Drawing behavior of melt-spun poly(vinyl alcohol) fibers
2017-08-30LiLiChenNingLiuQing
Li Li, Chen Ning, Liu Qing
(State Key Laboratory of Polymer Materials Engineering, Polymer Research Institute, Sichuan University, Chengdu 610065)
Drawing behavior of melt-spun poly(vinyl alcohol) fibers
Li Li, Chen Ning*, Liu Qing
(State Key Laboratory of Polymer Materials Engineering, Polymer Research Institute, Sichuan University, Chengdu 610065)
Poly(vinyl alcohol)(PVA) was plasticized by deionized water and was melt-spun into PVA fiber. The stress-strain curves of the melt-spun PVA fibers with 35% and 5% water by mass fraction at different drawing temperatures were measured as well as the activation energy. The results showed that the effect of drawing temperature on the apparent extensional viscosity of melt-spun PVA fibers containing 35% water by mass fraction could be divided into three zones: 30-100 ℃, 100-190 ℃ and 190-210 ℃, i.e. there were three different activation mechanisms and the fiber could be drawn at least through three steps; the stretching of the filaments was largely affected by the water content in the system; the motion ability of PVA molecular chains decreased and the apparent extensional viscosity of the melt-spun fibers increased with the reduction of the water content in system; the apparent extersional viscosity of the melt-spun PVA fiber with 5% water by mass fraction changed differently within two different temperature ranges, indicating two different activation mechanisms, so the as-spun fibers could be drawn through two steps.
poly(vinyl alcohol) fiber; melt-spinning; extensional viscosity; activation energy
Poly(vinyl alcohol) (PVA) fiber is widely used in many important high-tech areas owing to its excellent comprehensive properties, such as good mechanical properties, superior thermal properties and weather durability, excellent acid, alkaline and organic solvents resistance, etc. However, the melting point and decomposition temperature of PVA are so close that the melt spinning of PVA becomes very difficult due to the multi-hydroxyl structure. So far, PVA fiber has been produced by solution spinning[1], which is associated with low strength and modulus, high manufacturing cost caused by a large amount of energy consumption, long dissolving and drying time and recycle of coagulating bath.
Generally, the key point to realize the melt spinning of PVA is to obtain thermal processing window, i.e. decreasing the melting point of PVA and increasing its decomposition temperature, which can be performed by addition of plasticisers[2-3], chemical modification[4]and blending[5]. Among them, the plasticized melt spinning is simple, highly efficient and environmental friendly, which is the development direction of PVA melt spinning.
The author successfully realized the melt spinning of PVA according to the intermolecular complexation and plasticization.And the PVA as-spun fibers with uniform structure, circular cross-section and controllable diameter were obtained. Water, the key plasticizer for the melt spinning of PVA, exists in melt-spun PVA fibers in three different states[7], i.e., free water, freezable bound water and non-freezing water, which provides as-spun PVA fiber with good drawability at room temperature. However, excessive water would evaporate during the hot-drawing process of as-spun PVA fiber, resultting in the formation of internal defects. As a result, the drawability of PVA fibers was reduced. In this paper, the drawing behavior of melt-spun PVA fibers under different drawing temperature were studied; the apparent extensional viscosity as well as the activation energy were calculated, and the effect of water content on drawing behavior and drawing mechanism were analyzed, which provides theoretical basis for the preparation of PVA fibers at high draw ratio.
1 Experimental
1.1 Materials
PVA-1799F with an alcoholysis degree of 99.9% was commercially provided from Sichuan Vinylon Works,SINOPEC. The PVA raw materials were washed with deionized water until a pH value of 7 and dried at 80 ℃ to constant weight as the experimental sample in a vacuum oven.
1.2 Preparation of melt-spun PVA fibers
A modified PVA was obtained by adding pre-dried PVA into quantitative deionized water and then letting the solutions completely seep into PVA at 80 ℃ in a sealed vessel. Finally, the modified PVA was melt-spun in a melt spinning apparatus, which consisted of a single screw extruder with the diameter of 25 mm and length-diameter ratio of 25, a prefilter, a spin pump, spinnerets and a conventional take-up device. The capillary diameter of the spinnerets was 0.15 mm. The temperature of extruder and spin head was 120-150 ℃. The draw ratio was 1.5-2.0. The as-spun PVA fibers with 5% water by mass fraction were obtained by drying the melt-spun PVA fibers with 35% water by mass fraction at 200 ℃ for 3 min in an air-circulating oven.
1.3 Measurement of drawing behavior of melt-spun PVA fiber
The stress-strain curves of melt-spun PVA fibers were measured by a universal testing machine RGL-10(Shenzhen Reger Instrument Co., Ltd., China) at drawing temperature 30-220 ℃. The apparent extensional viscosity(ηε) was calculated by the following equations[8-9].
ηε=σε/ε
(1)
σε=F/At
(2)
ε=V/λtL0
(3)
Where,Atis cross-sectional area of PVA fiber at timet;Fis axial tensile force;Vis drawing speed;L0is initial length of melt-spun PVA fiber;λtis draw ratio at timet.
2 Results and discussion
2.1 Drawing behavior of melt-spun PVA fiber with 35% water by mass fraction
As shown in Fig.1, the drawability of melt-spun PVA fiber was sensitive to the drawing temperature. With the drawing temperature increasing, the hydrogen bonds interactions among PVA molecules were weakened, the motion ability of PVA molecule chain was enhanced, the tensile stress was decreased, and the stretch degree of PVA chains along the drawing direction and the elongation at break of the melt-spun PVA fiber were increased. However, the extremely high temperature made the water molecules move faster in melt-spun PVA fibers, which weakened the hydrogen bonds interaction between PVA and water. As a result, the water in melt-spun PVA fibers changed from bound water to free water and evaporated rapidly, causing internal defects to form in PVA fibers and the draw ratio to decrease. The transition temperature was 170-180 ℃.
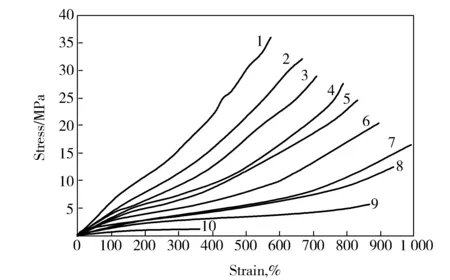
Fig.1 Stress-strain curves of melt-spun PVA fibers with 35% water by mass fraction drawn at different temperature1—30 ℃;2—60 ℃;3—80 ℃;4—110 ℃;5—120 ℃;6—140 ℃;7—170 ℃;8—180 ℃;9—200 ℃;10—220 ℃
From Fig.2, it can be found thatηεkept rising with the draw ratio(λ) increasing, but the rise slowed down when the drawing temperature increased. The main reason is that the increase ofλmade PVA molecule chains orient, the molecular distance decrease, free water evaporate and the motion ability of PVA molecules weaken. Furthermore, the orientation of PVA molecule chains made hydrogen bond rebuilt and restricted the stretchability of PVA fibers. As a result,ηεof melt-spun PVA fibers increased with the increase ofλ.
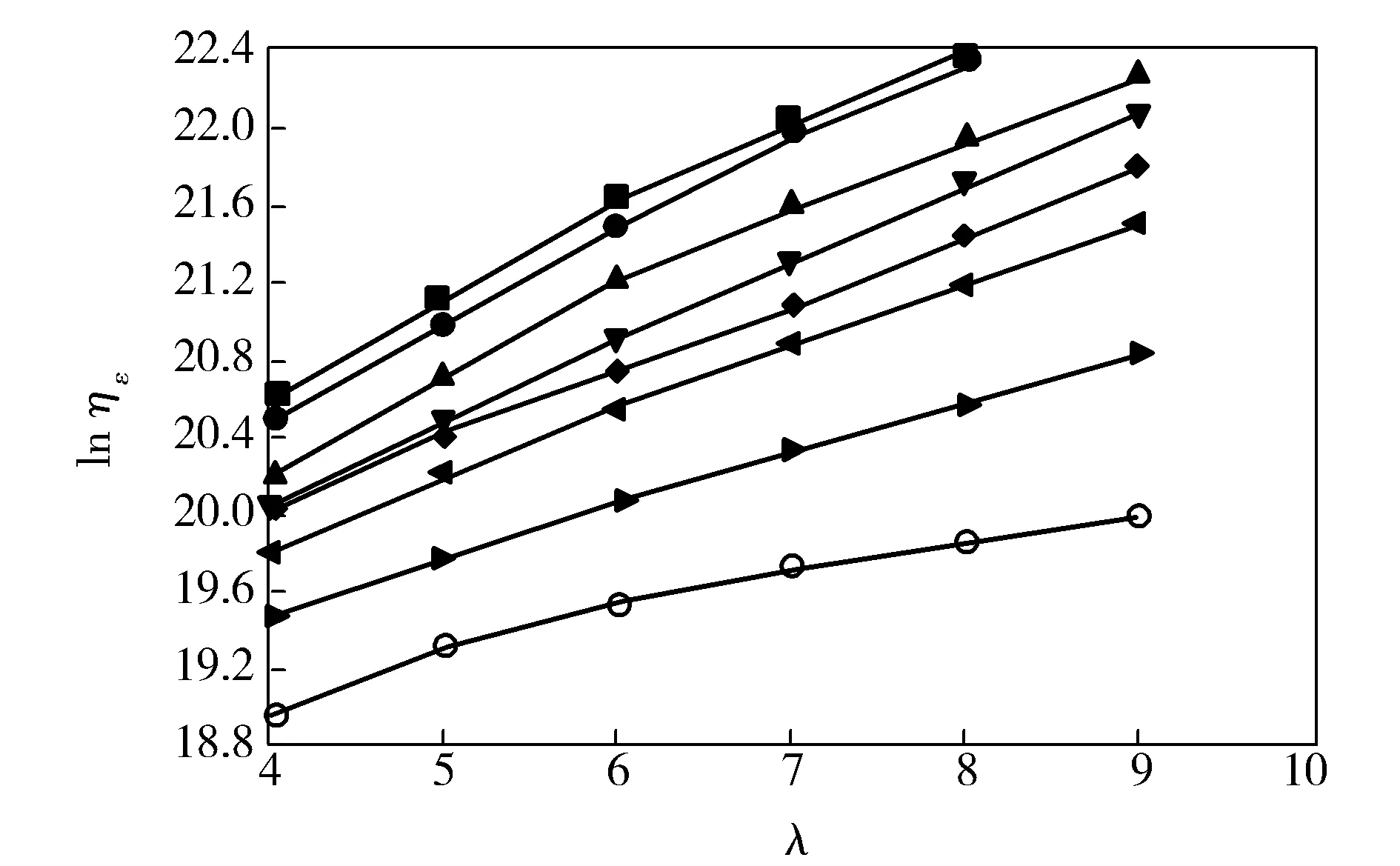
Fig.2 Relationship between ηε and λ of melt-spun PVA fibers with 35% water by mass fraction drawn at different temperature■—80 ℃;●—100 ℃;▲—120 ℃;▼—140 ℃;◆—160 ℃;◀—180 ℃;▶—200 ℃;○—210 ℃
The high drawing temperature weakened the hydrogen bonds interactions among PVA molecules and promoted the movement of PVA chain, which improved the stretchability of melt-spun PVA fibers. Soηεdecreased with the increase of drawing temperature, which indicated that the drawing temperature played an important role in the drawing process of melt-spun PVA fiber and the tensile stress could be profoundly decreased by increasing temperature.
The relation betweenηεand drawing temperature (T) can be described by Eyring-Frenkel equation. From a graphic representation (Fig.3) of the experimental data of lnηεagainst 1/T, the extensional activation energy (Ea) can be determined by the slope of the linear plot. The values ofEaare listed in Tab.1. From Fig.3 and Tab.1, it can be seen thatηεof PVA fibers decreased with the increase of drawing temperature. The relationship between lnηεand 1/Texhibited three-segment straight line with inflection point temperature of 100 ℃ and 190 ℃. The correspondingEawas divided into three regions, i.e. 11.5-12.5 kJ/mol in the lower-temperature region of 30-100 ℃,12.9-24.7 kJ/mol in the middle-temperature region of 100-190 ℃, and 69.5-141.0 kJ/mol in the higher-temperature region of 190-210 ℃ .
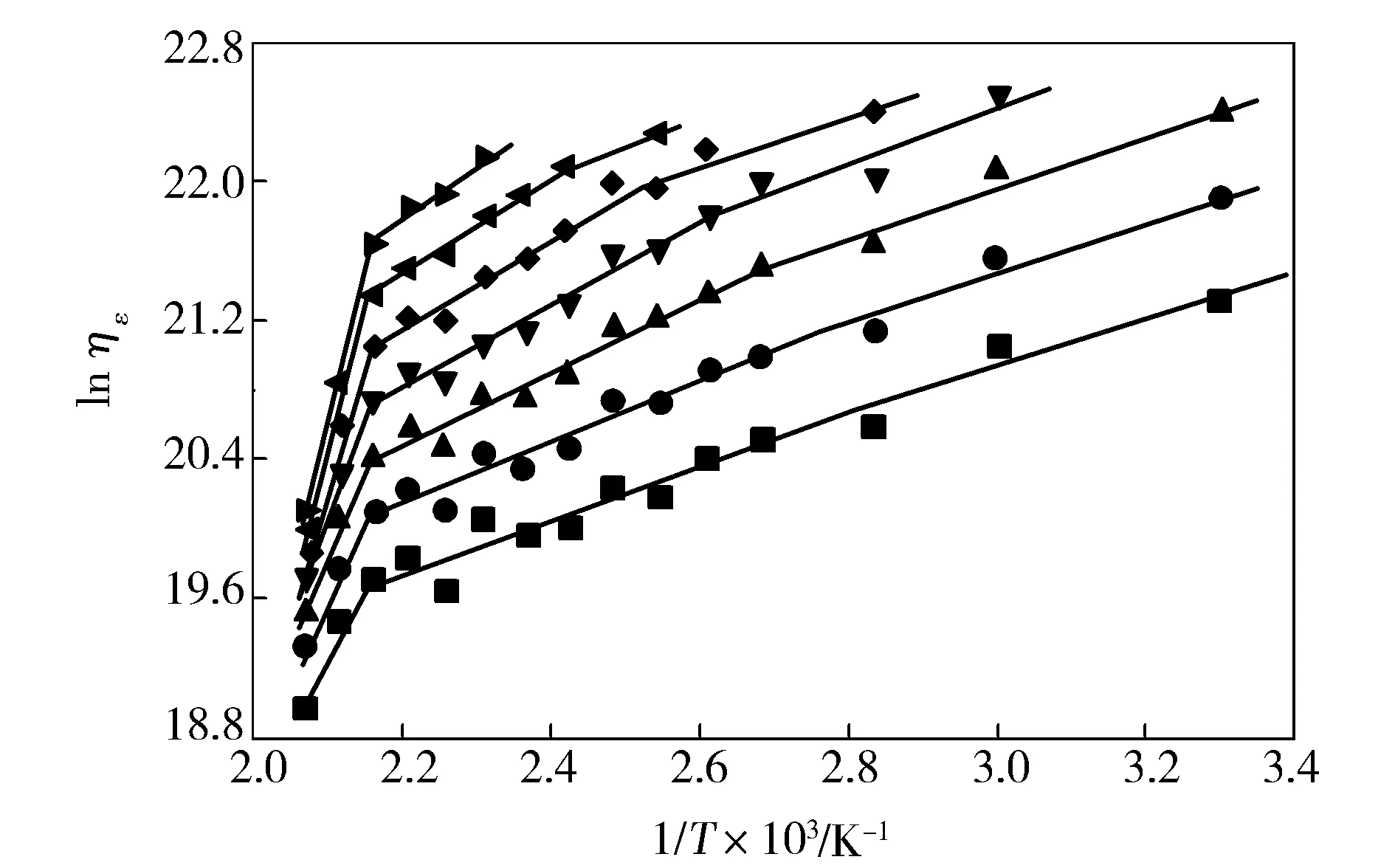
Fig.3 Relationship between ηε and 1/T of melt-spun PVA fibers at different λ ■—4;●—5;▲—6;▼—7;◆—8;◀—9;▶—10

λEa/(kJ·mol-1)30-100℃100-190℃190-210℃411.512.969.5512.014.871.2612.317.881.1713.120.393.0812.521.1109.5919.8125.41024.7141.0
In lower-temperature region, the melt-spun PVA fibers contained more water,which offered the plasticization and lubrication effect in the fiber,and was not sensitive to temperature during the drawing process. So it can be speculated that there mainly happened the shrink of free volumes and distortion of amorphous region in PVA fiber, resulting in lowEa.
In middle-temperature region, free water totally evaporated.The freezable bound water and drawing temperature exerted synergistic effect on the drawability of PVA fibers, which made the large deformation of PVA fiber and the increase ofEa.
In higher-temperature region, freezable bound water evaporated completely. The deformation of melt-spun PVA fibers was mainly affected by drawing temperature. The chain-folded lamellae was melted in a certain extent. The drawing temperature was close to the melt point of PVA fibers. The structure of PVA fibers changed distinctly and became more sensitive to drawing temperature, causingEato increase. In addition, PVA is a typical polar polymer, so the molecular chains would gradually get close to each other to make more chances for self-hydrogen bonds to rebuilt while increasing the draw ratio.Eawould increase with the increase of draw ratio because hydrogen bonds are sensitive to temperature. The change tendency ofEawith temperature indicated three different activation mechanisms and the fiber could be drawn at least by three steps.
2.2 Drawing behavior of melt-spun PVA fibers with 5% water by mass fraction
According to the comparison between Fig.1 and Fig.4, it can be found that the plasticization effect of water on PVA fibers was weakened, the self-hydrogen bonds of PVA were partly rebuilt, the motion ability of PVA chains was decreased and the tensile stress was increased when the water content decreased. In addition, the drawing behavior of PVA fibers changed and the yield phenomenon was clearly observed in the stress-strain curves of PVA fibers with 5% water by mass fraction. As compared with that of the melt-spun PVA fiber with 35% water by mass fraction, the draw ratio of the melt-spun PVA fibers with 5% water by mass fraction was lower in the drawing temperature below 100 ℃, but higher in the drawing temperature above 140 ℃.
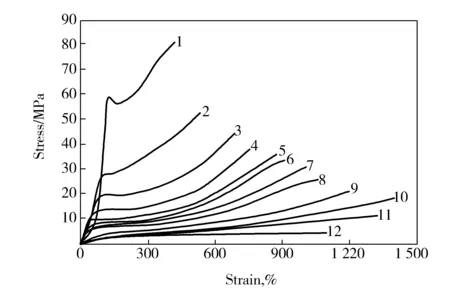
Fig.4 Stress-strain curves of melt-spun PVA fibers with 5% water by mass fraction drawn at different temperature 1—30 ℃;2—60 ℃;3—80 ℃;4—100 ℃;5—110 ℃;6—120 ℃;7—130 ℃;8—140 ℃;9—180 ℃;10—190 ℃;11—200 ℃;12—210 ℃
The main reason is that the motion ability of PVA molecular chains was mainly affected by water at lower temperature below 100 ℃, therefore, the higher water content in PVA fibers, the greater damage to PVA self-hydrogen bond, the more singnificant plasticization effect, the better drawability of PVA fibers, and the higher draw ratio of PVA fibers. What′s more, water evaporated constantly with the increase of temperature, making the fibers generate bubbles and defects and fracture finally. But the melt-spun PVA fiber with lower water content was effectively constrained by water, making water hardly evaporate. So it had few defects resulting from water evaporation, better high-temperature drawablity and higher draw ratio as well, as compared with the fiber with 35% water by mass fraction.
The effect of water on self-hydrogen bonds of PVA was weakened, the self-hydrogen bonds were partly rebuilt and the structure of PVA fiber became denser as the water content in melt-spun PVA fibers was decreased. So the motion ability of PVA molecular chains was depressed andηεwas increased. The change tendency ofηεwith the draw ratio and temperature was similar to that of fiber with 35% water by mass fraction.
The experimental data of lnηεagainst 1/Twere showed in Fig.5, which exhibited two-segment straight line with inflection point temperature being about 200 ℃ which was higher than that of fiber with 35% water by mass fraction.
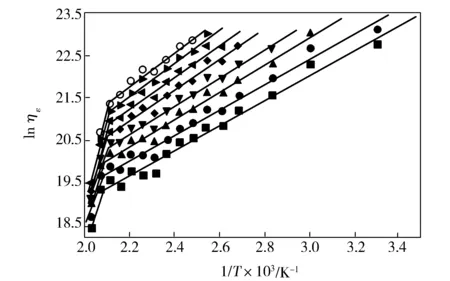
Fig.5 Relationship between ηε and 1/T of melt-spun PVA fibers with 5% water by mass fraction at different λ■—4;●—5;▲—6;▼—7;◆—8;◀—9;▶—10;○—11
As shown in Tab.2,the correspondingEaof PVA fibers with 5% water by mass fraction showed two regions,i.e.24.6-33.2 kJ/mol at 30-200 ℃,higher than that of PVA fiber with 35% water by mass fraction in the lower temperature and middle temperature regions,and 111.3-140.6 kJ/mol at 200-220 ℃,also higher than that of PVA fiber with 35% water by mass fraction in higher temperature region. Therefore, there existed two different activation mechanisms,so at least two steps should be adopted during drawing process.The increase ofEaand the change of activation mechanisms of melt-spun PVA fiber with 5% water by mass fraction was due to self-hydrogen bonds rebuilding and dense structure of PVA fibers caused by the decrease of water content.
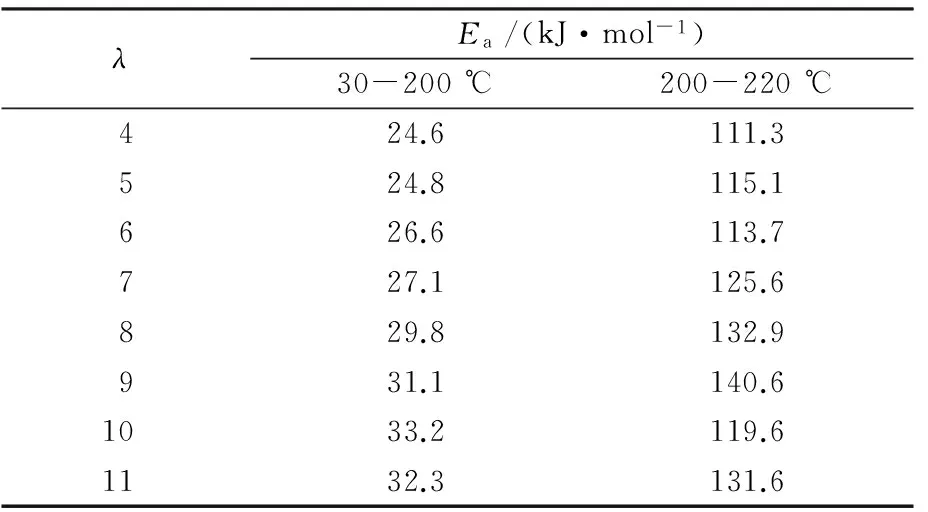
Tab.2 Ea of melt-spun PVA fibers with 5% water by mass fraction drawn in different temperature regions
3 Conclusions
a. The effect of temperature onηεof melt-spun PVA fiber with 35% water by mass fraction can be divided into three regions, lower-temperature region of 30-100 ℃, middle-temperature region of 100-190 ℃ and higher-temperature region of 190-210 ℃, which indicated that there were three different activation mechanisms and the fiber could be drawn at least with three steps.
b. When the water content of melt-spun PVA fibers was 5% by mass fraction, water could hardly evaporate during hot drawing, the self-hydrogen bonds of PVA were partly rebuilt, the structure of PVA became dense, the motion ability of PVA molecular chains decreased andηεincreased in two regions, i.e. lower-temperature region of 30-200 ℃ and higher-temperature region of 200-220 ℃. The activation mechanisms were different in these two regions.
c. The lower water content was beneficial to the oriented stretch of PVA molecules and stabilization of oriented structure. The water content of the system should be rationally decreased during drawing process to acquire the better stretchability at high temperatures and more outstanding mechanical properties of the melt-spun fibers.
[1] Zhao Huan. Poly(vinyl alcohol) fibers[M]. Beijing: Chemical Industry Press, 2014:7-8.
[2] Bai Hongjun, Yang Zhongkai, Tang Chuanjiang, et al. Rheological behavior of plasticized poly(vinyl alcohol) melt[J]. Chin Syn Fiber Ind, 2015,38(2):43-47.
[3] Lin C A, Ku T. Shear and elongational flow properties of thermoplastic polyvinyl alcohol melts with different plasticizer contents and degrees of polymerization[J]. J Mater Proc Tech, 2008, 200: 331-338.
[4] Hiroshi N, Nobuo D, Takeaki M. Preparation and thermal properties of thermoplastic poly(vinyl alcohol) complexes with boronic acids[J]. J Polym Sci, Part A: Polym Chem, 1998,36(17):3045-3050.
[5] Ku T H, Lin C A. Elongational flow properties of thermoplastic polyvinyl alcohol/polypropylene from the melt spinning method[J]. Text Res J, 2014,84(9):932-940.
[6] Wang Qi, Li Li, Chen Ning. Thermal processing of poly(Vinyl alcohol) [J]. Polym Mater Sci Eng, 2014,30(2):192-197.
[7] Wang Ning, Zhao Lipeng, Zhang Chuhong,et al. Water states and thermal processability of boric acid modified poly (vinyl alcohol) [J]. J Appl Polym Sci,2016,133(13):43246-43252.
[8] Pan Lijun, Hu Zuming, Liu Zhaofeng. A study of elongational rheological behavior of UHME-PE gel fiber[J]. J Chin Text Univ, 1993,19(6): 67-72.
[9] Smook J, Pennings A. The effect of temperature and deformation rate on the hot-drawing behavior of porous high-molecular-weight polyethylene fibers[J]. J Appl Polym Sci, 1982, 27(6): 2209-2228.
◀国内外动态▶
80 kt/a 高性能再生聚酯纤维项目落户宜宾
2017年7月12日,由浙江亿兴达纺织有限公司、绍兴新纶机械制造有限公司、佛山中泰光进出口公司共同出资建设的80 kt/a 高性能再生聚酯纤维及纺纱织布产业化项目正式落户宜宾屏山县工业园区。据了解,该项目总投资5.2亿元,用地约100亩,分二期建设,其中,一期项目选址石盘产业园区,用地约45亩,预计总投资2亿元;二期项目拟选址王场产业园区,用地约55亩,预计总投资3.2亿元。该项目全部建成并投产后将形成 80 kt/a 高性能再生聚酯纤维及纺纱织布生产能力,实现年产值10亿元、利税1.5亿元,新增就业300人以上。目前,该项目正在开展厂房设计等前期工作。
(通讯员 杨 朝)
圣泉集团首创石墨烯功能纤维
2017年6月3日,济南圣泉集团股份有限公司“生物质石墨烯宏量制备及石墨烯在功能纤维中的产业化应用”成果鉴定发布,该项目是中国纺织工业联合会科技指导性项目,由圣泉集团承担,并由工信部、中国化学纤维工业联合会、中国石化联合会共同鉴定完成。
由中国工程院院士孙晋良等权威专家组成的鉴定委员会一致认为:该成果发明了天然生物质纤维素制备生物质石墨烯的“基团配位组装析碳法”,在国际上首次实现了生物质石墨烯材料的宏量制备;研发出具有远红外、抗菌抑菌、抗静电、防紫外等多功能的石墨烯改性纤维,开发其在服饰、家纺、军工、轻工、医疗、精细化工等领域的应用;该成果对提高纺织工业科技创新能力、纺织行业技术升级和高附加值产品开发具有深远意义;在国际上首次实现了生物质石墨烯宏量制备及石墨烯在功能纤维中的产业化应用,达到了国际领先水平,属国际首创。
圣泉集团此次成功突破生物质石墨烯宏量制备及石墨烯在功能纤维中的产业化应用,开发了具有低温远红外、防紫外线、改善微循环、抗菌抑菌、抗静电、增温保温等功能性纺织品,高性能化、多功能化、智能化的纺织纤维材料体系初现。此外,圣泉集团正研发和储备石墨烯在军民融合领域、医疗用品、防腐涂料、电池材料、增强材料等领域的技术。
(通讯员 钱伯章)
齐鲁石化制成石墨烯腈纶
2017年7月,齐鲁石化腈纶厂与山东济南圣泉集团合作,成功完成石墨烯腈纶试样的制备工作。经国家纺织制品质量监督检验中心对使用该原料制作成织物的试样进行检测,产品的抗静电性、抗菌抑菌性、远红外性能及耐水洗色牢度等指标全部达到并超过国家标准。该厂与济南圣泉集团协商,确定了放大实验设备的技术需求和具体参数,开始了石墨烯实验设备采购程序。该项目是提升腈纶产品附加值的有效途径。
(通讯员 郑宁来)
上海石化原液着色腈纶销量增长
2017年1~6月,上海石化腈纶部原液着色腈纶的销量同比增长49%。原液着色腈纶是该部自行开发的新产品,填补了国内空白,丰富了国内腈纶的品种,使腈纶生产技术水平得到了提升,逐步为国内客户所接受。该部原液着色腈纶销售量的扩大,得益于品种的增加。2015年,该部还只能生产单一的黑色纤维,而现在,已有十多种颜色的腈纶进入正常生产序列,另外还有二十多种颜色的腈纶产品进入技术储备序列。颜色增多,客户选择的余地也大了。另外,该部生产的原液着色腈纶原来仅用来做户外产品,现在则已进入了服用领域。
(通讯员 钱伯章)
聚乙烯醇熔纺初生纤维的拉伸行为研究
李莉 陈宁 刘庆
(1.四川大学 高分子材料工程国家重点实验室,高分子研究所,四川 成都 610065)
采用去离子水溶胀聚乙烯醇(PVA),通过熔融纺丝法制备PVA纤维。研究了水质量分数35%和5%的PVA熔纺初生纤维在不同拉伸温度下的应力-应变曲线,以及其拉伸活化机制。结果表明:拉伸温度对水质量分数35%的PVA熔纺初生纤维表观拉伸黏度的影响分为3个区,即30~100 ℃,100~190 ℃,190~210 ℃,纤维在热拉伸时存在3个不同机制的活化过程,至少可采用三级拉伸;初生纤维拉伸受体系中水含量的影响,水含量减少,PVA分子链运动能力降低,表观拉伸黏度增大,水质量分数5%的PVA熔纺初生纤维的表观拉伸黏度随温度变化呈现两个区,活化机制改变,可采用两步拉伸。
聚乙烯醇纤维 熔融纺丝 拉伸黏度 活化能
date:04-05-2017; revised date: 20- 06- 2017.
National Natural Science Foundation of China(51433006) and Independent Project of State Key Laboratory of Polymer Materials Engineering of China(sklpme2014-3-15)
TQ342+.41 Document code:A Article ID: 1001- 0041(2017)04- 0040- 05
Biography: Li Li(1977-), female,Ph. D,is engaged in the structure and properties of polymers. E-mail:powerlily@scu.edu.cn.
* Corresponding author: ningchen@scu.edu.cn.
