Hidden Relaxation Channels in Aqueous Methylene Blue after Functionalization of Graphene Oxide Probed by Transient Absorption Spectroscopy
2017-09-03DaKeLaizhiSuiDunliLiuYusuWangSuyuLiYuanfeiJiangAnminChenMingxingJin
Da Ke,Lai-zhi Sui,Dun-li Liu,Yu-su Wang,Su-yu Li,Yuan-fei Jiang,An-min Chen, Ming-xing Jin
Institute of Atomic and Molecular Physics,Jilin University,Changchun 130012,China;Jilin Provincial Key Laboratory of Applied Atomic and Molecular Spectroscopy,Jilin University,Changchun 130012, China
Hidden Relaxation Channels in Aqueous Methylene Blue after Functionalization of Graphene Oxide Probed by Transient Absorption Spectroscopy
Da Ke†,Lai-zhi Sui†,Dun-li Liu,Yu-su Wang,Su-yu Li,Yuan-fei Jiang,An-min Chen∗, Ming-xing Jin∗
Institute of Atomic and Molecular Physics,Jilin University,Changchun 130012,China;Jilin Provincial Key Laboratory of Applied Atomic and Molecular Spectroscopy,Jilin University,Changchun 130012, China
The mixture of graphene oxide(GO)and dye molecules may provide some new applications due to unique electronic,optical,and structural properties.Methylene blue(MB),a typical anionic dye,can attach on GO via π-π stacking and electrostatic interaction,and the molecule removal process on GO has been observed.However,it remains unclear about the ultrafast carrier dynamics and the internal energy transfer pathways of the system which is composed of GO and MB.We have employed ultrafast optical pump-probe spectroscopy to investigate the excited dynamics of the GO-MB system dispersed in water by exciting the samples at 400 nm pump pulse.The pristine MB and GO dynamics are also analyzed in tandem for a direct comparison.Utilizing the global analysis to fit the measured signal via a sequential model, five lifetimes are acquired:(0.61±0.01)ps,(3.52±0.04)ps,(14.1±0.3)ps, (84±2)ps,and(3.66±0.08)ns.The ultrafast dynamics corresponding to these lifetimes was analyzed and the new relaxation processes were found in the GO-MB system,compared with the pristine MB.The results reveal that the functionalization of GO can alter the known decay pathways of MB via the energy transfer from GO to MB in system,the increased intermediate state,and the promoted energy transfer from triplet state MB to ground state oxygen molecules dissolved in aqueous sample.
Transient absorption spectroscopy,Methylene blue,Functionalization of graphene oxide,Relaxation channels
I.INTRODUCTION
Graphene is a two-dimensional array material consisting of sp2hybridized carbon atoms arranged in a hexagonal lattice.Owing to its distinctive structure, graphene exhibits many unusual properties and potential applications in a variety of fields such as electronics, composites,sensors,and energy related systems[1−6]. Following the exciting research of graphene,in recent years,graphene oxide(GO)has attracted increasing research attention due to the candidate status instead of graphene in some aspects and promising applications [7−11].As the analogue of graphene with high carrier transport mobility,GO is more suitable for some electronics and optics applications because of the plentiful oxygen-containing functional groups which can interact with electron donors and acceptors such as dyes,polymers,and nanoparticles,than graphene that lacks sp3hybridized carbon atoms[12−15].Thus,recently,GO and dye composites have been widely explored for the removal of dye molecules and the investigations of the absorption behavior of the dye molecules onto the GO have been reported by different groups[16−22].The optical features of GO will alter evidently after mixing some dyes and meanwhile the photochemical properties of the dye molecules will also change due to the different GO functionalized processes.Till now,although much progresses have been achieved in related studies, the ultrafast carrier dynamics and the internal energy transfer pathways of the system which is composed of GO and the dye molecules are still a controversial issue.
Methylene blue(MB),a typical anionic dye,is used widely in biological and industrial applications such as assay for nucleic acids,protein determination,controlled drug release,color fiber,paints textiles,and so forth[23−26].In MB and GO composites,the removal process of the dye molecules at the GO has been observed and the mechanism of the adsorption behavior was finally explicated.MB molecules can attach on GO via π-π stacking and electrostatic interaction,andthe Benesi-Hildebrand method was adopted to study the interaction of MB and GO in the water system[27, 28].However,the mechanism of ultrafast carrier relaxation of GO and MB composites has still not been completely interpreted.Transient absorption spectroscopy (TAS)has been provn to be one of the most versatile techniques for studying ultrafast processes in physics, chemistry,and biology[29].Transient absorption properties can expose some hidden information on GO and dye composites to help us understand their mysterious ultrafast carrier relaxation dynamics and explore a new application prospect in some fields.
In the present work,we focus on the ultrafast carrier kinetic process of the system which is composed of GO and MB molecules studied by femtosecond timeresolved transient absorption spectroscopy.The transient absorption spectra of GO and MB composites in water,including both positive and negative absorbance changes,are observed via a broad probe region.Steady state absorption spectra measurements have also been conducted in order to help us interpret the related mechanism.We adopt the sequential kinetic model to globally fit the experiment data.The related electronic transition channels responsible for ground state bleaching,excited state absorption,and stimulated emission processes are discussed in detail.
II.EXPERIMENTS
The aqueous GO sample with a concentration of 1 mg/mL is purchased from Hengqiu Tech.Inc.The MB sample is purchased from Aladdin and is diluted in water to a concentration of 0.1 mmol/L.The third sample is prepared by mingling the above aqueous GO and the aqueous MB with the equal proportion.The pH value of both the aqueous GO and the aqueous MB is 5.
Steady-state absorption spectra are measured by a spectrometer(AvaSpec-1650F-USB2).The system of time-resolved transient absorption measurement[30]is carried out using a regeneratively ampli fied Ti:sapphire laser system(Coherent Libra)to provide the fundamental light source. The output of the ampli fier of 2 mJ pulse energy,50 fs pulse width,1000 Hz repetition rate,at 800 nm wavelength is split into two parts. The stronger beam is used to generate the pump pulses (400 nm)by using a beta barium borate(BBO)crystal.The broadband white light continuum probe pulses from 450 nm to 750 nm are generated by focusing another beam into a 2 mm thick sapphire plate.The pump and probe beams are overlapped on the sample which is put into quartz cuvette(1 mm).The pump beam at the sample has a diameter of 1 mm and the diameter of the probe beam is 300µm.The signals of probe pulse are collected by a fiber-coupled spectrometer(AvaSpec-1650F-USB2)connected to a computer after passing through the sample.A neutral density optical filter is used to adjust the energy of the 400 nm pump pulse to about 3µJ per pulse and the excitation pulse is chopped at 500 Hz.The width of IRF is 100 fs and the group velocity dispersion effect of the transient spectra is compensated by a home-made chirp program. All of the experimental measurements are performed at room temperature.
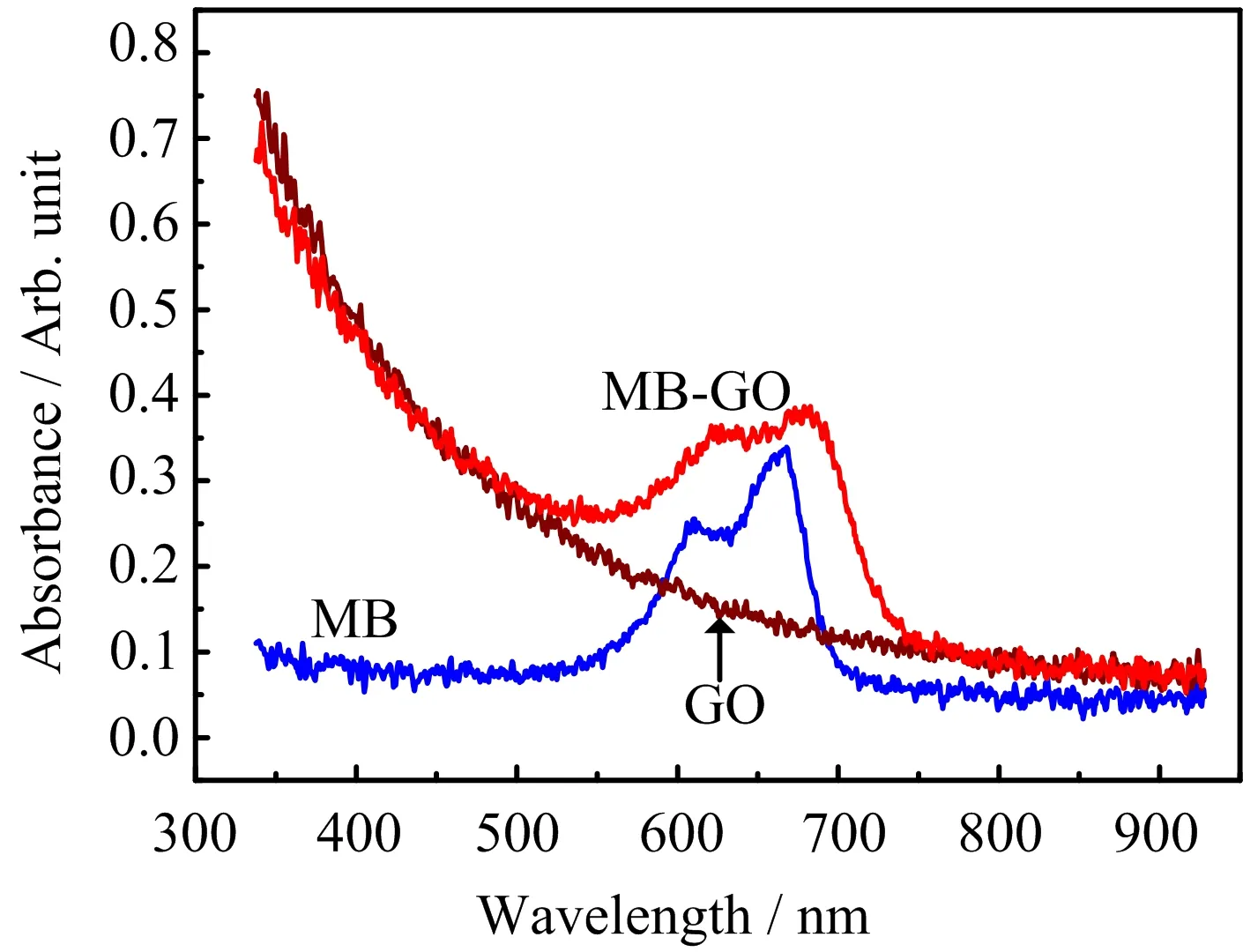
FIG.1 Absorption spectra of aqueous MB,aqueous GO, aqueous MB,and GO composites in a 1 mm cuvette.
III.RESULTS AND DISCUSSION
FIG.1 presents the absorption spectra of three samples in 1 mm cuvtte ranging from the UV to near infrared range.For MB,the main peak at~670 nm comes from the n-π∗transition in dilute aqueous solution[31],this is exactly consistent with the characteristic of the MB monomer in solution.The shoulder peak at 610 nm is assigned to MB dimerization in solution and the absorbance of MB almost disappears before 550 nm and after 700 nm[32].Different from MB,the absorption spectrum of GO has a relatively wide absorption band and is peaked at~300 nm,corresponding to n-π∗transition of the C=O bond in sp3hybrid regions of carbon based materials[33,34].As shown in FIG.1,the absorbance of GO decreases gradually after 300 nm and these absorption features are typical for as-prepared aqueous GO[8].In addition,the absorption spectra of the composites exhibit two maxima at~630 and 690 nm,which are assigned to adsorbed MB dimer and monomer respectively[32].Here,it can been seen that the composites show red-shifted absorption peaks,compared to the aqueous MB,it indicates that new aggregations form after mixing MB and GO. The new aggregations mainly include two types:the aggregation of the MB monomer(MB-GO)and the MB dimer(MB2-GO)on the GO surface,they are in full agreement with the findings from the UV-Vis measurements and related study[31].
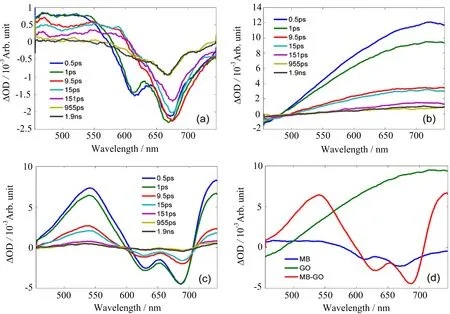
FIG.2 Transient absorption spectra of(a)aqueous MB,(b)aqueous GO,(c)aqueous MB and GO composites with different delay times after 400 nm excitation,(d)the above samples at 1 ps.
The transient absorption spectra of aqueous MB and aqueous GO recorded in the 450−750 nm spectral region and in the 0−2 ns time window are shown in FIG.2(a)and(b). There is an apparent negative signal at about 670 nm and a broad absorption band in the 450−560 nm region in FIG.2(a).The former agrees well with the UV-visible absorption spectra of MB and can be mainly attributed to the ground state bleaching.For GO,a broad excited state absorption signal from around 470 nm to 750 nm is observed in FIG.2(b),which indicates that the photo-excited carriers absorb probe light being promoted into higher excited state levels and produce an excitation of higher energy.As shown in FIG.2(c),the transient absorption spectra of aqueous MB and GO composites exhibit two obvious features,a broad excited state absorption peak at 540 nm and a broad bleaching band at about 670 nm.It has already been con firmed that MB molecules can attach on the surface of GO by π-π stacking and electrostatic interaction to form the aggregations,and the energy transfer process can occur in the aggregation system[35−37].In order to expediently see the change after mixing MB and GO,the transient absorption spectra of the above three samples at 1 ps are shown in FIG.2(d).Here,it can be found that MB and GO composites show an enhanced absorption signal around 540 nm,compared to the cumulative signal of pure aqueous MB and GO in FIG.2(a)and(b). It is suggested that MB molecules acquire the energy from GO molecules in these aggregations after 400 nm excitation to increase the yield of the excited carriers, which are promoted to excited state levels from ground state levels,thus more excited state absorption signals are observed in the measurement and the results agree well with the previous studies[32,35,49].Furthermore, MB-GO composites also exhibit more intense bleaching signals around 670 nm because of the enhanced efficiency of ground state to excited state transition of MB in the aggregations.This phenomenon reveals the fact that the negative signal of ground state bleaching of MB is much more intense than the positive signal of excited state absorption of GO in the aggregations.
In order to grasp further kinetic traces of the system which is composed of GO and MB molecules,the dynamic decay curves of aqueous MB,aqueous GO,and aqueous MB and GO composites at the center wavelength(540 nm)are shown in FIG.3(a).These curves have been normalized to unity at 2 ns in logarithmic scale for a better comparison of their changes over time. As known from FIG.1(a)and FIG.2(a),the excited state absorption of MB at 540 nm is much weaker than GO due to the negligible absorption of the 400 nm pump pulse,and the absorption signals of MB should be covered approximately by the signals of GO in the MB and GO composites.However,the curve that represents the excited state absorption recovery of the MB and GO composites becomes slower than the one of the GO in FIG.3(a).It indicates that new aggregation molecules are formed by mixing MB and GO,thus MB molecules make a much greater contribution to the overall excited state absorption signals of the composites at around 540 nm via acquiring the energy from the aggregation system than pure aqueous MB.The intersystem energy transfer processes occur after being excited by the 400 nm laser pulse and this is in agreement with our former explanation.The recovery kinetics traces at 670 nm for the three specimens are compared in FIG.3(b).It can be found that the recovery of the MB and GO composites is obviously faster thanthe bleach recovery of MB,indicating that not all signals in the dimer system derive from the ground state bleaching of the MB in the aggregation.The signals of the composites at 670 nm mainly include,the excited state absorption of the GO,the ground state bleaching of the MB which absorbs energy from pump pulse directly and harvests energy from the aggregation system indirectly.
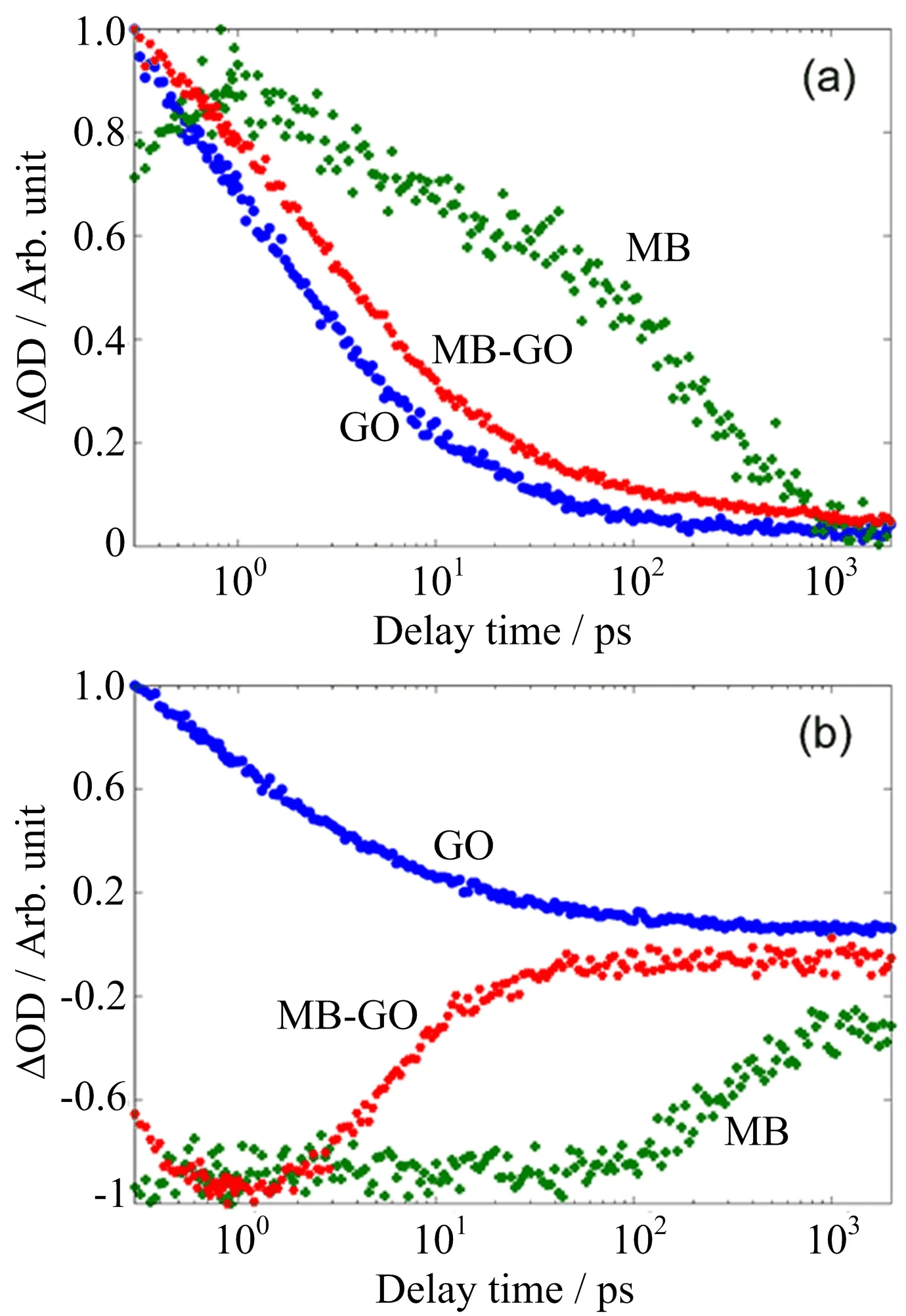
FIG.3 Normalized transient decay dynamics of aqueous MB,aqueous GO and aqueous MB and GO composites at (a)540 nm and(b)670 nm after being excited by the 400 nm laser pulse.
To investigate the evolution of the excited or intermediate states of the system more clearly,the transient absorption signals are analyzed within a global fitting framework[38,39].As shown in FIG.4(a)−(c),the evolution associated difference spectra(EADS)of the three samples are achieved by using a sequential kinetic scheme with increasing time to fit globally the experiment data.In the case of the aqueous MB, five components are necessary to fit the data with lifetimes of(0.19±0.01)ps,(2.84±0.04)ps,(51±3)ps, (261±4)ps,and>1 ns.It has been proven that S1state splits into the upper excitonic state(S+)and the lower state(S−)in the MB dimer due to the addition of the in-phase transition dipole moments of constituent monomers[40−43].The first EADS correspond to MB dimers S∗+and the second EADS correspond to S∗−. The time constant of 0.19 ps corresponds torelaxation,in excellent agreement with the previous study which speculates internal conversion within exciton state to occur in extremely rapid time(<1 ps)[44]. The later three component represent S∗1→S1,S1→T1, and T1→T0of MB monomer respectively[26,45,46]. Previously,the triplet state lifetime of MB system had been shown(>1.0µs)in Ref.[26].And the experimental data are in full agreement with another recent report[44].For GO,the transient data can be fitted globally with four time constants of(0.72±0.01)ps, (4.8±0.1)ps,(48±1)ps,>1 ns and these results correspond to the related reports in the previous pump-probe studies on GO[47,48].Furthermore,we still utilize the five population sequential reaction model to resolve the detailed relaxation process of the excited state in the MB-GO aggregation.Five lifetimes are acquired via fitting of(0.61±0.01)ps,(3.52±0.04)ps,(14.1±0.3)ps, (84±2)ps,and 3.66±0.08 ns.In FIG.4(c),the first EADS corresponds to MB2-GO S∗−and the S∗+→S∗−relaxation occur within 0.61 ps,which is almost three times slower than the same process in the pure MB2.It is inferred that a large proportion of energy which promotes carriers from S0into S∗+in MB2-GO,is derived from GO of MB2-GO,since GO can directly absorb the most energy from 400 nm excitation.And the process that energy transfer from GO to MB in MB2-GO system is accomplished about within 0.61 ps.The second lifetime of 3.52 ps almost is only longer 0.68 ps than MB2due to intersystem energy transfer,indicating that the decay pathways of MB2in MB2-GO system is the same as the pure MB2,both are internal conversion from excited state to the ground state.The third and fourth components represent S∗1→S1,S1→T1of MB-GO respectively,they are signi ficantly faster than MB and it implies the other potential channels that can increase the rate of decay of the singlet state of MB in MB-GO system.The phenomenon might be explained as the increased intermediate state between the highest occupied molecular orbital and the lowest unoccupied molecular orbital(HOMO-LUMO)of the MB-GO due to interaction between MB and GO containing a mixture of sp2and sp3carbons[49].And it is also in agreement with the red-shifted absorption peaks of MB-GO in comparison to MB in the absorption spectra measurement. The dynamic models of MB and MB-GO are shown in FIG.5.The last component corresponds to the decay of triplet state MB in MB-GO system,it is much faster than in pure MB system,this reveals that the decay pathways of triplet state MB also cause changes after combining functionalized GO.For triplet state MB,two major photochemical pathways have been observed:the triplet energy is transferred to oxygen forming singlet oxygen(1O2)and the triplet decay to the ground state via intersystem crossing[26,50−52].Wojtoniszak et al.have found that GO functionalized with MB(GOMB)shows enhanced efficiency in singlet oxygen generation compared to pristine MB[34].But they still have not come up with enough convincing explanation of this phenomenon.By comparing the decay lifetimes of triplet state MB in two samples,we speculate that adsorption of MB on GO can promote the energy transfer from triplet state MB to ground state oxygen molecules dissolved in aqueous sample and increase the proportion of this decay channel.
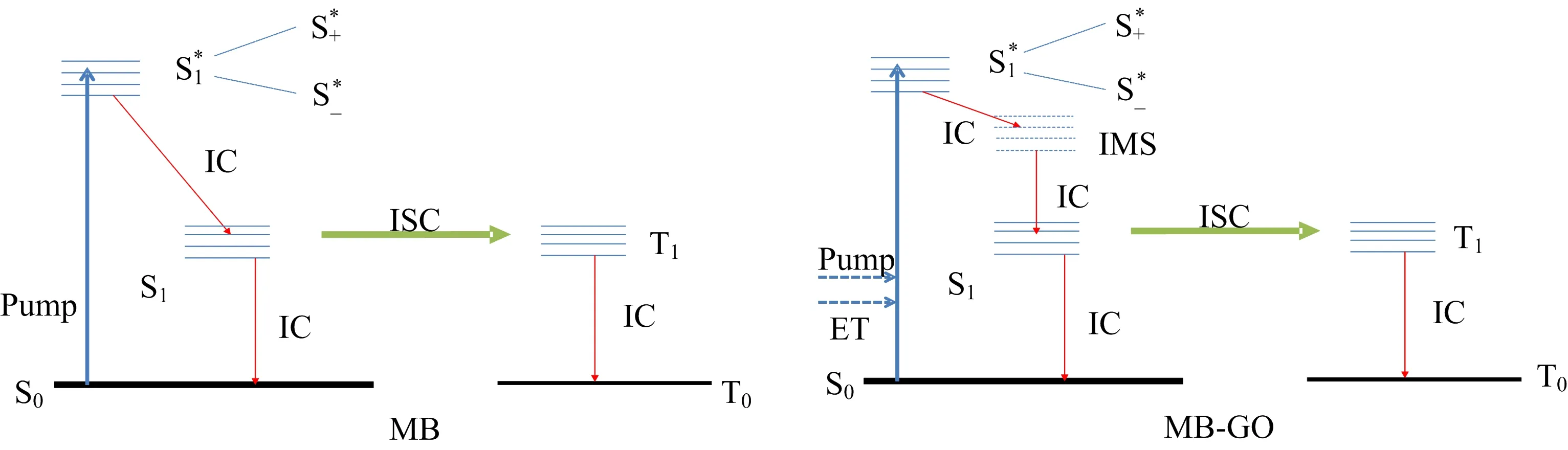
FIG.5 Dynamic models of MB and MB-GO.Pump:400 nm excitation,IC:internal conversion,ISC:intersystem crossing, ET:energy transfer from GO to MB,IMS:intermediate state.
IV.CONCLUSION
We have demonstrated the ultrafast carrier relaxation of MB and GO composites by the femtosecond time-resolved transient absorption spectra.Because of electrostatic interaction and π-π stacking,MB and GO form a new aggregation system in water,which changes the properties of dye molecules.The alterations in the aggregation system are monitored by steady-state and transient absorption spectroscopy measurements.The relaxation process and energy transfer channels are analyzed by using the sequential kinetic model to globally fit the experiment data.It is found that the relaxation rate of MB2-GO is slower than that of MB2, which is attributed to the internal energy transfer in the aggregation system.In addition,the increased intermediate state between the HOMO-LUMO results in the new photochemical pathways and increase the rate of decay of the singlet state of MB in MB-GO system. We infer that GO functionalized with MB may promote the intersystem energy transfer from triplet state MB to ground state oxygen.And it can be a reasonable explanation to this observation that GO-MB shows enhanced efficiency in singlet oxygen generation compared with pristine MB.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Basic Research Program of China(No.2013CB922200), the National Natural Science Foundation of China (No.11674128,No.11474129,and No.11504129),Jilin Province Scienti fic and Technological Development Program,China(No.20170101063JC),the Thirteenth Five-Year Scienti fic and Technological Research Project of the Education Department of Jilin Province,China (No.400).
[1]D.B.Lu,C.G.Luo,Y.L.Song,Q.N.Pan,and C.Y. Pu,Chin.J.Chem.Phys.29,205(2016).
[2]J.H.Chen,X.Y.Feng,W.F.Chen,Y.Q.Song,and L.F.Yan,Chin.J.Chem.Phys.30,112(2017).
[3]L.J.Liang,Q.Wang,T.Wu,J.W.Shen,and Y.Kang, Chin.J.Chem.Phys.22,627(2009).
[4]L.S.Li and X.Yan,J.Phys.Chem.Lett.1,2572 (2010).
[5]A.J.Du and S.C.Smith,J.Phys.Chem.Lett.2,73 (2011).
[6]P.V.Kamat,J.Phys.Chem.Lett.2,242(2011).
[7]D.R.Dreyer,S.Park,C.W.Bielawski,and R.S.Ruo ff, Chem.Soc.Rev.39,228(2009).
[8]G.Eda and M.Chhowalla,Adv.Mater.22,2392 (2010).
[9]C.Mattevi,G.Eda,S.Agnoli,S.Miller,K.A. Mkhoyan,O.Celik,D.Mastrogiovanni,G.Granozzi, E.Garfunkel,and M.Chhowalla,Adv.Funct.Mater. 19,2577(2009).
[10]D.A.Dikin,S.Stankovich,E.J.Zimney,R.D.Piner, G.H.B.Dommett,G.Evmenenko,S.T.Nguyen,and R.S.Ruo ff,Nature 448,457(2007).
[11]R.R.Nair,H.A.Wu,P.N.Jayaram,I.V.Grigorieva, and A.K.Geim,Science 335,442(2012).
[12]X.Wang,L.J.Zhi,and K.M¨ullen,Nano Lett.8,323 (2008).
[13]C.M.Hill,Y.Zhu,and S.L.Pan,ACS Nano 5,942 (2011).
[14]I.V.Lightcap and P.V.Kamat,J.Am.Chem.Soc. 134,7109(2012).
[15]G.Katsukis,J.Malig,C.Schulz-Drost,S.Leubner,N. Jux,and D.M.Guldi,ACS Nano 6,1915(2012).
[16]X.Y.Peng and F.Gong,E-J.Chem.5,802(2008).
[17]P.Bradder,S.K.Ling,S.B.Wang,and S.M.Liu,J. Chem.Eng.Data 56,138(2011).
[18]G.K.Ramesha,A.V.Kumara,H.B.Muralidhara,and S.Sampath,J.Colloid Interface Sci.361,270(2011).
[19]T.H.Liu,Y.H.Li,Q.J.Du,J.K.Sun,Y.Q.Jiao, G.M.Yang,Z.H.Wang,Y.Z.Xia,W.Zhang,K.L. Wang,H.W.Zhu,and D.H.Wu,Colloids Surf.BBiointerfaces 90,197(2012).
[20]H.Liu,J.Gao,M.Q.Xue,N.Zhu,M.N.Zhang,and T.B.Cao,Langmuir 25,12006(2009).
[21]D.Wang,Y.G.Li,P.Hasin,and Y.Y.Wu,Nano Res. 4,124(2011).
[22]D.D.Zhang,L.Fu,L.Liao,B.Y.Dai,R.Zou,and C. X.Zhang,Electrochim.Acta 75,71(2012).
[23]P.R.Ginimuge and S.D.Jyothi,J.Anaesthesiol.Clin. Pharmacol.26,517(2010).
[24]R.H.Schirmer,B.Coulibaly,A.Stich,M.Scheiwein, H.Merkle,J.Eubel,K.Becker,H.Becher,O.M¨uller, T.Zich,W.Schiek,and B.Kouyat´e,Redox Rep.8,272 (2003).
[25]J.P.Tardivo,A.Del Giglio,C.S.De Oliveira,D.S. Gabrielli,H.C.Junqueira,D.B.Tada,D.Severino, R.D.F.Turchiello,and M.S.Baptista,Photodiagn. Photodyn.Ther.2,175(2005).
[26]J.Chen,T.C.Cesario,and P.M.Rentzepis,Chem. Phys.Lett.498,81(2010).
[27]D.Chen,H.B.Feng,and J.H.Li,Chem.Rev.112, 6027(2012).
[28]A.Wojcik and P.V.Kamat,ACS Nano 4,6697(2010).
[29]G.R.Fleming,Chemical Application of Ultrafast Spectroscopy,New York:Oxford University Press, 1986.
[30]L.Z.Sui,W.W.Jin,S.Y.Li,D.L.Liu,Y.F.Jiang, A.M.Chen,H.Liu,Y.Shi,D.J.Ding,and M.X.Jin, Phys.Chem.Chem.Phys.18,3838(2016).
[31]C.P´ark´anyi,C.Boniface,J.J.Aaron,and M.Maa fi, Spectrochim.Acta Part A 49,1715(1993).
[32]K.Haubner,J.Murawski,P.Olk,L.M.Eng,C.Ziegler, B.Adolphi,and E.Jaehne,ChemPhysChem.11,2131 (2010).
[33]T.V.Cuong,V.H.Pham,Q.T.Tran,S.H.Hahn,J. S.Chung,E.W.Shin,and E.J.Kim,Mater.Lett.64, 399(2010).
[34]Z.T.Luo,Y.Lu,L.A.Somers,and A.T.C.Johnson, J.Am.Chem.Soc.131,898(2009).
[35]M.Wojtoniszak,D.Rogi´nska,B.Machali´nski,M. Drozdzik,and E.Mijowska,Mater.Res.Bull.48,2636 (2013).
[36]H.C.Junqueira,D.Severino,L.G.Dias,M.S. Gugliotti,and M.S.Baptista,Phys.Chem.Chem. Phys.4,2320(2002).
[37]J.E.Huang,Z.Q.Huang,Y.Yang,H.M.Zhu,and T. Q.Lian,J.Am.Chem.Soc.132,4858(2010).
[38]I.H.M.van Stokkum,D.S.Larsen,and R.van Grondelle,Biochim.Biophys.Acta 1657,82(2004).
[39]J.J.Snellenburg,S.Laptenok,R.Seger,K.M.Mullen, and I.H.M.van Stokkum,J.Stat.Software 49,1 (2012).
[40]M.Kasha,Radiat.Res.20,55(1963).
[41]F.C.Spano,Acc.Chem.Res.43,429(2010).
[42]S.Verma,A.Ghosh,A.Das,and H.N.Ghosh,J.Phys. Chem.B 114,8327(2010).
[43]H.Yamagata and F.C.Spano,J.Chem.Phys.136, 184901(2012).
[44]J.C.Dean,D.G.Oblinsky,S.Ra fiq,and G.D.Scholes, J.Phys.Chem.B 120,440(2016).
[45]M.Enescu,L.Krim,L.Lindqvist,and T.Q.Wu,J. Photochem.Photobiol.B 22,165(1994).
[46]D.A.Dunn,V.H.Lin,and I.E.Kochevar,Photochem. Photobiol.53,47(1991).
[47]Z.B.Liu,X.Zhao,X.L.Zhang,X.Q.Yan,Y.P.Wu, Y.S.Chen,and J.G.Tian,J.Phys.Chem.Lett.2, 1972(2011).
[48]S.Kaniyankandy,S.N.Achary,S.Rawalekar,and H. N.Ghosh,J.Phys.Chem.C 115,19110(2011).
[49]G.Eda,Y.Y.Lin,C.Mattevi,H.Yamaguchi,H.A. Chen,I.S.Chen,C.W.Chen,and M.Chhowalla,Adv. Mater.22,505(2010).
[50]J.L.Ravanat,J.Cadet,K.Araki,H.E.Toma,M.H. G.Medeiros,and P.Di Mascio,Photochem.Photobiol. 68,698(1998).
[51]N.Kosui,K.Uchida,and M.Koizumi,Bull.Chem.Soc. Jpn.38,1958(1965).
[52]D.Harmatz and G.Blauer,Photochem.Photobiol.38, 385(1983).
ceived on April 13,2017;Accepted on June 10,2017)
†These authors contributed equally to this work.
∗Authors to whom correspondence should be addressed.E-mail: amchen@jlu.edu.cn,mxjin@jlu.edu.cn
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- γ-Ray Irradiation-Derived MnO/rGO Composites for High Performance Lithium Ion Batteries
- Identi fication of Superoxide O2−during Thermal Decomposition of Molten KNO3-NaNO2-NaNO3Salt by Electron Paramagnetic Resonance and UV-Vis Absorption Spectroscopy
- Binding Mechanism and Molecular Design of Benzimidazole/Benzothiazole Derivatives as Potent Abl T315I Mutant Inhibitors
- Highly Responsive and Selective Ethanol Gas Sensor Based on Co3O4-Modi fied SnO2Nano fibers
- Geometric Design of Anode-Supported Micro-Tubular Solid Oxide Fuel Cells by Multiphysics Simulations
- Laser-Assisted Stark Deceleration of Polar Molecules HC2n+1N(n=2,3,4) in High-Field-Seeking State
