靶向S1P2基因的siRNA有效片段慢病毒载体的筛选*
2017-08-07夏纪毅朱永生
晏 萧, 夏纪毅, 程 波, 朱永生, 姜 睿△
(西南医科大学附属医院 1泌尿外科, 2医学实验中心, 四川 泸州 646000)
·实验技术·
靶向S1P2基因的siRNA有效片段慢病毒载体的筛选*
晏 萧1, 夏纪毅2, 程 波1, 朱永生1, 姜 睿1△
(西南医科大学附属医院1泌尿外科,2医学实验中心, 四川 泸州 646000)
目的: 筛选能显著抑制原代培养的自发性高血压大鼠(spontaneously hypertensive rats,SHR) 阴茎海绵体平滑肌细胞内1-磷酸鞘氨醇受体2(sphingosine-1-phosphate receptor 2,S1P2)基因表达的siRNA慢病毒载体。方法: SHR及SD大鼠各5只用于原代培养阴茎海绵体平滑肌细胞并分成6组,每组5个样本,每个样本约1.0×105个细胞,分别为携带靶向S1P2基因的siRNA-1~3号靶点的SHR慢病毒转染组(SHR siRNA-1、SHR siRNA-2和SHR siRNA-3组)、 携带慢病毒空载体的SHR转染组(SHR GFP组)、SHR未转染对照组(SHR control组)和SD大鼠对照组(SD control组)。将靶向S1P2的siRNA片段的慢病毒载体,以感染复数(MOI)= 60转染各组SHR阴茎海绵体平滑肌细胞,于转染72 h后荧光显微镜下观察细胞荧光表达情况,用Western blot分析各组S1P2、ROCK1、ROCK2和eNOS在阴茎海绵体平滑肌细胞内的表达变化,RT-PCR检测各组阴茎海绵体平滑肌细胞S1P2、ROCK1和ROCK2的mRNA 的表达。结果: 荧光显微镜下观察SHR siRNA-1、SHR siRNA-2、SHR siRNA-3和SHR GFP组细胞转染效率均>80%;SHR GFP组与SHR control组相比,S1P2、ROCK1和ROCK2的mRNA和蛋白表达无明显差异, SHR siRNA-1、SHR siRNA-2、SHR siRNA-3和SD control组均显著低于SHR control组(P<0.05)。而SHR siRNA-1、SHR siRNA-2、SHR siRNA-3和SHR GFP组eNOS蛋白表达与SHR control组相比均无显著差别,但SD control组显著高于SHR control组(P<0.05)。结论: 3组针对S1P2的siRNA慢病毒载体均能显著抑制SHR阴茎海绵体平滑肌细胞内S1P2的表达,下调ROCK1和ROCK2表达,其中靶向S1P2的siRNA-1抑制效率最高。
自发性高血压大鼠; 1-磷酸鞘氨醇受体2; 小干扰RNA; 慢病毒载体
阴茎勃起是一个由血管、神经共同调节的复杂生理过程,涉及多条信号通路,其中一氧化氮(nitric oxide,NO)/环磷酸鸟苷(cyclic guanosine monophosphate,cGMP)信号通路障碍、RhoA/Rho激酶信号通路的激活等原因都可导致勃起功能障碍(erectile dysfunction,ED)的发生。40岁以上男性中有近40%患有不同程度的ED,且ED患病率随着年龄增加[1]。此外,心血管疾病(cardiovascular disease,CVD)危险因素(如血脂异常、高血压、糖尿病、吸烟、肥胖、代谢综合征等)与ED的发生显著相关[2]。ED与CVD是2种具有不同表现形式的相同的系统性疾病,ED也是心血管疾病的早期临床表现和导致心血管事件发生的独立危险因素[3-4]。
高血压作为常见的CVD危险因素,高血压人群中ED的发病率超过正常血压人群发病率的2倍[5]。高血压可通过下调阴茎海绵体内一氧化氮合酶(nitric oxide synthase, NOS)表达,使NO合成减少,抑制内皮型一氧化氮(endothelial nitric oxide synthase, eNOS)/NO 信号通路,以及通过激活RhoA/Rho激酶信号通路,增加阴茎海绵体平滑肌张力,从而导致ED[6-8]。5型磷酸二酯酶抑制剂(phosphodiesterase type 5 inhibitor, PDE5I)作为当前治疗ED的一线用药,具有与cGMP类似的结构,可通过竞争性结合PDE5的催化位点从而抑制eNOS/NO 信号通路中cGMP降解,以维持阴茎的勃起[9]。但部分糖尿病、心血管疾病引起的难治性ED在单独应用PDE5I治疗时无效,其中西地那非在治疗高血压性ED时有效率仅为83.2%[5],表明包括RhoA/Rho激酶信号通路在内的其它分子信号在高血压性ED的发生中具有重要作用。我们前期研究发现自发性高血压大鼠(Spontaneously hypertensive rats,SHR)阴茎海绵体平滑肌细胞中RhoA/Rho激酶信号通路激活,导致阴茎海绵体平滑肌张力增加[6-7]。此外,还发现高血压导致的ED与1-磷酸鞘氨醇受体2(sphingosine-1-phosphate receptor 2,S1P2)上调相关[8],认为高血压可通过上调S1P2激活RhoA/Rho激酶信号通路,从而引起ED。因此抑制SHR的S1P2表达,有望改善勃起功能,成为治疗高血压引起的ED新的治疗方式。本研究通过构建3条针对大鼠S1P2的小干扰RNA(small interfering RNA, siRNA)慢病毒载体,转染至SHR阴茎海绵体平滑肌细胞中,筛选出能够显著抑制S1P2表达的siRNA慢病毒载体,并观察S1P2基因沉默对RhoA/Rho激酶信号通路的影响。
材 料 和 方 法
1 实验动物
10周龄健康雄性SHR、SD大鼠各5只,分别购自北京维通利华实验动物技术有限公司[许可证号为SCXK(京)2012-0001]、西南医科大学实验动物中心[许可证号为SCXK(川)2013-17]。
2 材料
携带靶向S1P2基因的siRNA慢病毒载体(上海吉凯基因化学技术有限公司);兔抗大鼠S1P2多克隆抗体和兔抗大鼠ROCK2多克隆抗体(Santa Cruz);兔抗大鼠ROCK1多克隆抗体和小鼠抗大鼠eNOS多克隆抗体(Abcam);小鼠抗大鼠GAPDH多克隆抗体、辣根过氧化物酶(horseradish piroxidase,HRP)标记的山羊抗兔IgG和山羊抗小鼠IgG(碧云天公司);总RNA提取试剂盒(北京天根生化科技有限公司);RT-PCR 试剂盒(TaKaRa);DMEM 高糖培养液、胎牛血清、青-链霉素混合液和0.25%胰蛋白酶液(HyClone);倒置荧光显微镜(Olympus);恒温细胞孵箱(Thermo);紫外可见分光光度计(上海精密科学仪器有限公司); PCR 扩增仪(MyCycler);凝胶成像系统(Bio-Rad)。
3 方法
3.1 大鼠阴茎海绵体平滑肌培养与鉴定 采用本课题组前期实验方法对SHR和SD大鼠阴茎海绵体平滑肌进行原代培养[10],培养并纯化至第4代,采用α-平滑肌肌动蛋白(α-smooth muscle actin, α-SMA)标记阴茎海绵体平滑肌细胞,应用免疫荧光法与流式细胞术进行细胞的性质及纯度鉴定。用台盼蓝染色计数法对细胞存活率进行细胞活力测定。
3.2 siRNA 慢病毒载体的制备 由上海吉凯基因化学技术有限公司针对大鼠S1P2基因合成3条特异性siRNA。siRNA序列1为5’-GCTCTACGGCAGTGACAAA-3’,siRNA序列2为5’-GTGCCATCGTGGTGGAGAA-3’,siRNA序列3为5’-TGGAGAGAGGCTTGCATAT-3’,空载体为5’-TTCTCCGAACGTGTCACGT-3’。并以此构建、包装3种慢病毒载体及空病毒载体。
3.3 慢病毒转染 将5只SHR和5只SD大鼠阴茎海绵体平滑肌细胞随机分为6个组,分别为携带靶向S1P2基因siRNA-1~3号靶点的SHR慢病毒转染组(SHR siRNA-1、SHR siRNA-2和SHR siRNA-3组)、 携带慢病毒空载体的SHR转染组(SHR GFP组)、SHR未转染对照组(SHR control组)和SD大鼠对照组(SD control组)。在病毒转染前1 d消化细胞,并制成密度为5.0×107/L的细胞悬液,以2 mL每孔接种在6孔板上。接种后第2天,观察细胞若细胞生长良好,长至约40%融合时可行慢病毒转染。-80 ℃冰箱取出病毒,于冰上融化,按照每孔病毒转染液最终体积1 mL,在完全培养基中加入Polybrene,使其最终浓度为50 mg/L, 以感染复数(MOI)=60加入病毒原液。用吸管洗尽每孔中培养基,将制备好的病毒转染液混匀后加入相应孔中,转染12 h后吸出病毒转染液,用完全培养基洗2次,加入含20%胎牛血清的完全培养基2 mL后继续于5% CO2培养箱中继续培养。转染72 h后荧光显微镜下观察绿色荧光蛋白表达情况,转染效率低于80%的孔重新进行转染,转染效率大于80%的孔继续培养4 d后进行下一步实验。
3.4 RT-PCR检测胞内S1P2、ROCK1和ROCK2的mRNA水平 (1)引物设计合成:由上海生工生物有限公司采用Beacon Designer 2软件设计并合成S1P2、ROCK1、ROCK2和GAPDH引物,序列见表1。(2)总RNA提取:病毒转染细胞后第7天,按照RNAsimple总RNA提取试剂盒(北京天根生化科技有限公司)说明书提取各组细胞总RNA。每个样本取1 μL,用紫外分光光度仪检测RNA纯度和浓度,吸光度(A)值介于1.8~2.0,浓度>84.22 μg/L为合格,并行逆转录反应。(3)逆转录:按RT-PCR试剂盒说明书,加入dNTP Mixture 1 μL、Oligo dT Primer(2.5 μmol/L)1 μL和总RNA 1 μg,加RNase-free dH2O至10 μL,在PCR仪上进行65 ℃ 5 min变性、退火反应;再向以上变性退火反应液中加入5× PrimeScript buffer 4 μL、RNase inhibitor 0.5 μL、PrimeScript RTase 0.5 μL和RNase-free dH2O 5 μL,在PCR仪上按30 ℃ 10 min、42 ℃ 25 min、95 ℃ 5 min进行反转录反应,将总RNA逆转录为稳定的cDNA。(4)PCR扩增反应及凝胶电泳:配制10× PCR buffer Ⅱ 5 μL、dNTP Mixture 2 μL、TaKaRa Ex Taq HS 0.5 μL、反转录反应液5 μL、上游引物和下游引物各0.5 μL、H2O 36.5 μL的50 μL体系,94 ℃ 1 min、57 ℃ 30 s、72 ℃ 30 s,30个循环进行扩增反应。配制2%的琼脂糖凝胶,加入0.1 mg/L Godview,PCR扩增反应后在110 V恒压下电泳30 min。使用凝胶成像系统照相,并分析各组指标灰度值。

表1 用于RT-PCR的引物序列
3.5 Western blot测定S1P2、ROCK1、ROCK2和eNOS在大鼠阴茎海绵体平滑肌细胞中的表达 吸出6孔板内培养液后,PBS洗涤3次,每孔加入200 mL提前配置好的裂解液,将6孔板置于冰上充分裂解30 min,用刮棒将细胞刮于每孔一侧,吸入EP管。4 ℃下15 000 r/min离心5 min,取上清液并加入5×上样缓冲液后开水煮沸5 min。制10%分离胶,取上述蛋白液上样,电泳初始电压80 V,当溴酚蓝染料的前缘进入分离胶上缘后提高电压至120 V,继续电泳至溴酚蓝到分离胶的下缘。0.4 A 恒流转膜120 min,PVDF 膜以5%脱脂牛奶室温封闭1 h,兔抗大鼠ROCK1(1∶1 000)、ROCK2 (1∶500),以及小鼠抗大鼠S1P2(1∶500)、eNOS(1∶1 000)4 ℃孵育过夜,羊抗兔IgG及羊抗小鼠IgG(1∶2 000)室温孵育1 h。ECL化学发光,以Quantity One软件进行分析,结果以S1P2、ROCK1、ROCK2和eNOS灰度值与GAPDH灰度值进行比较。
4 统计学处理
统计分析用SPSS 19.0软件处理。实验数据采用均数±标准差(mean±SD)表示,各组间均数比较采用单因素方差分析,S1P2与ROCK1、ROCK2表达量的相关性采用Pearson相关性分析,以P<0.05为差异有统计学意义。
结 果
1 大鼠阴茎海绵体平滑肌鉴定
培养、纯化至第4代的SHR及SD大鼠阴茎海绵体平滑肌细胞用α-SMA免疫荧光鉴定。在荧光显微镜下观察,SHR和SD大鼠的细胞绿色荧光染色阳性分别为(94.140±1.640)%和(94.180±1.281)%,见图1。流式细胞术纯度鉴定显示,SHR和SD大鼠阴茎海绵体平滑肌细胞纯度分别达(95.382±1.383)%和(94.620±2.910)%,见图2。台盼蓝进行细胞染色,镜下可见SHR和SD大鼠活细胞率分别为(98.280±0.691)%和(97.760±0.832)%。

Figure 1.Observation of the corpus cavernosum smooth muscle cells of the SHR and SD rats by immunofluorescence staining (×200). The experiments were repeated for 5 times.
图1 SHR和SD大鼠的阴茎海绵体平滑肌细胞免疫荧光鉴定

Figure 2.Identification of the corpus cavernosum smooth muscle cells of the SHR and SD rats by flow cytometry. The purity of the SHR and SD rat corpus cavernosum smooth muscle cells was 98.49% and 97.04%, respectively. Mean±SD.n=5.
图2 SHR和SD大鼠阴茎海绵体平滑肌细胞的FCM鉴定
2 病毒转染后胞内绿色荧光蛋白表达
在病毒转染后72 h于荧光显微镜下观察细胞绿色荧光蛋白表达情况,可见SHR siRNA-1、SHR siRNA-2、SHR siRNA-3和SHR GFP组细胞均能激发绿色荧光,且荧光阳性细胞大于80%,说明本实验构建的慢病毒载体能成功转入SHR阴茎海绵体平滑肌细胞内。SHR control和SD control组细胞不能激发绿色荧光,此外,可见SHR siRNA-1、SHR siRNA-2、SHR siRNA-3和SHR GFP组细胞间杂质增多,部分细胞出现皱缩,细胞呈多角形,形态不规则,少量细胞脱落,以SHR siRNA-3组最为明显,见图3。
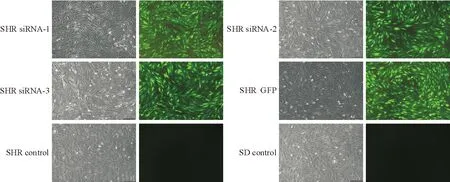
Figure 3.Green fluorescence of the corpus cavernosum smooth muscle cells after transfection (×100).
图3 转染后细胞内绿色荧光表达情况
3 RT-PCR测定各组大鼠阴茎海绵体平滑肌细胞胞内S1P2、ROCK1和ROCK2的mRNA表达
RT-PCR所测得SHR GFP组与SHR control组间S1P2、ROCK1和ROCK2的mRNA表达水平差异无统计学显著性,但均显著高于SHR siRNA-1、SHR siRNA-2、SHR siRNA-3及SD control组,SHR siRNA-1组被抑制效率最高(P<0.05),见图4。
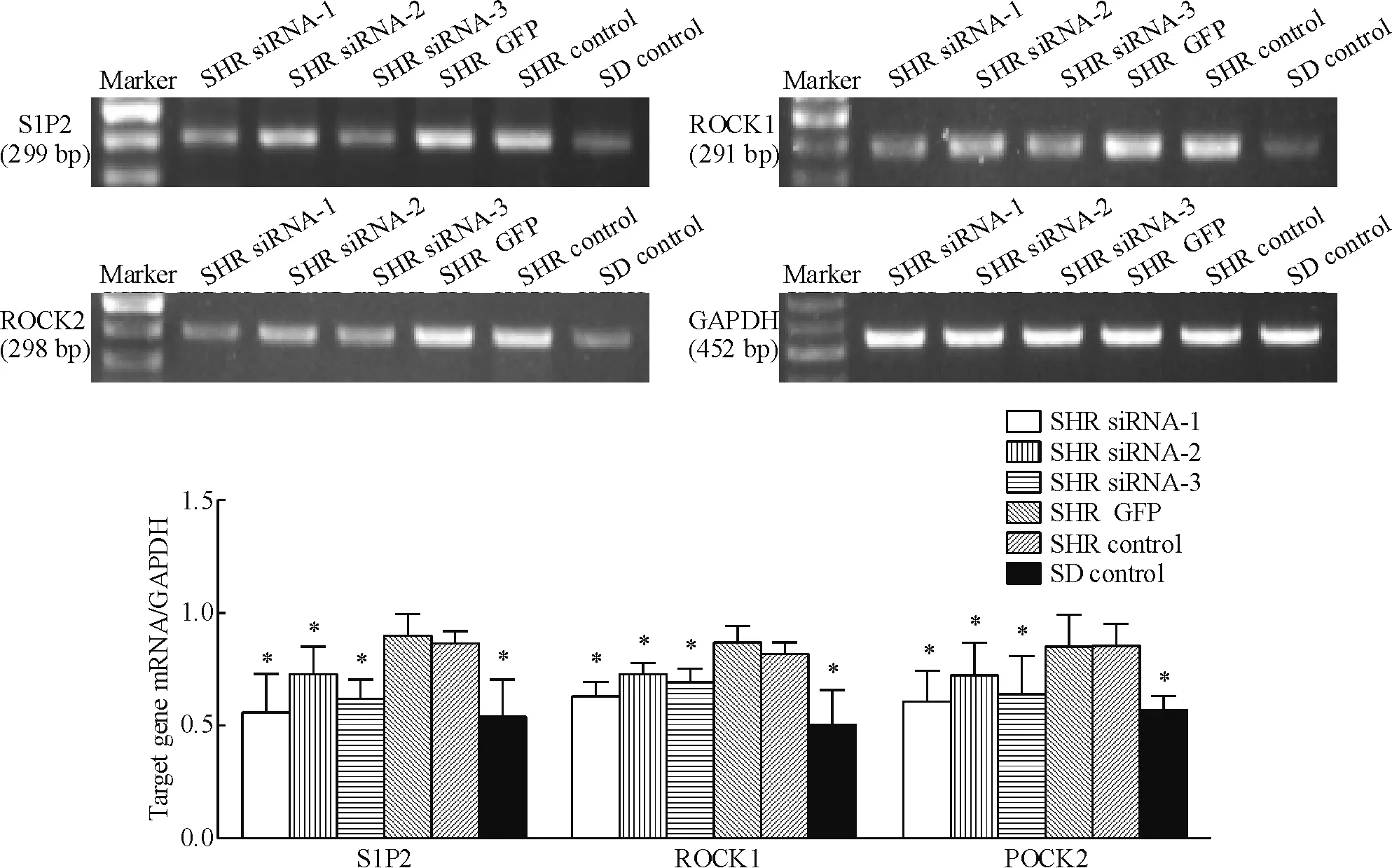
Figure 4.The mRNA expression of S1P2, ROCK1 and ROCK2 in the corpus cavernosum smooth muscle cells after transfection. Mean±SD.n=5.*P<0.05vsSHR control.
图4 转染后阴茎海绵体平滑肌细胞内S1P2、ROCK1和ROCK2 mRNA表达的变化
4 Western blot测定S1P2、ROCK1、ROCK2和eNOS在各组大鼠阴茎海绵体平滑肌细胞中的表达
S1P2、ROCK1、ROCK2和eNOS蛋白在6组细胞中均有表达,其中S1P2、ROCK1和ROCK2在SHR GFP组与SHR control组间表达水平的差异无统计学显著性,但显著高于SHR siRNA-1、SHR siRNA-2、SHR siRNA-3及SD control组,SHR siRNA-1组抑制效率最高(P<0.05)。与SHR control组相比,eNOS在SHR siRNA-1、SHR siRNA-2、SHR siRNA-3和SHR GFP组表达水平的差异无统计学显著性,但显著低于SD control组(P<0.05),见图5。
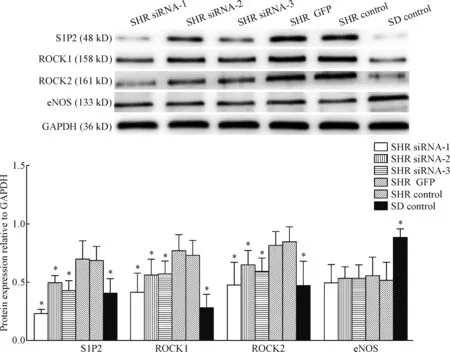
Figure 5.The protein expression of S1P2, ROCK1, ROCK2 and eNOS in the corpus cavernosum smooth muscle cells after transfection. Mean±SD.n=5.*P<0.05vsSHR control.
图5 转染后阴茎海绵体平滑肌细胞内S1P2、ROCK1、ROCK2和eNOS蛋白表达的变化
5 Western blot中S1P2与ROCK1、ROCK2表达量的相关性分析
S1P2与ROCK1的蛋白表达量进行相关性分析,相关系数r=0.887(P<0.05)。S1P2与ROCK2的蛋白表达量进行相关性分析,相关系数r=0.894(P<0.05)。
讨 论
在高血压人群中ED发病率高于正常血压人群,约为35%~50.6%,且高血压引起的ED比正常血压人群ED严重程度更严重,ED的发病率也随高血压病程的增加而增加[5, 11-12]。高血压引起ED的机制包括:海绵体动脉的部分功能和结构异常,管壁增厚和胶原沉积使管腔直径的减小是常见的结构变化[13];海绵体血管平滑肌细胞增殖和纤维化[13];高血压导致阴部内动脉狭窄或闭塞导致其对阴茎的供血减少[14];抗高血压药物的使用也是导致高血压患者ED患病率增加的因素[11];高血压引起NO/cGMP、RhoA/Rho激酶、HO/CO等信号通路障碍是导致ED的重要机制[7-8, 15]。同时,ED也是高血压患者血管损伤的临床征兆,还意味着未来心血管事件的发生风险增加[16]。
RhoA/Rho激酶是种丝氨酸/苏氨酸激酶,其有2种亚型:ROCKα[Rho关联含卷曲螺旋蛋白激酶2(Rho-associated coiled-coil containing protein kinase 2, ROCK2)]和ROCKβ[Rho关联含卷曲螺旋蛋白激酶1(Rho-associated coiled-coil containing protein kinase 1, ROCK1)]。平滑肌细胞收缩主要是通过增加胞内Ca2+浓度,使胞内Ca2+与钙调蛋白结合,活化肌球蛋白轻链激酶(myosin light chain kinase, MLCK)使肌球蛋白轻链(myosin light chain, MLC)磷酸化,导致肌球蛋白与肌动蛋白发生交联,从而引起平滑肌的收缩。而肌球蛋白轻链磷酸酯酶(myosin light chain phosphatase, MLCP)使磷酸化的MLC脱磷酸,转化为MLC,引起平滑肌的舒张[17]。RhoA/Rho激酶可通过Ca2+增敏途径抑制MLCP,增加MLC磷酸化,使平滑肌维持收缩状态[18]。此外,动脉粥样硬化和血管炎的发展也涉及到调节平滑肌张力的RhoA/Rho激酶途径[19]。我们前期研究发现在SHR阴茎海绵体平滑肌细胞中RhoA/Rho激酶通路激活,参与高血压性ED发生机制[6-7]。我们采用Rho激酶抑制剂法舒地尔通过抑制RhoA/Rho 激酶信号通路表达和降血压作用,使SHR勃起功能得到改善[20],还使用针对RhoA/Rho激酶亚型ROCK2基因的siRNA转染至SHR海绵体平滑肌细胞后Rock2蛋白的表达受到抑制,显著改善SHR勃起功能[6]。另一方面,我们还发现在SHR阴茎海绵体平滑肌细胞中S1P2表达上调,而S1P2可通过激活RhoA/Rho激酶信号通路导致ED[8]。S1P2是一种与S1P高度亲和的G蛋白偶联受体,其最初从大鼠主动脉血管平滑肌细胞中克隆分离出来[21],主要参与炎症、肿瘤、动脉粥样硬化、平滑肌收缩及血管通透性等病理生理过程[22]。S1P2同时也是介导RhoA/Rho激酶信号通路激活的主要S1P受体[23],S1P与S1P2结合后可通过诱导细胞内钙离子浓度增加和激活RhoA/Rho激酶信号通路引起不同类型的平滑肌收缩,包括血管、支气管、肠和膀胱平滑肌[24-26]。敲除S1P2基因的小鼠与正常小鼠相比平均动脉压和左心室的功能及血管形态无明显改变异,但肾脏和肠系膜血管阻力降低,使血流增加[27],说明S1P2能增加血管平滑肌的收缩性。S1P2同样在阴茎海绵体组织中表达,且主要表达在阴茎海绵体平滑肌和血管内皮细胞胞膜[8,16]。海绵体神经损伤后阴茎组织纤维化就与S1P2活化,导致RhoA/ROCK1途径上调相关[28]。在低雄激素状态下,海绵体内S1P2/RhoA/Rho激酶信号通路上调,引起大鼠ICPmax/MAP下降,导致ED[29]。血管内皮细胞中S1P2抑制可通过操作磷酸化调节eNOS活性的蛋白激酶B(protein kinase B, Akt),使通过eNOS产生的NO减少,从而降低血管屏障的破坏[30]。
在本研究中,SHR较SD大鼠对照组阴茎海绵体平滑肌细胞中S1P2、ROCK1和ROCK2的mRNA和蛋白表达均显著增加, eNOS蛋白表达显著降低,通过针对大鼠S1P2设计的3条不同的siRNA慢病毒转染SHR阴茎海绵体平滑肌细胞,发现均能显著抑制S1P2表达,而eNOS表达无显著差异,表明高血压对NO/cGMP通路影响不通过S1P2途径;同时ROCK1和ROCK2表达均受到显著抑制,并且S1P2 与ROCK1、ROCK2具有正相关性,表明高血压可通过上调S1P2的表达而激活RhoA/Rho激酶信号,并且采用针对大鼠S1P2基因的siRNA可显著抑制SHR海绵体平滑肌细胞S1P2/RhoA/Rho激酶信号通路,其中靶向S1P2基因的siRNA1慢病毒抑制效率最高,可作为抑制RhoA/Rho激酶信号通路而改善高血压引起的ED的潜在靶点。
[1] Johannes CB, Araujo AB, Feldman HA, et al. Incidence of erectile dysfunction in men 40 to 69 year old: longitudinal results from the Massachusetts male aging study[J]. J Urol, 2000, 163(2):460-463.
[2] Jackson G, Boon N, Eardley I, et al. Erectile dysfunction and coronary artery disease prediction: evidence-based guidance and consensus[J]. Int J Clin Pract, 2010, 64(7):848-857.
[3] Gandaglia G, Briganti A, Jackson G, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease[J]. Eur Urol, 2014, 65(5):968-978.
[4] Vlachopoulos C, Ioakeimidis N, Terentes-Printzios D, et al. The triad: erectile dysfunction-endothelial dysfunction-cardiovascular disease[J]. Curr Pharm Des, 2008, 14(35):3700-3714.
[5] Aranda P, Ruilope LM, Calvo C, et al. Erectile dysfunction in essential arterial hypertension and effects sildenafil: results of Spanish national study[J]. Am J Hypertens, 2004, 17(2):139-145.
[6] Zhu X, Lin H, Jiang R, et al. Improving erectile function of spontaneously hypertensive rats by silencingROCK2[J]. Urology, 2014, 84(4):983.e11-983.e18.
[7] 朱平宇, 姜 睿, 邓青富, 等.Rho激酶及血红素氧合酶在自发性高血压大鼠阴茎海绵体中的表达[J]. 中华男科学杂志, 2008, 14(3):215-219.
[8] Wang B, Jiang J, Fan Z, et al. Expression of sphingosine 1-phosphate 1-3 on penile cavernous tissue in hypertensive and normotensive rats[J]. Urology, 2014, 84(2):490.e7-490.e13.
[9] Eardley I, Donatucci C, Corbin J, et al. Pharmacotherapy for erectile dysfunction[J]. J Sex Med, 2010, 7(1 Pt 2):524-540.
[10]马福年, 姜 睿.自发性高血压大鼠阴茎海绵体平滑肌细胞培养及鉴定[J]. 泸州医学院学报, 2013, 36(1):10-14.
[11]Artom N, Pinna G, Musso NR, et al. Prevalence of erectile dysfunction in a cohort of Italian hypertensive subjects[J]. Clin Exp Hypertens, 2016, 38(2):143-149.
[12]Doumas M, Tsakiris A, Douma S, et al. Factors affecting the increased prevalence of erectile dysfunction in Greek hypertensive compared with normotensive subjects[J]. J Androl, 2006, 27(3):469-477.
[13]Nunes KP, Labazi H, Webb RC. New insights into hypertension-associated erectile dysfunction[J]. Curr Opin Nephrol Hypertens, 2012, 21(2):163-170.
[14]Hale TM, Hannan JL, Carrier S, et al. Targeting vascular structure for the treatment of sexual dysfunction[J]. J Sex Med, 2009, 6(Suppl 3):210-220.
[15]di Villa BR, Sorrentino R, Sorrentino R, et al. Sphingosine 1-phosphate induces endothelial nitric-oxide synthase activation through phosphorylation in human corpus cavernosum[J]. J Pharmacol Exp Ther, 2006, 316(2):703-708.
[16]Okazaki H, Ishizaka N, Sakurai T, et al. Molecular cloning of a novel putative G protein-coupled receptor expressed in the cardiovascular system[J]. Biochem Biophys Res Commun, 1993, 190(3):1104-1109.
[17]Chitaley K, Webb RC, Mills TM. RhoA/Rho-kinase: a novel player in the regulation of penile erection[J]. Int J Impot Res, 2001, 13(2):67-72.
[18]Jin L, Burnett AL. RhoA/Rho-kinase in erectile tissue: mechanisms of disease and therapeutic insights[J]. Clin Sci (Lond), 2006, 110(2):153-165.
[19]Nunes KP, Rigsby CS, Webb RC. RhoA/Rho-kinase and vascular diseases: what is the link?[J]. Cell Mol Life Sci, 2010, 67(22):3823-3836.
[20]马志鹏, 姜 睿, 程 勇. 法舒地尔对高血压大鼠勃起功能的影响[J]. 中华男科学杂志, 2012, 18(1): 11-15.
[21]Blankenbach KV, Schwalm S, Pfeilschifter J, et al. Sphingosine-1-phosphate receptor-2 antagonists: therapeutic potential and potential risks[J]. Front Pharmacol, 2016, 7:167.
[22]Cui H, Okamoto Y, Yoshioka K, et al. Sphingosine-1-phosphate receptor 2 protects against anaphylactic shock through suppression of endothelial nitric oxide synthase in mice[J]. J Allergy Clin Immunol, 2013, 132(5):1205-1214.
[23]Means CK, Brown JH. Sphingosine-1-phosphate receptor signalling in the heart[J]. Cardiovasc Res, 2009, 82(2):193-200.
[24]Aydin M, Downing K, Villegas G, et al. The sphingosine-1-phosphate pathway is upregulated in response to partial urethral obstruction in male rats and activates RhoA/Rho-kinase signalling[J]. BJU Int, 2010, 106(4):562-571.
[25]Ohmori T, Yatomi Y, Osada M, et al. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P[J]. Cardiovasc Res, 2003, 58(1):170-177.
[26]Hu W, Mahavadi S, Huang J, et al. Characterization of S1P1 and S1P2 receptor function in smooth muscle by receptor silencing and receptor protection[J]. Am J Physiol Gastrointest Liver Physiol, 2006, 291(4):G605-G610.
[27]Lorenz JN, Arend LJ, Robitz R, et al. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice[J]. Am J Physiol Regul Integr Comp Physiol, 2007, 292(1) R440-R446.
[28]Cho MC, Park K, Chai JS, et al. Involvement of sphingosine-1-phosphate/RhoA/Rho-kinase signaling pathway in corporal fibrosis following cavernous nerve injury in male rats[J]. J Sex Med, 2011, 8(3):712-721.
[29]陈学勤, 夏纪毅, 程 波, 等. 1-磷酸鞘氨醇受体1-3(S1P1-3)在雄性去势大鼠阴茎海绵体组织中的表达[J]. 中华男科学杂志, 2016, 22(5):393-400.
[30]Burchardt M, Burchardt T, Baer L, et al. Hypertension is associated with severe erectile dysfunction[J]. J Urol, 2000, 164(4):1188-1191.
(责任编辑: 卢 萍, 罗 森)
Screening of lentiviral vectors carrying effective siRNA targetingS1P2 gene
YAN Xiao1, XIA Ji-yi2, CHENG Bo1, ZHU Yong-sheng1, JIANG Rui1
(1DepartmentofUrology,2CenterofExperimentalMedicine,TheAffiliatedHospitalofSouthwestMedicalUniversity,Luzhou646000,China.E-mail:jiangrui@126.com)
AIM: To screen the lentiviral vector carrying siRNA with higher efficiency of suppressing the sphingosine-1-phosphate receptor 2 (S1P2) gene expression in the primarily cultured corpus cavernosum smooth muscle cells of spontaneously hypertensive rats (SHR). METHODS: SHR and SD rats (n=5 each) were used for primarily culturing corpus cavernosum smooth muscle cells. The cells were randomly divided into 6 groups: SHR siRNA-1, SHR siRNA-2, SHR siRNA-3, SHR GFP, SHR control (SHR non-transfection group), and SD control (SD rat control group). Each group had 5 samples with 1.0×105cells of each sample. At 72 h after transfection (MOI=60) with lentiviral vectors carryingS1P2 siRNA into the SHR corpus cavernosum smooth muscle cells, the expression of GFP was observed under fluorescence microscope. The protein expression of S1P2, ROCK1, ROCK2 and eNOS in the corpus cavernosum smooth muscle cells, and the mRNA expression of S1P2, ROCK1 and ROCK2 were determined by by Western blot and RT-PCR. RESULTS: The transfection efficiency of the corpus cavernosum smooth muscle cells in SHR siRNA-1, SHR siRNA-2, SHR siRNA-3 and SHR GFP groups were>80%. Compared with SHR control group, the mRNA levels and the protein expression of S1P2, ROCK1 and ROCK2 in SHR GFP group showed no remarkable changes, while those in SHR siRNA-1, SHR siRNA-2, SHR siRNA-3 and SD control groups were significantly lower than those in SHR control group (P<0.05). The protein expression of eNOS in SHR siRNA-1, SHR siRNA-2, SHR siRNA-3 and SHR GFP groups were not significantly changed as compared with SHR control group, but that in SD control group was significantly higher than that in SHR control group. CONCLUSION: Three groups of siRNA lentiviral vectors targetingS1P2 inhibit the expression of S1P2 in the corpus cavernosum smooth muscle cells of SHR, and by silencing theS1P2 expression, the expression of ROCK1 and ROCK2 is inhibited. Among them, siRNA-1 has the highest inhibitory efficiency.
Spontaneously hypertensive rats; Sphingosine-1-phosphate receptor 2; Small interfering RNA; Lentiviral vector
1000- 4718(2017)07- 1338- 07
2016- 11- 18
2017- 05- 05
四川省科技厅基金资助项目(No. 14JC0803);四川省教育厅基金资助项目(No. 15ZA0164);四川省人力资源和社会保障厅留学回国人员科技项目(川人社办发[2016-64]号)
R698: R393; Q-33
A
10.3969/j.issn.1000- 4718.2017.07.031
杂志网址: http://www.cjpp.net
△通讯作者 Tel: 0830-3165411; E-mail: jiangrui@126.com
