneling microscopy,Cu adatoms,Hydrogen bonding
I.INTRODUCTION
Molecular self-assemblies(SAM)on metals have retained large research interests for decades due to its spontaneity,designability and functionality,which constitute a fascinating strategy for constructing variously functionalized nanostructures[1−3].Generally,on the active metals the ordering of the molecules is governed by the strong adsorbate-substrate interactions, whereas on the inert metals by various intermolecular interactions including van der Waals interaction,hydrogen bonding[4−6],metal coordination[7,8],halogen bonding[9,10],as well as indirect intermolecular interactions mediated by the strong surface states of the substrate[11].The metal adatoms,either intrinsically existing on the substrate or dosed intentionally, are frequently found participating and tailoring the selfassembly structures[8,12,13].On these occasions, metal adatoms are strongly involved in the chemical reactions of the molecules by initiating the covalent bond breaking as well as the coordinating bond formation [14−19].In other cases,they may also be trapped by the assembly frameworks,leaving no apparent in fluence on the assembly structures[5,20].Here we report another example wherein the dosed metal shows relatively weak interaction with the assembled molecules,but can signi ficantly change the assembly pattern by tailoring the existing intermolecular interactions.
Melamine(1,3,5-triazine-2,4,6-triamine)contains a triazine core with three terminal amino groups,and is widely applied as building block of hydrogen bonding networks[5,21].On coinage metals such as Au(111) [22,23]and Ag(111)[24],melamine has been reported to form highly ordered honeycomb structures,mediated by the ideal hydrogen bonding networks between the flat-lying molecules.Whereas on more active transition metals such as Ni(111)[25]and Pd(111)[26],dehydrogenation occurs upon depositing melamine at room temperature thus the assembly structure is governed by the π-π stacking of the vertically adsorbed radicals.Cu substrate is somehow an intermediate case which shows physisorption-to-chemisorption transition around room temperature[27−30].In a recent work of our group,we have carefully investigated the adsorption and assembling of melamine on the evaporated Cu/Au(111) films as a function of film thickness[30].A gradual transition of melamine from physisorption to chemisorption was clearly demonstrated and the effects of Cu adatoms were discussed in tuning the melamine assemblies on the exposed Au(111)surface.
In this work,we intentionally dosed Cu onto the Au(111)surface which was pre-covered with melamine assembly,and evidenced the changes of the molecular arrangements as functions of the amount of the dosed Cu.We found the interactions between Cu adatom and melamine molecule were moderate and could not lead to coordinative assembly on the Au(111)surface,but their incorporation already caused the changes of the hydrogen bonding patterns of melamine.These findings areexpected to shed new lights on the role of metal adatoms in organic self-assemblies.
II.EXPERIMENTS
All experiments were carried out on a Createc lowtemperature scanning tunneling microscope(LT-STM) housed in an ultra-high vacuum(UHV)system with base pressure of 1×10−10mbar. The Au(111)single crystal was cleaned by repeated Ar+sputtering and subsequent radiative heating at 800 K.Melamine (>98%)molecule was purchased from Sigma Aldrich and used without further puri fication before passed into the UHV chamber.Evaporation of melamine was conducted at~100◦C with a pre-degassing for 10 h before depositing onto the surface which was held at room temperature(RT).The home-made copper evaporator was constructed by wrapping a high-purity copper wire (99.99%,1 mm thick,Sigma-Aldrich)around the tungsten filament of a commercial bulb(Phillip). Electrochemically etched tungsten tips were used to obtain constant current images at liquid nitrogen(LN2) temperature with the bias voltage applied to the sample.All STM images were treated with WSxM software (ver.5.0)[31].
III.RESULTS AND DISCUSSION
The self-assembly of melamine on Au(111)at room temperature has been extensively studied previously [15,16].It is revisited as a reference for revealing the doping effect of the Cu adatom.FIG.1(a)shows the clean Au(111)surface with herringbone structure far before doping with any metals.A clear and periodicreconstruction pattern can be viewed.The inserted atomic resolution image also demonstrates that the surface is free of contaminations.After depositing 0.7 ML of melamine at RT,the highly ordered honeycomb structure is immediately developed with large domain size of tens to hundreds of nanometers.Only few antiphase domain boundaries can be observed,which is actually corresponding to close-packed structure.At the meantime,the substrate herringbone textures can still be visible,without any disturbance in comparison with the clean surface,manifesting the weak interactions between melamine and the Au(111)surface.The zoom-in image in FIG.1(c)in combination with the model in FIG.1(d)shows clearly that all the melamine molecules lie flat on the surface and assemble into honeycomb structure via the N−H···H hydrogen bonds between neighboring molecules.The rhombic unit cell is measured as a=b=1.19±0.05 nm,θ=60◦.Notice here we only show the clockwise assembly.Its enantiomer can be found on the surface with equal probability.
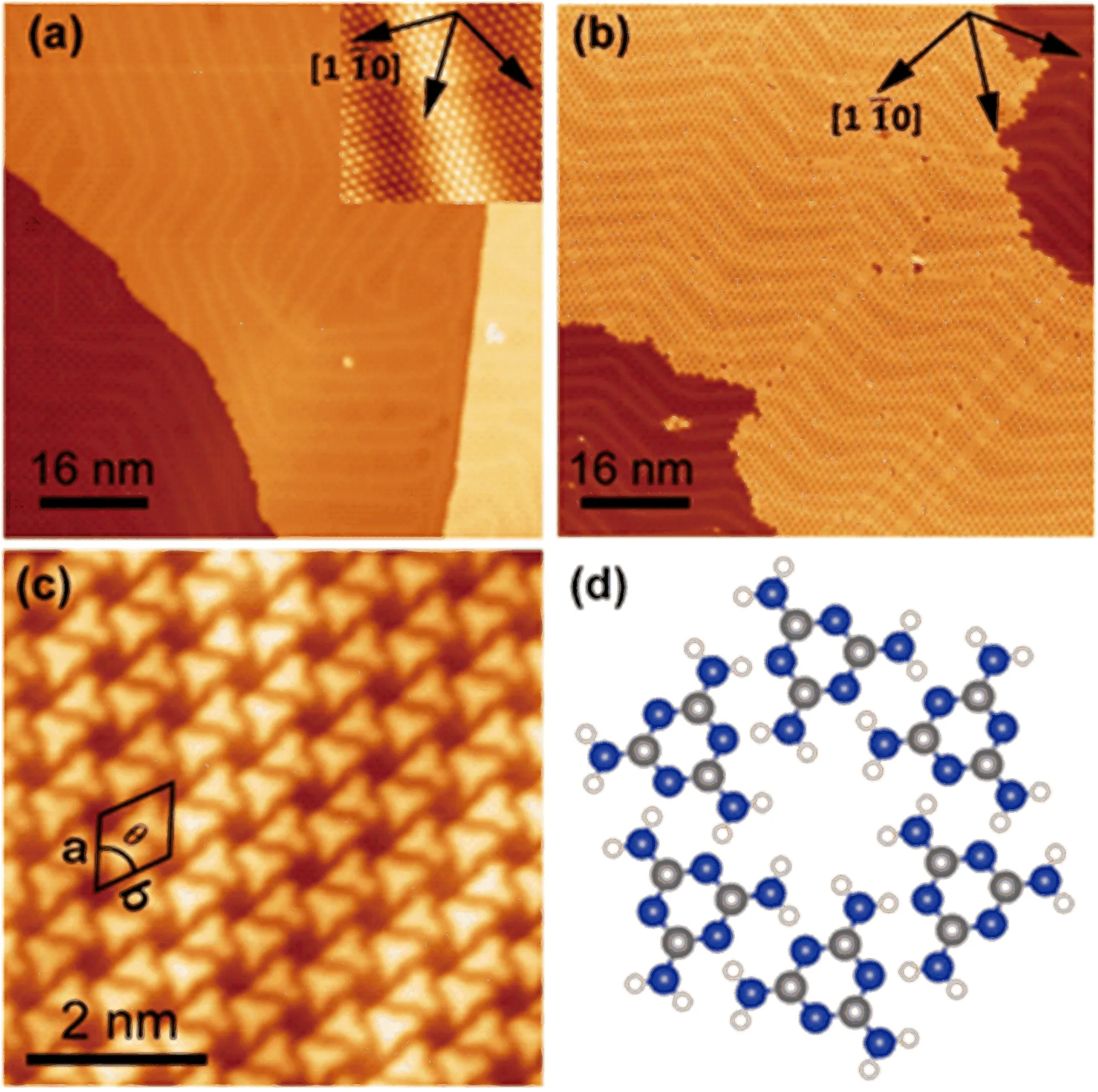
FIG.1(a)Clean surface of Au(111).Inserted is the atomic resolution image.(b)STM image of 0.7 ML melamine on Au(111)deposited at room temperature.(c)High resolution STM image of honeycomb structure of melamine.The lattice parameters outlined in inset of(c)were measured to be a=b=(1.19±0.05)nm,θ=60◦.(d)Molecular model of the honeycomb structure.In the model N,H and C are respectively represented by blue,white and grey balls.Tunneling parameters:(a)I=730 pA,U=−1.08 V;(b)I=140 pA, U=−1.01 V;(c)I=170 pA,U=−0.80 V.
On the basis of the clean Au(111)substrate,we prepared a Cu-doped surface by depositing small amount of Cu followed by annealing to~400◦C.With this treatment,a lightly Cu-doped Au(111)surface can be obtained. As shown in FIG.2(a),there are many shallow depressions observed,mostly positioned at the ridges(bright lines)of the reconstructed Au(111)surface,while the whole herringbone pattern retains unchanged.In the atomically resolved image as shown in FIG.2(b),these atomic-sized depressions are found occupying one to a few atomic lattice.According to previous studies of Cu films on Au,we assign these dark species as the doped Cu atoms that are positioned at the subsurface region of Au(111)[32,33].Here we notice that the local empty states around the Cu dopants may be suppressed based on the dark contrast under positive biases.However,their local filled states seem to be signi ficantly enhanced thus presenting as bright spots under negative biases,as shown in FIG.S1 in supplementary materials.Upon depositing melamine onto this surface,exactly the same honeycomb assembly structure as on the undoped Au(111)was formed,as shown in FIG.2(c)and the inserted image.Additionally many bright spots were observed positioning at the ridges of the Au substrate.We propose that these bright spots are formed due to the residing of melamine on top of the subsurface Cu atoms[20,34].Apparently,thesedoped Cu atoms did not cause any disturbance to the HB networks of the assembly,indicating a weak interaction with surface melamine molecules.On the other hand,if these Cu atoms are located at the surface layer, a stronger interaction with melamine can be expected which should affect the assembly structure of melamine [35].
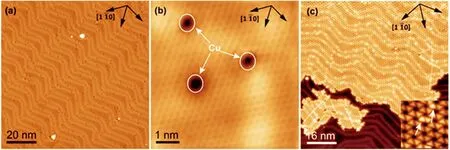
FIG.2 Cu-doped Au(111)surface before(a,b)and after(c)depositing 0.7 ML melamine.The doped Cu atoms are imaged as dark species under positive biases but bright spots under negative biases.Their combination with melamine lead to the formation of brighter triangles imbedded in the honeycomb network and seen at negative biases.Tunneling conditions: (a)I=790 pA,U=1.08 V;(b)I=8 nA,U=−13.6 mV;(c)I=370 pA,U=−1.50 V.

FIG.3 STM images of the emergence of the Track-like structure upon depositing(a)0.005 ML and(b)0.01 ML of Cu onto the honeycomb assembly of melamine at RT.The black lines de fine the three equivalent orientations of the TK domains. (c)High resolution STM image of the TK structure.The unit cell is de fined by the black parallelogram.a=(2.92±0.03)nm, b=(2.79±0.04)nm,θ=83◦.(d)Tentative model of the repeating building block de fined by the shadowed parallelogram in (c).Four triangular molecular clusters are de fined by the dashed triangles.N,H,C and Cu atoms are represented by blue, white,grey and yellow balls,respectively.Scanning conditions:(a)I=90 pA,U=1.16 V;(b)I=150 pA,U=0.40 V;(c) I=240 pA,U=0.71 V.
With these fundamental knowledge in mind,we start to consider the effect of dosing Cu atoms on the assembled melamine film on Au(111).As shown in FIG.3(a), upon depositing about 0.005 ML Cu onto the 0.7 ML honeycomb film of melamine at room temperature,a new track-like structure(termed as TK structure)immediately emerged coexisting with the remained honeycomb structure. Continuing dosing Cu to about 0.01 ML,the honeycomb network completely disappeared and only the TK structure was left on the surface,as shown in FIG.3(b).Meantime coexisting were the scattered large holes that are imbedded in the assembled film. The primary axes(the black lines in FIG.3(b)of the TK structure)are basically parallel to three equivalent〈1¯10〉directions of the Au(111)surface, thus forming three differently orientated domains based on the symmetry.A high-resolution image is shown in FIG.3(c),wherein the periodic unit cell is marked by the black parallelogram and the repeating molecular cluster is highlighted by the shadowed parallelogram.The unit cell is measured as a=(2.92±0.03)nm, b=(2.79±0.04)nm,θ=83◦,and contains 16 protrusions. Accordingly,the repeating unit also contains 16 protrusions,12 of which can be clearly identi fied with triangular shape and the other four basically triangular but with greater brightness than the others.We tentatively attribute the well-shaped triangular protrusions to the flat-lying melamine molecules which are analogous to those in the honeycomb structure,whereas those brighter asymmetric triangular species to the complex of a melamine molecule with a Cu adatom.An arbitrary model is shown in FIG.3(d),wherein each melamine molecule also forms three N−H···H HB pairs with neighboring molecules but the HBs are arranged in the unsymmetrical pattern.More importantly,four Cu adatoms are arbitrarily positioned under the amino groups, fitting with the observed four brighter protrusions.Such assumption can find supports from our recent work[21]where the Cu-underneath-NH2con figuration is a metastable position for isolated molecules but largely favored for the construction of HB network. However,why only four Cu adatoms can be incorporated in the structure cannot be fully understood.The repeating unit shown in FIG.3(d)can be further divided into four triangular melamine clusters(TMCs), each containing six melamine molecules and one Cu adatom. As marked by the dashed triangles,these TMCs connect each other by sharing their sides or the vortexes, finally spreading into the TK structure.
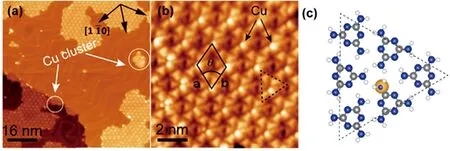
FIG.4(a)STM image of the coexisting Triangle structure and the Cu islands formed upon depositing 0.05 ML Cu onto the honeycomb assembly of melamine at RT.(b)High resolution STM image of Triangle structure.The unit cell is marked by black rhombus,and measured as a=b=(1.92±0.03)nm,θ=60◦.Dashed triangle highlights the building block.(c)Arbitrary model of the triangular molecular cluster.In the model N,H,C and Cu are respectively represented by blue,white,grey and yellow balls.Tunneling conditions:(a)I=140 pA,U=−1.68 V;(b)I=540 pA,U=−0.93 V.
Increasing the Cu dosage to about 0.05 ML,the assembly structure changes again.As shown in FIG.4(a), a new structure with hexagonal symmetry was formed and aggregated into large islands.Coexisting with these islands are the small Cu patches formed by the excessive Cu atoms.Their existence also implies that no other new assembly structures would be formed with even more Cu deposited,which is exactly what we found at higher Cu dosages.FIG.4(b)shows the high resolution STM image of this new assembly structure,wherein dim triangular species together with brighter protrusions can be clearly identi fied.The triangles can be assigned as flat-lying melamine molecules as in other assembly structures while the brighter protrusions as complexes of melamine and Cu adatoms.Obviously here the bright protrusions have arranged into a hexagonal pattern which naturally de fine the periodic unit cell,as shown by the black rhombus in FIG.4(b).The measured unit cell(marked by black rhombus)is superimposed on the high resolution STM image and the lattice parameters are measured as a=b=1.92±0.03 nm, θ=60◦.A closer look at the image reveals that the repeating building block of the assembly is actually the molecular cluster enveloped by the dashed triangle in FIG.4(b).Therefore,we term this new structure as Triangle structure(shorted as TA).FIG.4(c)shows the arbitrary model that we have proposed,where six melamine molecules and one Cu adatom are involved. In such cluster three melamine molecules are symmetrically placed at the inner shell with their amino groups pointing to the center,whereas the other three molecules are orientated antiparallel and symmetrically placed at the outer shell.The Cu adatom is proposed to be located under one of the three amino groups of the inner melamine molecules.Such proposition is based on similar arguments to those for the TK structure discussed above.However,an unavoidable question remains for the number of the Cu adatoms in the cluster, which is still beyond our understanding at the current stage.On the other side,we frequently observed more than one bright spots in the triangular molecular cluster,as can be found at the left bottom of FIG.4(b) (please also see FIG.S2 in supplementary materials). Such two-or three-bright-spots may imply that more Cu adatoms are incorporated in the assembly.Nevertheless,the majority of the film is still dominated by the domains consisting one Cu adatom per cluster.
IV.DISCUSSION
The above all experimental results have clearly demonstrated that upon dosing Cu onto the half monolayer melamine on Au(111)the assembly structure has drastically changed and presented an obvious dependence on the dosing amount of Cu.The newly emerged structures,i.e.the TK and TA types of structures are therefore proposed to be the co-assemblies of melaminewith different number of Cu adatoms.Such proposition was based on the consideration of the potential interactions between melamine and the Cu adatoms.It also found concreate supports from our experimental observations of the same assembly structures of melamine on a Cu/Au(111) film[21].On the basis of these models,we may brie fly discuss the formation mechanism of these assembly structures as well as the connections between them.
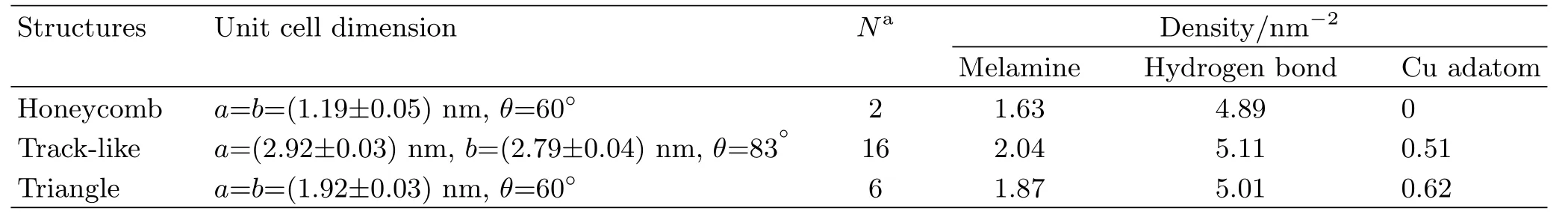
TABLE I Structural parameters of the three assembly structures of melamine.
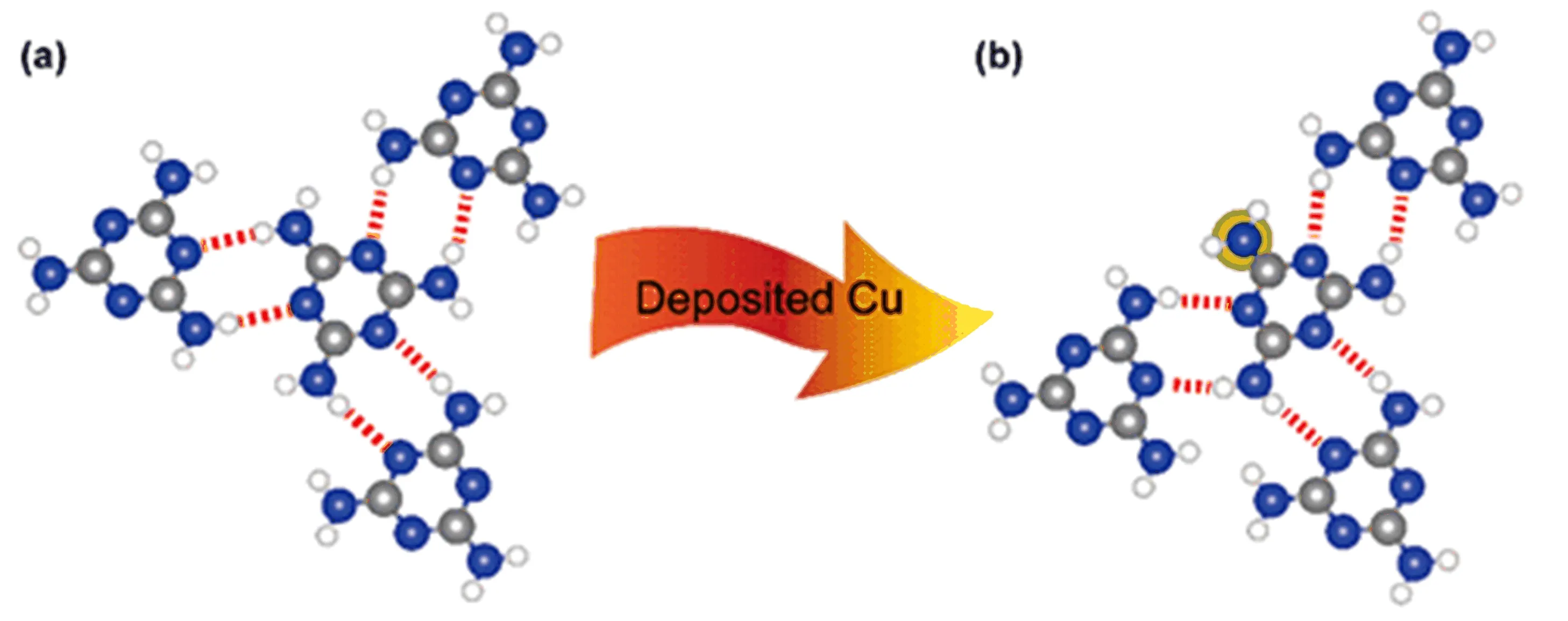
FIG.5 Illustrative cartoon for the mechanism of phase transition from the pure melamine assembly to the Cu-melamine co-assembly.The white,blue,grey and yellow balls represent the hydrogen,nitrogen,carbon and Cu atoms,respectively. The dashed red lines represent the intermolecular hydrogen bonds.
Firstly,we look at the interaction between Cu adatom and melamine molecule.Previous studies of melamine on Cu(111)and Cu(100)have demonstrated a strong interaction between melamine and bulk Cu surfaces. Upon depositing at room temperature,melamine would dehydrogenate and form strong chemical bonds with the Cu surfaces.Such chemisorption is so strong that the adsorbed molecules would not desorb even after heated to above 700 K.However,for the singly dispersed Cu atoms,the situation may be different.The STM images in FIG.2 clearly demonstrate that the melamine molecules maintain their honeycomb structure on an Au(111)surface doped with diluted Cu atoms.This fact manifests that melamine has little tendency to drag Cu atoms from the subsurface to the top surface.In addition,our thermal treatments of the Cu-melamine co-assemblies(TK and TA structures)lead to the same desorption behavior upon mild heating,indicating that the interactions between Cu adatom and the melamine cannot be that strong.Both arguments would support our assumption of the complex of Cu adatom with the melamine molecules,among which the latter keeps undissociated state.
Concerning the phase transition of the assembly films,in most cases it is driven by formation of a thermodynamically more stable structure.The principle may be applied to the co-assembly of melamine and Cu adatom in the present study as well.Table I lists the structural parameters of the three types of assembly structures of melamine with and without Cu adatoms. It can be found that upon forming the co-assembled structure the melamine density increased from 1.63 nm−2in the honeycomb structure to 2.04 nm−2in the TK structure and 1.87 nm−2in the TA structure,respectively.The density of hydrogen bonds is also increased correspondingly since in all three assemblies each melamine molecule forms six hydrogen bonds with three neighboring molecules.Such increment demonstrates that both TK and TA structures have lower energy than the honeycomb structure, thus being the favored phases with the presence of Cu adatoms.It is noticed that the TA structure was formed on the basis of TK structure with more Cu adatoms deposited,yet it has slightly lower densities of both melamine and hydrogen bond compared to the latter. We assume such disadvantage may be compensated by the number of density of Cu adatoms in the assembly structure considering that the incorporation of each Cu adatom with the amino group would induce an en-ergy drop.In FIG.4 we present a model with only one Cu adatom in a melamine triangular cluster.As a matter of fact,we frequently found that two or three bright protrusions aggregate together close to the middle of the triangular cluster(see FIG.S2 in supplementary materials),suggesting more than one Cu adatoms can be co-assembled in the structure.As a result,the averaged Cu adatom density in the TA structure can be estimated around 0.62 nm−2(corresponding to two Cu adatoms per triangular molecular cluster),becoming slightly larger than 0.51 nm−2in the TK structure. And the induced energy gain should be able to compensate the loss caused by the dilution of the melaminesubstrate interactions and the hydrogen bonds.
Finally,let us brie fly discuss the formation mechanism of the Cu-melamine co-assembly.As shown in FIG.4,the TA structure is composed of many identical triangular melamine clusters.Actually,the repeating unit of the TK structure can also be divided into similar triangular cluster each containing one Cu adatom, as shown in FIG.3(d).In this regard,in TK structure the triangles have to share their sides with neighboring triangles,making large difference from the TA structures.In addition,the detailed structure of the triangular cluster of TK is not identical with that in TA. Nevertheless,both TK and TA structures contain similar hydrogen bonding network,particularly around the melamine molecule with one Cu adatom underneath,as shown in FIG.5(b).Such common substructure of Cumelamine co-assemblies presents drastic difference from that in the honeycomb structure of solely melamine.
As shown in FIG.5,in the honeycomb structure the hydrogen bonds around each melamine form a pin-wheel pattern with three-fold rotational symmetry.Upon depositing Cu onto the honeycomb film,the Cu adatoms may attach to the N atom of either the triazine cycle or the amino group.But the former would only take place at the periphery melamine molecule of the assembly domain,as the tiazine-N is fully occupied by the hydrogen bonds.While the latter becomes more feasible since the bonded melamine can just shift by one N atom and form new hydrogen bonds,as shown in FIG.5(b).By doing so,the molecular interdistances become reduced while keeping the number of hydrogen bonds.As a result,the density of the melamine molecule as well as the hydrogen bonds is increased,in combination with the added Cu-melamine interactions.In this way,the honeycomb structure can be gradually transformed into the tracklike or triangular structures,depending on the number of the incorporated Cu adatoms as well as the spreading pattern of the triangular melamine clusters.
V.CONCLUSION
We have researched the regulation effect of Cu adatoms on the self-assembly structures of melamine on an Au(111)surface with STM.It is found that the evaporated Cu adatoms incorporate into the melamine assembly by accommodating the underneath positions of the amino groups. The interaction between the Cu adatom and melamine molecule is moderate,yet it signi ficantly modulates the hydrogen bond patterns from a three-fold rotationally symmetrical pattern to an unsymmetrical pattern.Such metal-incorporated selfassembly may potentially serve as a reservoir for metal atoms,the latter may get involved and thus play important roles in various surface reactions.
Supplementary materials:Additional STM images of the Cu-doped bare Au(111)surface(FIG.S1),the TA structure incorporating two or three Cu adatoms (FIG.S2),and the coexistence of TK and TA structures (FIG.S3)are given.
VI.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.91545128,No.21333001, No.91227117)and Ministry of Science and Technology of China(No.2011CB808702),the Fundamental Research Funds for the Central Universities and the Thousand Talent Program for Young Outstanding Scientists of the Chinese Government,and the“Strategic Priority Research Program”of the Chinese Academy of Sciences (XDB01020100).
[1]X.H.Liu,Y.P.Mo,J.Y.Yue,Q.N.Zheng,H.J.Yan, D.Wang,and L.J.Wan,Small 10,4934(2014).
[2]L.Niu,X.Ma,L.Liu,X.Mao,D.Wu,Y.Yang,Q. D.Zeng,and C.Wang,Phys.Chem.Chem.Phys.12, 11683(2010).
[3]H.Shi,Y.Liu,Q.Zeng,Y.Yang,C.Wang,and X.Lu, Phys.Chem.Chem.Phys.19,1236(2017).
[4]R.Madueno,M.T.R¨ais¨anen,C.Silien,and M.Buck, Nature 454,618(2008).
[5]L.Wang,Q.Chen,H.Shi,H.Liu,X.Ren,B.Wang,K. Wu,and X.Shao,Phys.Chem.Chem.Phys.18,2324 (2016).
[6]Y.Ye,W.Sun,Y.Wang,X.Shao,X.Xu,F.Cheng, and K.Wu,J.Phys.Chem.C 111,10138(2007).
[7]J.Liu,T.Lin,Z.Shi,F.Xia,L.Dong,P.N.Liu,and N.Lin,J.Am.Chem.Soc.133,18760(2011).
[8]T.Lin,X.S.Shang,J.Adisoejoso,P.N.Liu,and N. Lin,J.Am.Chem.Soc.135,3576(2013).
[9]Q.N.Zheng,X.H.Liu,T.Chen,J.H.Yan,T.Cook, D.Wang,P.J.Stang,and L.J.Wan,J.Am.Chem. Soc.137,6128(2015).
[10]J.Shang,Y.Wang,M.Chen,J.Dai,X.Zhou,J.Kuttner,G.Hilt,X.Shao,J.M.Gottfried,and K.Wu, Nat.Chem.7,389(2015).
[11]Y.Wang,X.Ge,C.Manzano,J.Kr¨oger,R.Berndt,W. A.Hofer,H.Tang,and J.Cerda,J.Am.Chem.Soc. 131,10400(2009).
[12]M.A.Lingenfelder,H.Spillmann,A.Dmitriev,S. Stepanow,N.Lin,J.V.Barth,and K.Kern,Chem. Eur.J.10,1913(2004).
[13]K.J.Shi,X.Zhang,C.H.Shu,D.Y.Li,X.Y.Wu, and P.N.Liu,Chem.Commun.52,8726(2016).
[14]M.Chen,J.Shang,Y.Wang,K.Wu,J.Kuttner,G. Hilt,W.Hieringer,and J.M.Gottfried,ACS Nano.11, 134(2017).
[15]A.Dmitriev,H.Spillmann,N.Lin,J.V.Barth,and K. Kern,Angew.Chem.Int.Ed.115,2774(2003).
[16]S.Stepanow,M.Lingenfelder,A.Dmitriev,H.Spillmann,E.Delvigne,N.Lin,X.B.Deng,C.Z.Cai,J. V.Barth,and K.Kern,Nat.Mater.3,229(2004).
[17]H.Spillmann,A.Dmitriev,N.Lin,P.Messina,J.V. Barth,and K.Kern,J.Am.Chem.Soc.125,10725 (2003).
[18]R.Zhang,G.Lyu,D.Y.Li,N.P.Liu,and N.Lin, Chem.Commun.53,1731(2017).
[19]Y.L.Zhao,W.Wang,F.Qi,J.Li,G.Kuang,R.Zhang, R.Q.Zhang,N.Lin,and M.A.Van Hove,Langmuir 33,451(2017).
[20]V.Iancu,K.F.Braun,K.Schouteden,and C.Van Haesendonck,Phys.Rev.Lett.113,106102(2014).
[21]L.Wang,H.X.Shi,W.Y.Wang,H.Shi,and X.Shao, Acta Phys.Chim.Sin.33,393(2017).
[22]F.Silly,A.Q.Shaw,M.R.Castell,G.A.D.Briggs,M. Mura,N.Martsinovich,and L.Kantorovich,J.Phys. Chem.C 112,11476(2008).
[23]M.Mura,F.Silly,V.Burlakov,M.R.Castell,G.A.D. Briggs,and L.N.Kantorovich,Phys.Rev.Lett.108, 176103(2012).
[24]C.H.Schmitz,J.Ikonomov,and M.Sokolowski,Surf. Sci.605,1(2011).
[25]J.Greenwood,H.A.Fru¨chtl,and C.J.Baddeley,J. Phys.Chem.C 116,6685(2012).
[26]J.Greenwood,H.A.Fru¨chtl,and C.J.Baddeley,J. Phys.Chem.C 117,22874(2013).
[27]A.Ferral,P.Paredes-Olivera,V.A.Macagno,and E. M.Patrito,Surf.Sci.525,85(2003).
[28]J.C.Cook and E.M.McCash,Surf.Sci.356,445 (1996).
[29]M.C.Lu,R.B.Wang,A.Yang,and S.Duhm,J.Phys.: Condens.Matter 28,094005(2016).
[30]L.Wang,P.Li,H.Shi,Z.Li,K.Wu,and X.Shao,J. Phys.Chem.C 121,7977(2017).
[31]I.Horcas,R.Fern´andez,J.M.Gomez-Rodriguez,J. Colchero,J.W.S.X.M.G´omez-Herrero,and A.M. Baro,Rev.Sci.Instrum.78,013705(2007).
[32]X.Zhao,P.Liu,J.Hrbek,J.A.Rodriguez,and M. Perez,Surf.Sci.592,25(2005).
[33]F.Grillo,H.Fru¨chtl,S.M.Francis,and N.V.Richardson,New J.Phys.13,013044(2011).
[34]O.M.Magnussen,J.Hotlos,R.J.Nichols,D.M.Kolb, and R.J.Behm,Phys.Rev.Lett.64,2929(1990).
[35]Y.P.Lin,O.Ourdjini,L.Giovanelli,S.Clair,T.Faury, Y.Ksari,and M.Abel,J.Phys.Chem.C 117,9895 (2013).
ceived on April 21,2017;Accepted on May 22,2017)
∗Author to whom correspondence should be addressed.E-mail: shaox@ustc.edu.cn
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- γ-Ray Irradiation-Derived MnO/rGO Composites for High Performance Lithium Ion Batteries
- Identi fication of Superoxide O2−during Thermal Decomposition of Molten KNO3-NaNO2-NaNO3Salt by Electron Paramagnetic Resonance and UV-Vis Absorption Spectroscopy
- Binding Mechanism and Molecular Design of Benzimidazole/Benzothiazole Derivatives as Potent Abl T315I Mutant Inhibitors
- Highly Responsive and Selective Ethanol Gas Sensor Based on Co3O4-Modi fied SnO2Nano fibers
- Geometric Design of Anode-Supported Micro-Tubular Solid Oxide Fuel Cells by Multiphysics Simulations
- Laser-Assisted Stark Deceleration of Polar Molecules HC2n+1N(n=2,3,4) in High-Field-Seeking State
