Density Function Theory Study on Effects of Different Energetic Substituent Groups and Bridge Groups on Performance of Carbon-Linked Ditetrazole 2N-Oxides
2017-09-03QiongWuBoKouZewuZhangZushengHangWeihuaZhu
Qiong Wu,Bo Kou,Ze-wu Zhang,Zu-sheng Hang,Wei-hua Zhu
a.School of Materials Science and Engineering,Nanjing Institute of Technology,Nanjing 211167, China
b.Jiangsu Key Laboratory of Advanced Structural Materials and Application Technology,Nanjing 211167,China
c.Institute for Computation in Molecular and Materials Science and Department of Chemistry,Nanjing University of Science and Technology,Nanjing 210094,China
Density Function Theory Study on Effects of Different Energetic Substituent Groups and Bridge Groups on Performance of Carbon-Linked Ditetrazole 2N-Oxides
Qiong Wua,b∗,Bo Koua,b,Ze-wu Zhanga,b,Zu-sheng Hanga,b,Wei-hua Zhuc
a.School of Materials Science and Engineering,Nanjing Institute of Technology,Nanjing 211167, China
b.Jiangsu Key Laboratory of Advanced Structural Materials and Application Technology,Nanjing 211167,China
c.Institute for Computation in Molecular and Materials Science and Department of Chemistry,Nanjing University of Science and Technology,Nanjing 210094,China
Based on the parent tetrazole 2N-oxide,six series of novel carbon-linked ditetrazole 2N-oxides with different energetic substituent groups(-NH2,-N3,-NO2,NF2,-NHNO2)and energetic bridge groups(-CH2-,-CH2−CH2-,-NH-,-N=N-,-NH−NH-)were designed.The overall performance and the effects of different energetic substituent groups and energetic bridge groups on the performance were investigated by density functional theory and electrostatic potential methods.The results showed that most of designed compounds have oxygen balance around zero,high heats of formation,high density,high energy,and acceptable sensitivity,indicating that tetrazole N-oxide is a useful parent energetic compound employed for obtaining high energy compounds,even only combined with some very common energetic substituent groups and bridge groups.Comprehensively considering the effects on energy and sensitivity,the-NO2,-NF2,-NH-and-NH−NH-are appropriate substituent groups for combining tetrozale N-oxide to design new energetic compounds,while-NH2, -N3,-CH2−CH2-,and-N=N-are inappropriate.
Tetrazole,N-oxide,High energy,Bridge group,Density functional theory
I.INTRODUCTION
Seeking for novel advanced energetic materials with better energy properties and sensitivity performance is an everlasting topic and challenge for researchers.In the past several years,many studies have been done to theoretically design and synthesize new kinds of energetic molecules[1−4],salts[5−7],co-crystals[8−10], and metal-organic frameworks[11−13].Among them, tetrazole N-oxide-based compounds[14−19]attracted lots of attention lately,because of their high oxygen balance(OB),high detonation properties(detonation velocity D and detonation pressure P),good thermal stability or low sensitivity. For instance,Fischer et al.[14]synthesized the hydroxylammonium and ammonium salts of aminotetrazole 1N-oxide,these compounds have obvioulsy lower impact sensitivity,friction sensitivity,and electrostatic discharge sensitivity than two very famous high explosives 1,3,5-trinitro-1,3,5-triazinane(RDX)and 1,3,5,7-tetranitro-1,3,5,7-tetrazocane(HMX),and their energies are close to RDX.Then,a series of 5,5′-bis(tetrazole 1N-oxide)salts [15]were prepared,including a very powerful representative with low toxicity:dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate(TKX-50)[15−18],whose D and P are around 9.7 km/s and 42.4 GPa,respectively,which are obviously higher than HMX.Besides, TKX-50 also has lower impact sensitivity(20 J)and IF (120 N)than HMX.The excellent overall performance makes TKX-50 be a possible replacement for RDX and HMX,and it shows the high research worth of tetrazole N-oxide-based energetic compounds.Many 5,5′-bis(tetrazole 2N-oxide)salts[19]were also synthesized successfully,among them,3-amino-1-nitroguanidinium salt has comparable energy and sensitivity with HMX, the hydroxylammonium salt possesses close performance to RDX,while the guanidinium salt is high thermally stable and insensitive. Five azotetrazole-1,1′-dioxide salts with high energy were prepared through the oxidation of hydroxylammonium aminotetrazole 1-oxide[14],these compounds also have better detonation properties than RDX,especially for the dipotassium salt,whose D and P are close to TKX-50 but its IF and electrostatic discharge sensitivity are higher than TKX-50.By oxidizing the 5-azidotetrazolate an-ion at the mild aqueous conditions,a series of azidotetrazolate 2N-oxide salts were synthesized successfully, among them,the ammonium and aminoguanidinium salts have comparative D with RDX while the sodium salt is more insensitive than RDX.Furthermore,these salts possess better D than their corresponding azidotetrazolates that lack an N-oxide.
From these above analyses,it can be found that some good tetrazole N-oxide-based compounds with high energetic performance and low sensitivity have been synthesized successfully.The introduction of N-oxide into tetrazole are feasible. Because of the introduction of N-oxide,some of these tetrazole N-oxide-based compounds have OB close to zero without possessing too much oxygen-rich energetic substituent groups like -NO2or-ONO2.Generally,the most ideal OB value is zero,which could release the maximum energy when detonating.A negative OB value means that C and H atoms could not be oxidized completely,while a positive OB value indicates that extra O2would be formed from the needless oxygen.These two cases would both decrease the heat released,and may generate some toxic gases like CO too.Besides,the existing of N-oxide could be helpful for forming hydrogen-bond to increase the thermal stability and decrease the sensitivity,this is may be one reason why many of these synthesized tetrazole N-oxide-based compounds possess lower sensitivity than RDX.These above analyses show the high research worth of tetrazole N-oxides.However,the number of synthesized tetrazole N-oxides is limited and most of them are energetic slats in the past decade,more systematic studies are needed.
In the present study,based on the parent tetrazole 2N-oxide,we designed six series of novel carbonlinked ditetrazole 2N-oxides with different energetic substituent groups(-NH2,-N3,-NO2,NF2,-NHNO2) and energetic bridge groups(-CH2-,-CH2−CH2-,-NH-, -N=N-,-NH−NH-)with OB around zero.Molecular structures of six series of designed compounds are shown in FIG.1.The effects of different energetic substituent groups and energetic bridge groups on the performance were investigated.Density functional theory(DFT)and electrostatic potential(ESP)were employed to study the molecular and electronic structures,heats of formation(HOF),density,detonation properties,and sensitivity.And a good combination among the parent compound,bridge group,and substituent group with good overall properties was found.
II.COMPUTATIONAL METHODS
D and P are two important parameters used for judging the energy level of energetic materials,in this study, we employed Kamlet-Jacobs equations[20]to calculate them:
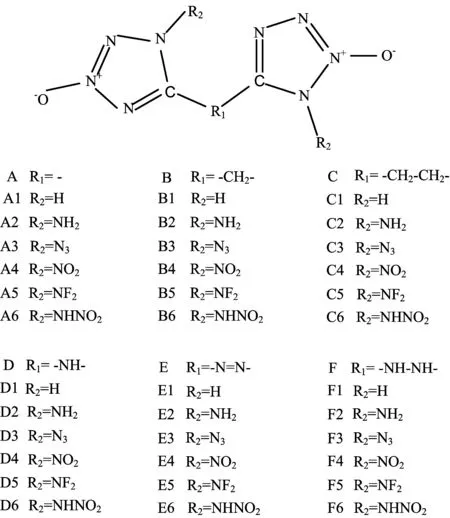
FIG.1 Molecular frameworks of six series of designed compounds.
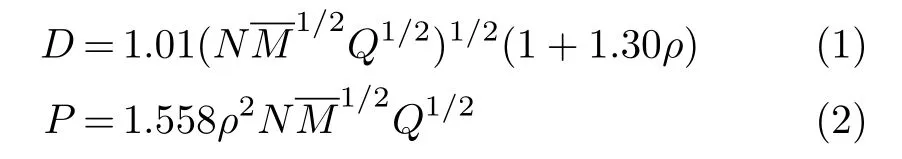
Density(ρ)and heats of formation(HOF)are two key parameters related with D and P,we calculated ρ (Eq.(3))in solid-phase using the ESP method[21]proposed by Politzer et al.at B3PW91/6-31G(d,p)level, and the HOF in solid-phase(∆Hf,solid)at 298 K by using a compositive method[22,23]based on the Hess’s law(Eq.(4)and Eq.(6))is used for predicting heat of sublimation(∆Hsub)at B3LYP/6-31G(d,p)level. Isodesmic reaction used for predicting gas-phased HOF:∆Hf,gas)is shown in Scheme 1.
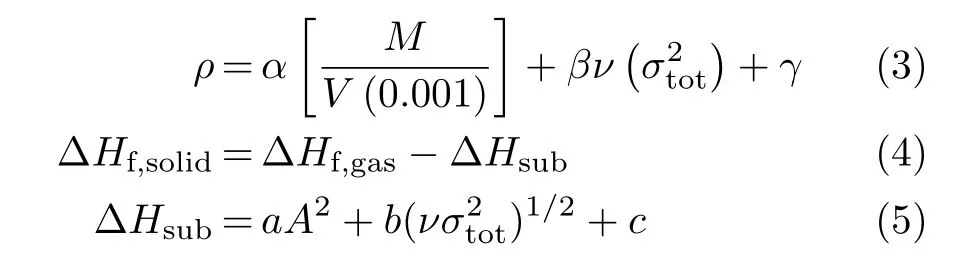
The free space per molecule in the unit cell(∆V)is an common ESP method[24,25]used for estimating the impact sensitivity of energetic materials,which is proposed by the Politzer group,calculated at B3PW91/6-31G(d,p):
Molecular and electronic structures calculations were performed on Gaussian program[26]at B3LYP/6-31G(d,p).
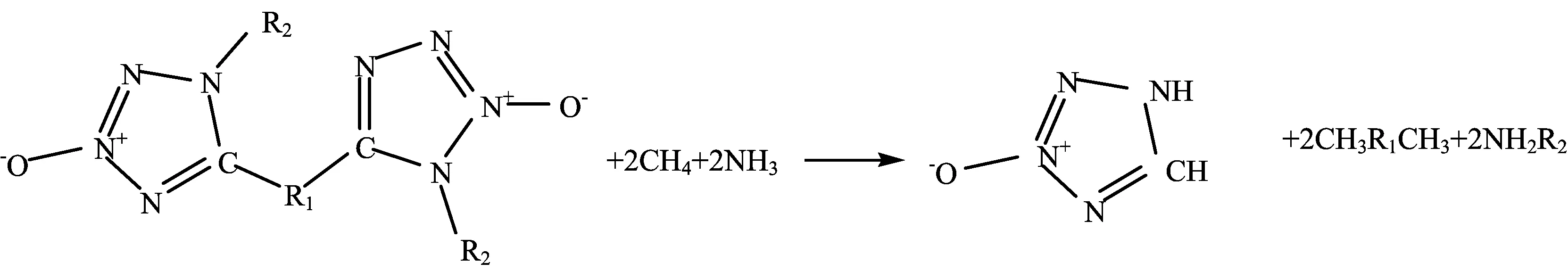
Scheme 1 Isodesmic reaction used for predicting gas-phased HOF.
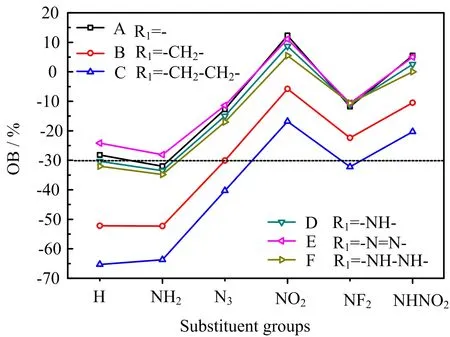
FIG.2 Oxygen balance of 36 designed compounds.
III.RESULTS AND DISCUSSION
A.Oxygen balance and heats of formation
OB is a parameter related with the energy of high explosives,generally,the more the OB close zero,the higher the energy.The OBs of designed compounds were calculated and displayed in FIG.2.It is found that among these 36 designed compounds,there are about 26 compounds(A1,A3−A6,B3−B6,C4,C6, D1,D3−D6,E1−E6,F3−F6)have OB between−30% and 30%,showing the relative ideal OB value is close to zero,which is a good basis for obtaining high detonation performance.The relative higher OB values of these compounds are mainly from the oxygen-rich tetrazole N-oxide,though all compounds only have no more than two energetic substituent groups.There are seven compounds with OB more than zero and one(F6)with the most ideal value 0%.In addition,series B(-CH2-bridged)and C(-CH2-CH2-bridged)have the lowest while-NO2and-NHNO2substituted derivatives possess the highest OB values.
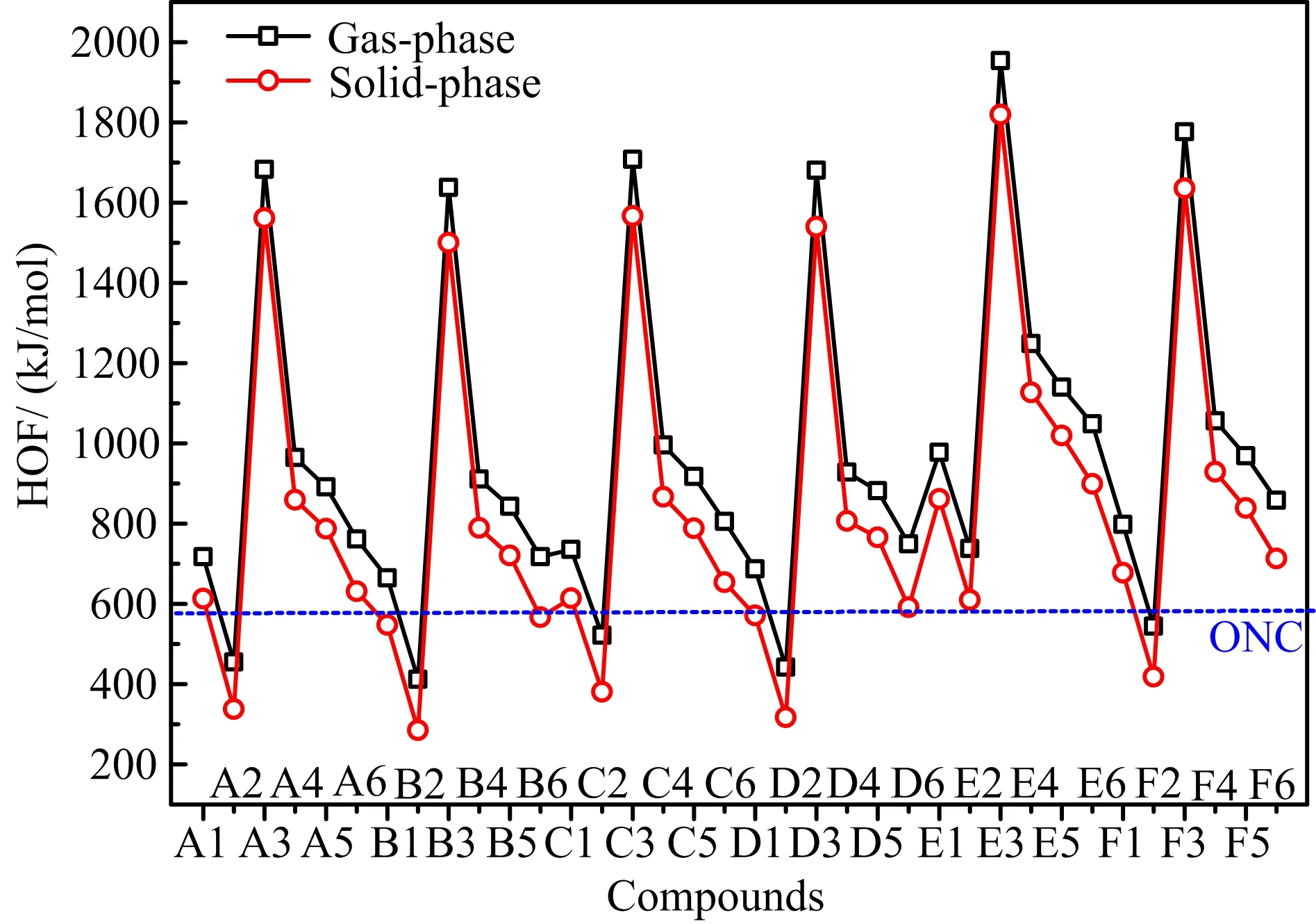
FIG.3 Comparison of heats of formation in gas-phase and solid-phase of 36 designed compounds.
HOF is another important parameter associated with energetic properties,usually,the higher the HOF,the better the energetic properties.The HOFs in gas-phase and solid-phase of designed compounds were predicted, as depicted in FIG.3.It is seen that gas-phase HOFs are higher than solid-phase HOFs in general,and their variation tendency is similar.27 designed compounds have higher solid-phase HOF than one of most powerful CHNO explosives octanitrocubane[27](ONC,solidphase HOF=594 kJ/mol),eight compounds even possess solid-phase HOF more than 1000 kJ/mol,and all compounds have obvious higher solid-phase HOF than RDX and HMX.These indicate the outstanding HOF property of designed compounds,which is also a good base for acquiring high energy.A comparison of the effects of substituent groups and bridge groups on solidphase HOF is displayed in FIG.4.It is seen that-N3, -NO2,-NF2and-NHNO2substituted derivatives possess higher HOF than those of unsubstituted ones while -NH2substituted compounds have the lowest value, showing that-N3,-NO2,-NF2,and-NHNO2groups are helpful for improving HOF,especially for-N3,while -NH2group just has the opposite effect.Then,series E has the highest HOF,followed by series F,while the other series have close value,indicating that-N=N-and-NH-NH-groups increase the HOF,especially for -N=N-group.
B.Density and detonation performance
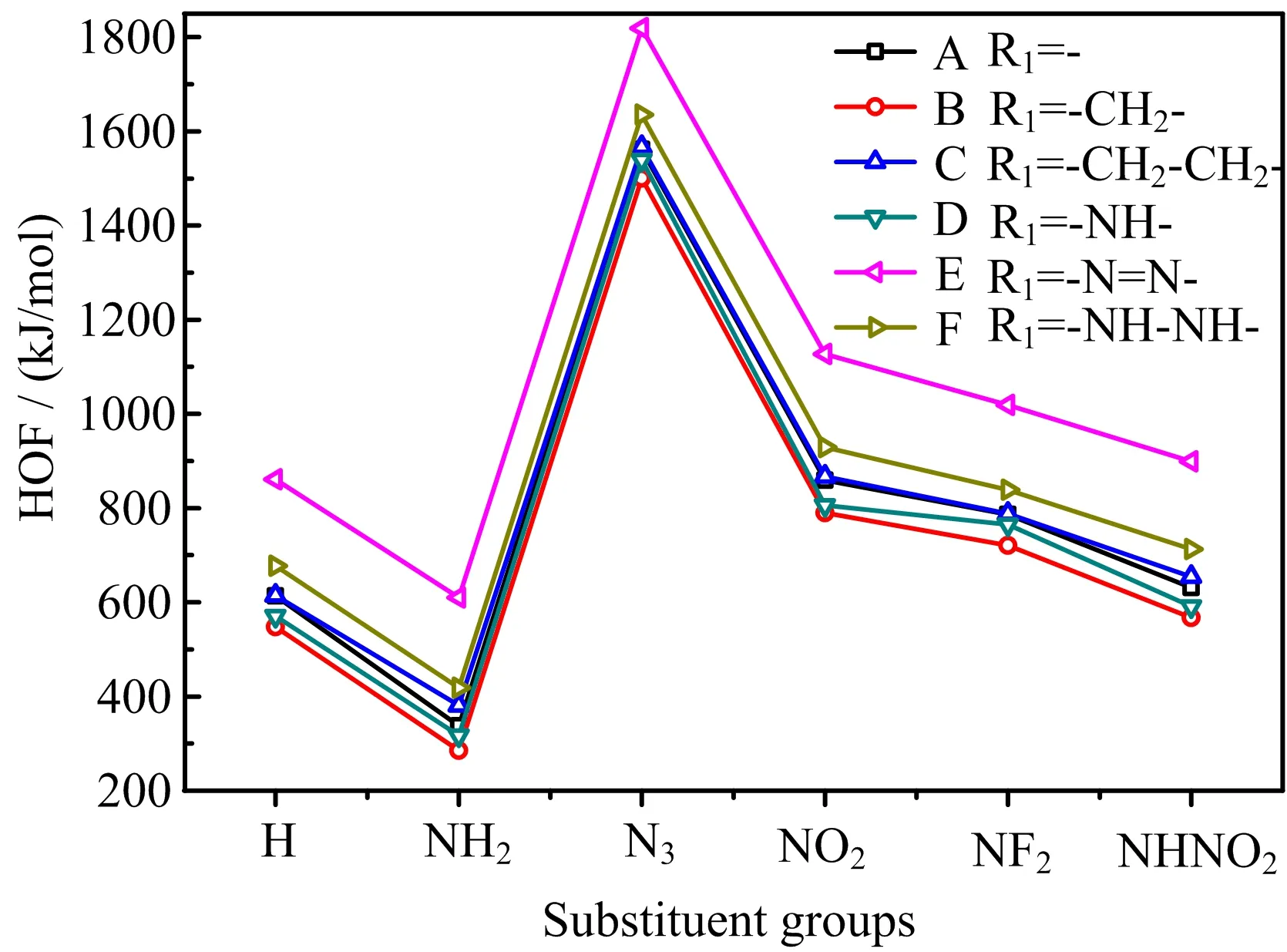
FIG.4 Comparison of solid-phase heats of formation of 36 designed compounds.
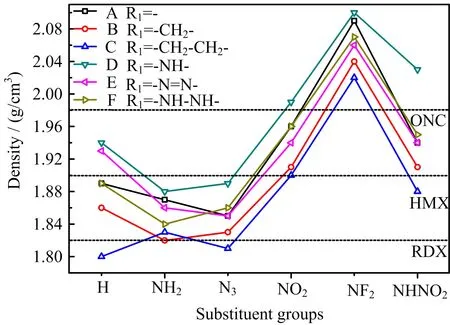
FIG.5 Density of 36 designed compounds.
Density is also a key parameter connected with detonation performance,a high density would be very helpful for enhancing the detonation performance.The densities in solid-phase of designed compounds were estimated and compared in FIG.5.First,there are 34, 19,and 8 compounds with higher density than RDX, HMX,and ONC,respectively,seven of them even possess density more than 2.00 g/cm3,indicating the good density property of designed molecules[28−30].Then, series D and series B/C have higher and lower density than series A,while series E/F have comparable density with series A,showing that-NH-group and-CH2-/-CH2−CH2-increase and reduce the density,respectively,while-NH-NH-and-N=N-groups have little effects on density.Finally,-NF2,-NHNO2,and-NO2substituted derivatives have higher density while-N3and -NH2substituted ones possess lower density than unsubstituted compounds,showing that the former groups enhance the density,especially for-NF2group,while -N3and-NH2have the opposite in fluence.
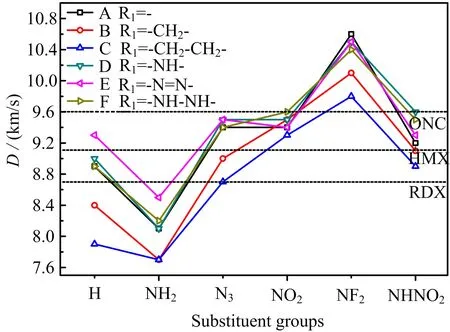
FIG.6 Detonation velocity of 36 designed compounds.
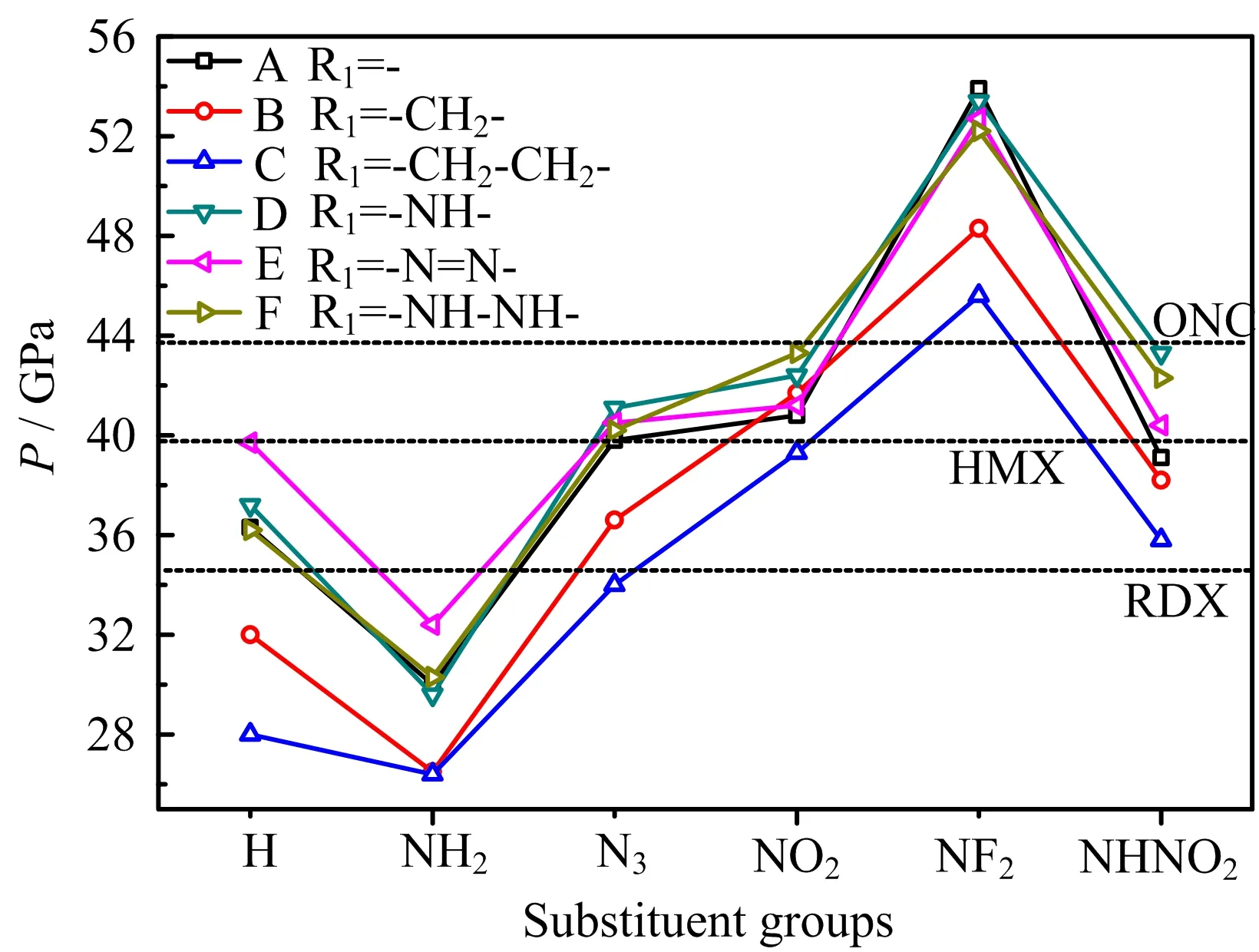
FIG.7 Detonation pressure of 36 designed compounds.
Since most of the designed compounds have relative ideal OB,high HOF,and high density,it may be expected that these molecules would have better detonation performance.D and P are two most common and important parameters used for judging the detonation properties of energetic compounds. They were calculated and depicted in FIG.6(D) and FIG.7(P). From them,it can be seen that, first of all,D and P decrease with the order of-NF2>-NHNO2≈-NO2>-N3>-H(unsubstituted)>-NH2substituted derivatives,showing that these former four energetic substituent groups are helpful for increasing the detonation performance while-NH2group has the opposite effect. Then,generally,D and P increase with the order of-CH2−CH2-,-CH2-, -(directly linked)≈-N=N-≈-NH−NH-<-NH-, indicating that-CH2−CH2-and-CH2-bridge groups decrease and-NH-bridge group increases the detonation performance,respectively,while-N=N-and-NH-NH-make little in fluence on them. Finally,27,21,and 8 designed compounds have better both D and P than RDX,HMX,and ONC,respectively,which shows the outstanding energy performance of designed molecules. This also indicates that tetrazole N-oxide is a useful parent energetic compound employed for obtaining high energy compounds,even only combined with some very common energetic substituent groups and bridge groups.
C.Sensitivity and electronic structure
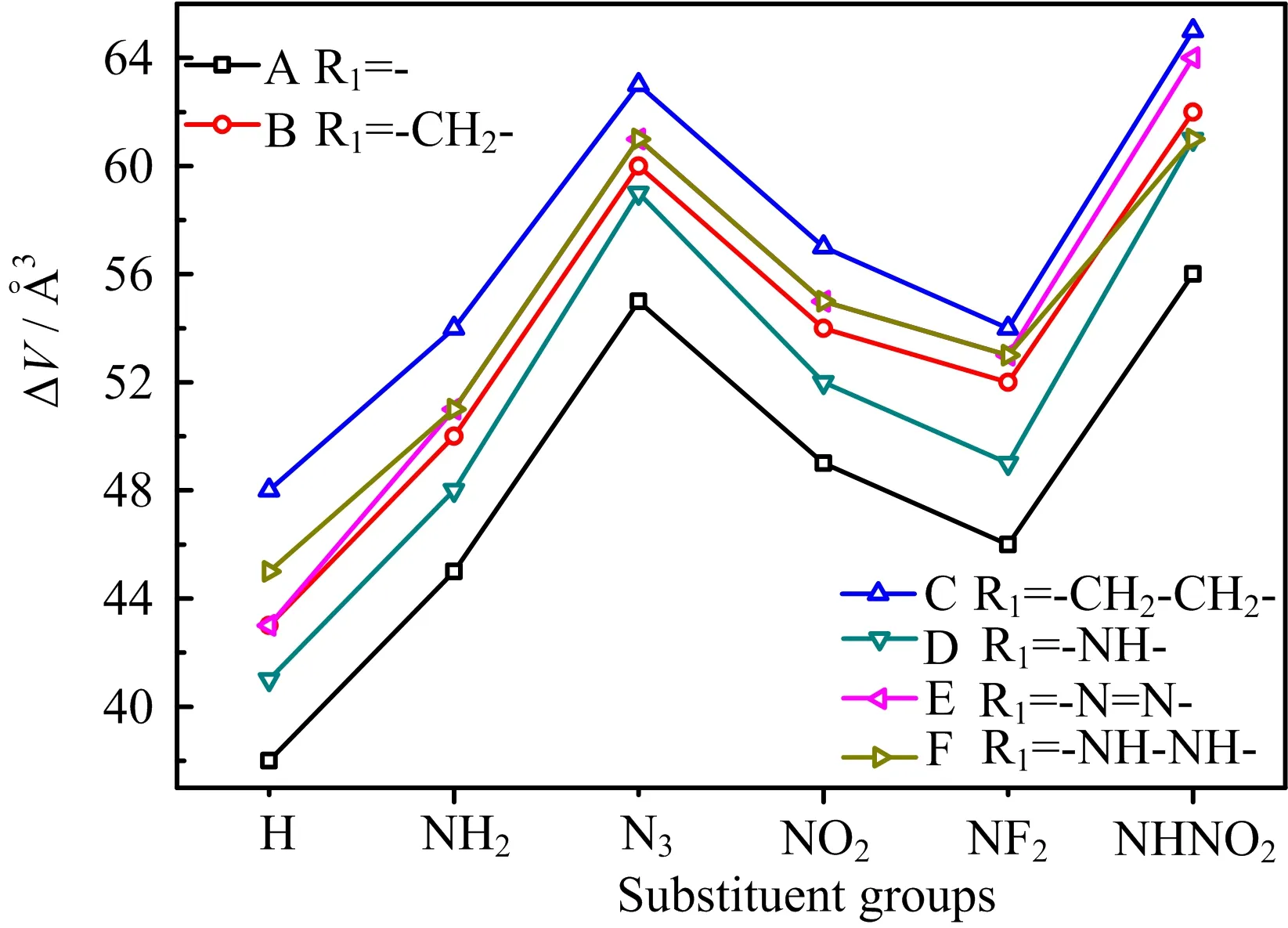
FIG.8 Free space per molecule in the unit cell of 36 designed compounds.
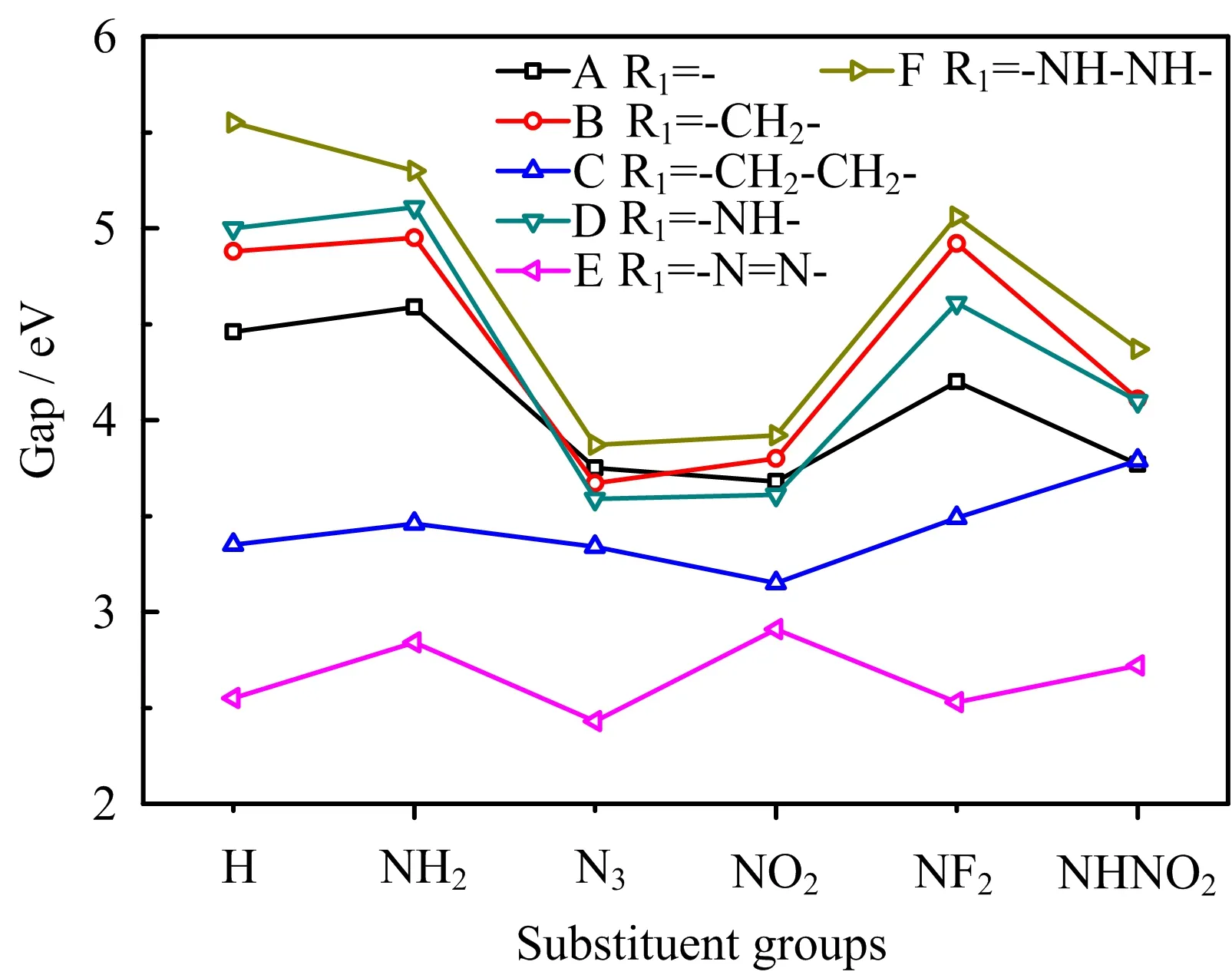
FIG.9 A comparison of energy gap of designed compounds.
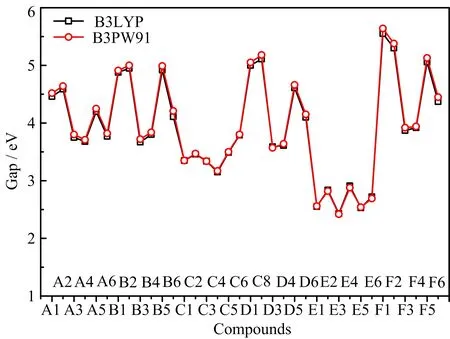
FIG.10 Energy gap of 36 designed compounds calculated by the B3LYP/6-31G(d,p)and B3PW91/6-31G(d,p)methods.
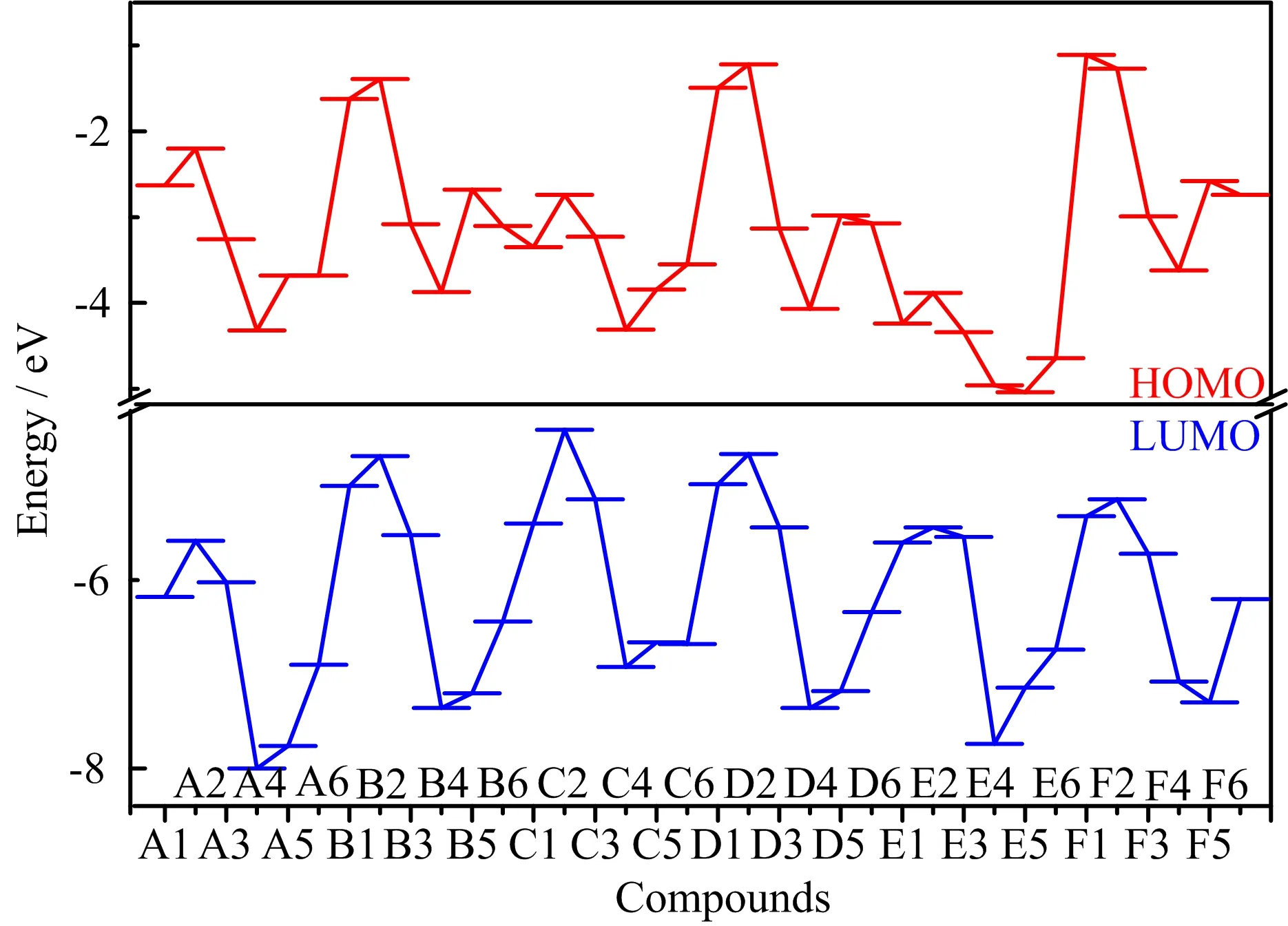
FIG.11 HOMO and LUMO of 36 designed compounds.
To study the sensitivity,the free space per molecule in the unit cell(∆V)is used[24,25].Generally,a larger∆V value means a higher sensitivity.For instance,the∆V values[25]of hexanitrohexaazaisowurtzitane(CL-20)and HMX are 86 and 49˚A3,respectively,while their h50(impact sensitivity)[31]are 14 and 29 cm,respectively. The∆V of designed compounds were calculated and displayed in FIG.8. First,it is seen that the∆V increases with the order of-H(unsubstituted)<-NH2<-NF2<<-NO2<-N3<-NHNO2substituted compounds,showing that all substituted groups increase the sensitivity,especially for -N3and-NHNO2group. Then,the∆Vincreases with the sequence of-(directly linked)<-NH-<-CH2-<-NH−NH-<-N=N-<-CH2-CH2-bridged compounds, suggesting that all bridge groups increase the sensitivity,especially for-N=N-and-CH2-CH2-bridge groups. Finally,these compounds have∆V values ranged from 38˚A3to 65˚A3,lower than that of CL-20 and close to HMX,showing the sensitivity of them is acceptable.
The energy gap between highest occupied molecular orbital(HOMO)and lowest unoccupied molecular orbital(LUMO)energies has been also used to investigate the activity and sensitivity of energetic compounds[32−34].Generally,the larger the gap is,the less activity or sensitivity the compound. The energy gap values of designed compounds were calculated and depicted in FIG.9.It can be seen that the gap decreases with the order of-N3>-NO2>-NHNO2>-NF2>-NH2≈-H substituted compounds,indicating that the energetic substituent groups increase the activity and sensitivity in general,which is consistent with the∆V result. Then,the gap decreases with the sequence of-NH−NH->-NH-≈-CH2-≈-(directly linked)>-CH2−CH2->-N=N-bridged compounds,showing that-CH2-CH2-and-N=N-bridge groups increase the activity and sensitivity obviously, which is in agreement with the∆V result mainly.This above analysis shows that-N3substituent group and -CH2-CH2-and-N=N-bridge groups may be not suitable for combining with tetrazole N-oxide to design new energetic compounds with good overall properties,since they would increase the sensitivity obviously.FIG.10 depicts the energy gap calculated by the B3LYP/6-31G(d,p)and B3PW916-31G(d,p)methods,it can be seen that predicted values of each compound by two methods are very close and the overall variation tendency are the same.A further comparison of the effects of different bridge groups on HOMO and LUMO is displayed in FIG.11.It is seen that-CH2-CH2-bridged compounds(series C)and-N=N-bridged groups(series E)have the highest HOMO and lowest LUMO,respec-tively,showing that the former bridge group increase the HOMO while the later one decrease the LUMO of tetrazole N-oxide obviously,this is a main reason why these two series have the lowest energy gap and highest activity and sensitivity.
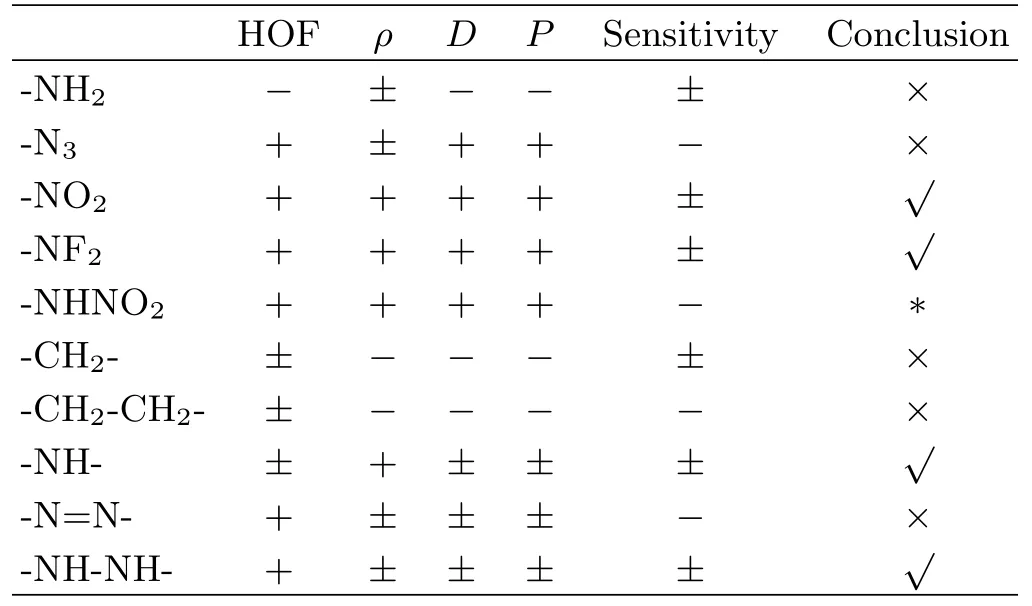
TABLE I The effects of different substituent groups and bridge groups on the HOF,ρ,D,P and sensitivity.
A overall summary for the effects of different substituent groups and bridge groups on the HOF,ρ,D, P and sensitivity compared to unsubstituted and directly linked compounds,is listed in Table I.Comprehensively considering,-NO2and-NF2are two appropriate substituent groups used for combining tetrozale N-oxide to design new energetic compounds while-NH2and-N3are inappropriate.For the-NHNO2group,it is a debatable group since it would both increase the sensitivity and detonation performance.The-NH-and -NH-NH-are two appropriate bridge groups used to link two tetrazole N-oxide rings,while-CH2-,-CH2-CH2-and-N=N-are inappropriate.In addition,the directly linked derivatives also possess good overall performance,thus,two tetrazole N-oxide rings being linked directly may be an available alternative.
IV.CONCLUSION
We designed six series of new carbon-linked ditetrazole 2N-oxides with different energetic substituent groups(-NH2,-N3,-NO2,NF2,-NHNO2)and energetic bridge groups(-CH2-,-CH2−CH2-,-NH-,-N=N-,-NH−NH-)with OB around zero.The effects of different energetic substituent groups and energetic bridge groups on the performance were studied by using the DFT and ESP methods. The results show that, first of all,most of designed compounds have OB between−30%and 30%and HOF higher than ONC; furthermore,-N3,-NO2,-NF2,-NHNO2,-N=N-and-NH−NH-groups are helpful for improving HOF.Then, most of them have higher density and better detonation performance than RDX and HMX,some of them even possess comparable energetic properties with ONC.The -NF2,-NHNO2,-NO2and-NH-groups are very helpful for increasing the detonation performance.Finally, the designed compound have receivable sensitivity and -N3,-N=N-and-CH2−CH2-could greatly increase the sensitivity and activity.In a word,-NO2,-NF2,-NH-and-NH−NH-are four appropriate substituent groups used for combining tetrozale N-oxide to design new energetic compounds while-NH2,-N3,-CH2−CH2-and -N=N-are inappropriate.
V.ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of Nanjing Institute of Technology (YKJ201507,CKJA201603)and the Youth Natural Science Foundation of Jiangsu Province(BK20160774), and Outstanding Scienti fic and Technological Innovation Team in Colleges and Universities of Jiangsu Province.
[1]X.X.Zhao,S.H.Li,Y.Wang,Y.C.Li,F.Q.Zhao, and S.P.Pang,J.Mater.Chem.A 4,5495(2016).
[2]M.Zheng,X.H.Li,H.L.Cui,and R.Z.Zhang,Chin. J.Chem.Phys.29,349(2016).
[3]Q.Wu,W.H.Zhu,and H.M.Xiao,J.Mater.Chem. A 2,13006(2014).
[4]J.H.Zhang and J.M.Shreeve,J.Am.Chem.Soc.136, 4437(2014).
[5]J.H.Zhang,Q.M.Zhang,T.T.Vo,D.A.Parrish,and J.M.Shreeve,J.Am.Chem.Soc.137,1697(2015).
[6]T.M.Klap¨otke,P.C.Schmid,S.Schnell,and J.Stierstorfer,J.Mater.Chem.A 3,2658(2015).
[7]P.Yin and J.M.Shreeve,Angew.Chem.Int.Ed.54, 14513(2015).
[8]C.B.Aaker¨oy,T.K.Wijethunga,and J.Despe,Chem. Eur.J.21,11029(2015).
[9]C.Y.Zhang,X.G.Xue,Y.F.Cao,J.H.Zhou,A. B.Zhang,H.Z.Li,Y.Zhou,R.J.Xu,and T.Gao CrystEngComm 16,5905(2014).
为了促进水产养殖行业的健康发展,生产出更多绿色无污染的产品,在实际工作中,养殖户要在自动化养殖的基础上,融入环保理念,减少药物的应用。此外,还可以研发和使用绿色环保型药物,减少水产养殖中水体污染问题的发生,保证水体质量。目前,我国已经在绿色环保型药物的研究上取得了一定的成绩,这将会进一步推动水产养殖行业健康发展[2]。
[10]D.Hong,Y.Li,S.Zhu,L.Zhang,and C.Pang,Cent. Eur.J.Energ.Mater.12,47(2015).
[11]Q.H.Zhang and J.M.Shreeve,Angew.Chem.Int.Ed. 53,2540(2014).
[12]Y.Shang,B.Jin,R.F.Peng,Q.Q.Liu,B.S.Tan,and Z.C.Guo,J.Zhao,and Q.C.Zhang,Dalton Trans. 45,13881(2016).
[13]Q.Yang,X.X.Song,G.W.Zhao,G.L.Yang,L.L. Yang,Q.Wei,G.Xie,S.P.Chen,and S.L.Gao,Eur. J.Inorg.Chem.31,5052(2016).
[14]D.Fischer,T.M.Klap¨otke,D.G.Piercey,and J. Stierst¨orfer,Chem.Eur.J.19,4602(2013).
[15]N.Fischer,D.Fischer,T.M.Klap¨otke,D.G.Piercey, and J.Stierst¨orfer,J.Mater.Chem.22,20418(2012).
[16]B.Yuan,Z.J.Yu,and E.R.Bernstein,J.Phys.Chem. A 119,2965(2015).
[17]Q.An,T.Cheng,W.A.Goddard III,and S.V.Zybin, J.Phys.Chem.C 119,2196(2015).
[18]V.P.Sinditskii,S.A.Filatov,V.I.Kolesov,K.O. Kapranov,A.F.Asachenko,M.S.Nechaev,V.V. Lunin,and N.I.Shishov,Thermochim.Acta 614,85 (2015).
[19]N.Fischer,L.Gao,T.M.Klap¨otke,and J.Stierst¨orfer, Polyhedron 51,201(2013).
[20]M.J.Kamlet and S.J.Jacobs,J.Chem.Phys.48,23 (1968).
[21]P.Politzer,J.Martinez,J.S.Murray,M.C.Concha, and A.Toro-Labb´e,Mol.Phys.107,2095(2009).
[22]P.W.Atkins,Physical Chemistry,Oxford:Oxford University Press,(1982).
[23]E.F.C.Byrd and B.M.Rice,J.Phys.Chem.A 110, 1005(2006).
[24]M.Posp´ıˇs´ıl,P.V´avra,M.C.Concha,J.S.Murray,and P.Politzer,J.Mol.Model.17,2569(2011).
[25]P.Politzer and J.S.Murray,J.Mol.Model.20,2223 (2014).
[26]M.J.Frisch,G.W.Trucks,H.B.Schlegel,G.E.Scuseria,M.A.Robb,J.R.Cheeseman,V.G.Zakrzewski,J. A.Montgomery,R.E.Stratmann,J.C.Burant,S.Dapprich,J.M.Millam,A.D.Daniels,K.N.Kudin,M.C. Strain,O.Farkas,J.Tomasi,V.Barone,M.Cossi,R. Cammi,B.Mennucci,C.Pomelli,C.Adamo,S.Clifford,J.Ochterski,G.A.Petersson,P.Y.Ayala,Q. Cui,K.Morokuma,D.K.Malick,A.D.Rabuck,K. Raghavachari,J.B.Foresman,J.Cioslowski,J.V.Ortiz,A.G.Baboul,B.B.Stefanov,G.Liu,A.Liashenko, P.Piskorz,I.Komaromi,R.Gomperts,R.L.Martin, D.J.Fox,T.Keith,M.A.Al-Laham,C.Y.Peng,A. Nanayakkara,C.Gonzalez,M.Challacombe,P.M.W. Gill,B.Johnson,W.Chen,M.W.Wong,J.L.Andres, C.Gonzalez,M.Head-Gordon,E.S.Replogle,and J. A.Pople,Gaussian 09,Revision A.01.Pittsburgh,PA: Gaussian,Inc.(2009).
[27]A.M.Astakhov,R.S.Stepanov,and A.Y.Babushkin, Combust.Explos.Shock Waves 34,85(1998).
[28]W.A.Trzcin´ski,S.Cudzilo,Z.Chylek,and L. Szyman´czyk,J.Hazard.Mater.157,605(2008).
[29]Q.Wu,W.H.Zhu,and H.M.Xiao,RSC Adv.4,3789 (2014).
[30]M.X.Zhang,P.E.Eaton,and R.Gilardi,Angew. Chem.Int.Ed.39,401(2000).
[31]B.M.Rice and J.J.Hare,J.Phys.Chem.A 106,1770 (2002).
[32]W.H.Zhu and H.M.Xiao,Struct.Chem.21,657 (2010).
[33]Q.Wu,W.H.Zhu,and H.M.Xiao,J.Mol.Model.19, 4039(2013).
[34]Q.Wu,W.H.Zhu,and H.M.Xiao,J.Phys.Chem.C 117,16830(2013).
ceived on March 27,2017;Accepted on June 3,2017)
∗Author to whom correspondence should be addressed.E-mail: clx@njit.edu.cn,Tel.:+86-25-86118274
猜你喜欢
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- γ-Ray Irradiation-Derived MnO/rGO Composites for High Performance Lithium Ion Batteries
- Identi fication of Superoxide O2−during Thermal Decomposition of Molten KNO3-NaNO2-NaNO3Salt by Electron Paramagnetic Resonance and UV-Vis Absorption Spectroscopy
- Binding Mechanism and Molecular Design of Benzimidazole/Benzothiazole Derivatives as Potent Abl T315I Mutant Inhibitors
- Highly Responsive and Selective Ethanol Gas Sensor Based on Co3O4-Modi fied SnO2Nano fibers
- Geometric Design of Anode-Supported Micro-Tubular Solid Oxide Fuel Cells by Multiphysics Simulations
- Laser-Assisted Stark Deceleration of Polar Molecules HC2n+1N(n=2,3,4) in High-Field-Seeking State
