High-Altitude Hypoxia Increased Neuroglobin Expression in Rat Cerebral Cortices
2017-08-07HANShufenBAIZhenzhongCAOYueLUOPengliJINGuoenYANGYingzhongGERiLi
HAN Shu-fen,BAI Zhen-zhong,CAO Yue,LUO Peng-li,JIN Guo-en,YANG Ying-zhong,GE Ri-Li
(1.Research Center of High Altitude Medical Sciences,Qinghai University Medical College,Xining 810001, Qinghai,China;2.School of Public Health,Soochow University,Suzhou 215123,Jiangsu,China; 3.Qinghai University Affiliated Hospital,Xining 810001,Qinghai,China)
High-Altitude Hypoxia Increased Neuroglobin Expression in Rat Cerebral Cortices
HAN Shu-fen1,2,BAI Zhen-zhong1,CAO Yue1,LUO Peng-li3,JIN Guo-en1,YANG Ying-zhong1,GE Ri-Li#
(1.Research Center of High Altitude Medical Sciences,Qinghai University Medical College,Xining 810001, Qinghai,China;2.School of Public Health,Soochow University,Suzhou 215123,Jiangsu,China; 3.Qinghai University Affiliated Hospital,Xining 810001,Qinghai,China)
Objective Many researchers have observed that NGB may play a protective role in hypoxic-ischemic brain damage.The present study aimed to explore the expression and change of neuroglobin(NGB)induced by acute and chronic high-altitude hypoxia in the cerebral cortices of rats.Methods According to the principle and method of animal experimental design and animal model replication,seventy SD rats were randomly divided into the control group(10 rats,housed in Xining at 2,260m altitude)and hypoxia groups(10 rats per time point,housed in Kekexili,at 4,600m altitude).The acute and chronic high-altitude hypobaric hypoxic rats were treated in Kekexili(4,600m)National Natural Reserve Areas for 12 h,24 h,48 h,72 h,1 week and 1 month(chronic group)before dissection.NGB gene expression was determined by semi-quantitative RT-PCR,and the protein levels by western blotting.Results After exposing for 12 hours in high altitude hypoxic condition,arterial oxygen saturation(SaO2)was the lowest and heart rate(HR)was the highest,but SaO2gradually rose and HR gradually decreased with prolonged exposure time in the high-altitude,hypoxic condition.NGB rapidly and significantly increased after 24 h of exposure(mRNA expression 128.01±19.82;protein expression 11.58±3.03)at high-altitude hypoxia compared with that in the control(mRNA expression 77.78±16.89;protein expression 6.17±1.45)and then gradually decreased to the normal level at one week(mRNA expression 72.52±16.02;protein expression 5.99±1.70).But NGB expression later increased significantly after hypoxia exposure for one month(mRNA expression 99.38±21.26;protein expression 8.50±2.98)at an altitude of 4,600 m compared with the control group.Conclusion The significant increases in NGB expression in cerebral cortices after acute and chronic hypoxia at an altitude of 4,600 m suggest that NGB may play an important role in the protection of hypoxic-ischemic brain injury caused by high-altitude hypoxia and increase the tolerance to hypobaric hypoxia.The data suggest that the changes in NGB levels may represent an endogenous protective mechanism in the nervous tissue of hypoxia-injured brains.
Neuroglobin Hypoxia Brain injury Reactive oxygen species
Introduction
Globins are small heme-proteins with a characteristic three-on-three α-helical sandwich structure that have the ability to bind oxygen and supply the cells with oxygen[1,2].Hemoglobin(HGB)and myoglobin(MGB)are the most prominent members of this globin family[3].The former,found in the blood,consists of four polypeptide chains that are used to transport oxygen in the red blood cells of the vertebrate animal′s circulatory system[4].The latter,located in striated and cardiac muscles,is a monomeric protein that acts as a temporary oxygen store that may facilitate oxygen diffusion[5].Recently,two newly discovered members have joined the globin family,cytoglobin(CGB)[6]and neuroglobin(NGB)[7].The latter oxygen-carrying globulin is found mainly in the vertebrate brain tissue and is also identified as an intracellular respiratory protein expressed in peripheral neurons and endocrine cells[8,9].NGB appears to have a characteristic three-on-three α-helical sandwich structure[10,11]and is the first of a hexacoordinated globin[12,13]that binds O2with a similar affinity to that of myoglobin and may function as a“neuronal myoglobin”[14],providing oxygen to the respiratory chain.The physiological role of NGB is not completely understood,but it may serve as an intracellular oxygen-carrier or oxygen-sensor[12],a terminal oxidase to regenerate NAD+under anaerobic conditions[15],or a regulator[16,17]of nitric oxide(NO)and reactive oxygen species(ROS)metabolism.
As the vertebrate nervous system is particularly sensitive to hypoxia, an intracellular protein that helps sustain cellular respiration would aid hypoxic survival. However, high-altitude hypoxia is a major physiological challenge and can be regarded as a stressful environmental condition to which organisms have the capacity to respond by adaptation[18].Several studies[19-26]have observed that NGB may play a protective role in hypoxic-ischemic brain damage.Nevertheless,the presence of an intracellular respiratory protein,which could enhance the uptake of oxygen in the high-altitude hypobaric hypoxic environment,is still unknown.In order to understand the pathogenesis and prevention of hypoxic brain injury at high altitude,we studied the expression and change of NGB in cerebral cortices after rats were exposed to an altitude of 4,600 m.
Material and Methods
Animal preparation
This animal study was pre-approved by the Ethics Committee of Qinghai University(Xining,China)and by China Zoological Society.The protocol of the investigation was in accordance with the principles outlined in China Practice for the Care and Use of Laboratory Animals.Male and healthy SD rats weighing between 180 g and 200 g were obtained from Experimental Animal Center of Qinghai Endemic Institute.
Hypoxic treatment
According to the principle and method of animal experimental design and animal model replication,SD rats were randomly assigned to hypoxia treatment(10 rats per time point,housed in Kekexili,at 4,600m altitude)or control group(10 rats,housed in Xining at 2,260m altitude).Hypoxia treatment was performed at an altitude of 4,600 m of Kekexili Natural Reservation in Qinghai Province,People′s Republic of China,in June of 2006.Levels of arterial oxygen saturation(SaO2)and heart rate(HR)in hypoxia groups were measured with Datex Ohmeda(Finland,Co.,Ltd)in 10 rats at 12 h,24 h,48 h,72 h,1 week,and 1month(chronic group)after hypoxia treatment and then sacrificed.The cerebral cortices were rapidly dissected and immediately stored in liquid nitrogen.All the instruments were treated in 180 ℃ for 8 h and all the reagents were dissolved with DEPC-treated water.The control animals were raised with the same method at an altitude of 2,260 m in Xining,Qinghai Province.
RNA extraction and primer design
RNAs were extracted from the SD rat cerebral cortices and purified using TRIzol regent(Invitrogen,Co.,Ltd.)following the Kit′s protocol and the RNAs were treated with DNAse I to eliminate DNA contamination.The concentration of RNAs in each sample were quantified with EPPENDORF 6131 nuclear detector(EPPENDORF,Co.,Ltd.),and the samples that showed a ratio of A260/A0280>1.8 were used for the experiment.The primers were designed according to SD rat NGB cDNA sequence using Dnaman 6.0 software by Aoke(Beijing,Co.,Ltd.).The forward primer was NGB-F:TGCTGCCCCTCTTCCAGTAC,the reverse primer was NGB-R:CTTCTCCAGCATGTAGAGCA,and the length of the amplified product was 245 bp.To ensure equal loading between samples,the forward primer of β-actin(GTACCACTGGCATCGTGATGGACT)and the reverse primer of β-actin(ATCCACACGGAGTACTTGCGCTCA)were used under the same conditions to amplify β-actin as a 583-bp product.
cDNA cloning and sequencing
Reverse transcription(RT)was performed using M-MLV Reverse Transcription Kit(Fermentas,Co.,Ltd)to obtain the cDNA.Briefly,2 μg of total RNAs were used in each reverse transcription reaction(20μL)to obtain the cDNA.For the subsequent PCR reaction,1 μL of diluted cDNAs was used as the template in a 30 μL reaction.The reaction was heated for 5 min at 94 ℃ followed by 35 cycles of a standard PCR protocol(94℃ 30″,58℃ 35″,72℃ 1′)and a final elongation at 72 ℃ for 8 min.The 245-bp PCR products were eluted from the agrose gel,purified with QIAquick Gel Extraction Kit(Clontech,Co.,Ltd.),and cloned into the pGEM-T Easy vector(Promega,Co.,Ltd.)using PCR-generated EcoRI restriction sites.The recombinants selected by the white-blue selection method were sequenced by an ABI 377 DNA Analyzer using the dye-terminator chemistry(Invitrogen,Co.,Ltd.).The positive clone was identified based on BLAST analyses.
Semi-quantitative RT-PCR
Equal amounts of total RNAs from each group were quantified with an EPPENDORF 6131 nuclear detector(EPPENDORF,Co.,Ltd.).The RT-PCR and PCR amplification reactions were the same as described above.The PCR amplification products were separated by 1.5% agarose gel electrophoresis,visualized by ethidium bromide,and photographed using a digital camera.The expression levels of NGB gene in the cerebral cortex from different groups of SD rats were measured by densitometry based on the specific bands.For semi-quantitative measurement of NGB transcript levels,the RT-PCR signal intensities were normalized to β-actin cDNA.Data were expressed as percent increase above the control.
Protein extraction and western blotting
After the hypoxic treatment,SD rat cerebral cortices used for protein analyses were cooled down on ice and immediately homogenized in 1% SDS,5% β-mercaptoethanol,10% glycine,and 65 mM Tris(pH 6.8).Total proteins were heat-denatured at 95 ℃ for 5 min and the concentration was determined using BCA Protein Analysis Kit(Pierce,Co.,Ltd.).Equal amounts of proteins were loaded to a 12% SDS-polyacrylamide gel and were transferred to PVDF membrane(Millipore,Co.,Ltd.) for 110 min at 60 V constant.Nonspecific binding sites were blocked by incubation for 1 h with 2% nonfat dry milk in TBST(10mM/L Tris-HCl,50mM/L NaCl,0.05 % Tween-20,pH 7.6).The membrane was incubated with a rabbit polyclonal anti-NGB antibody(Santa Cruz,Co.,Ltd.,diluted to 1:500)overnight at 4 ℃.The membrane was washed three times for 5 min in TBST and incubated for 2 h with a horseradish peroxidase-conjugated(HRP)anti-rabbit secondary antibody(Cell Signal,Co.,Ltd.)in room temperature.The membrane was washed in TBST as described above and the immunolabeled bands were detected with the NEN chemiluminescence substrate system(Pierce,Co.,Ltd.).
Image and statistical analysis
Signals of RT-PCR and western blotting were quantified after scanning of the bands with LabWorks 4.0 Image Analysis System and the mean grey values of the background of empty gel lanes were subtracted from the measurements and compared to beta-actin.Statistical analysis was performed using the SPSS statistical software version13.0.Results are expressed as means±SD.A one-way analysis of variance(ANOVA)was performed for the differences in these groups,followed by the LSD post hoc test.P values less than 0.05 were considered statistically significant.
Results
We first examined the Arterial Oxygen Saturation(SaO2)and heart rate(HR)of SD rats after exposure to high-altitude hypobaric hypoxia.We found that SaO2was the lowest and HR was the highest after 12 h of hypoxia and then SaO2increased slowly and HR gradually decreased.There were significant differences in both SaO2values and HR values of hypoxia-treated groups with that in the control group(P<0.05)(Table 1).
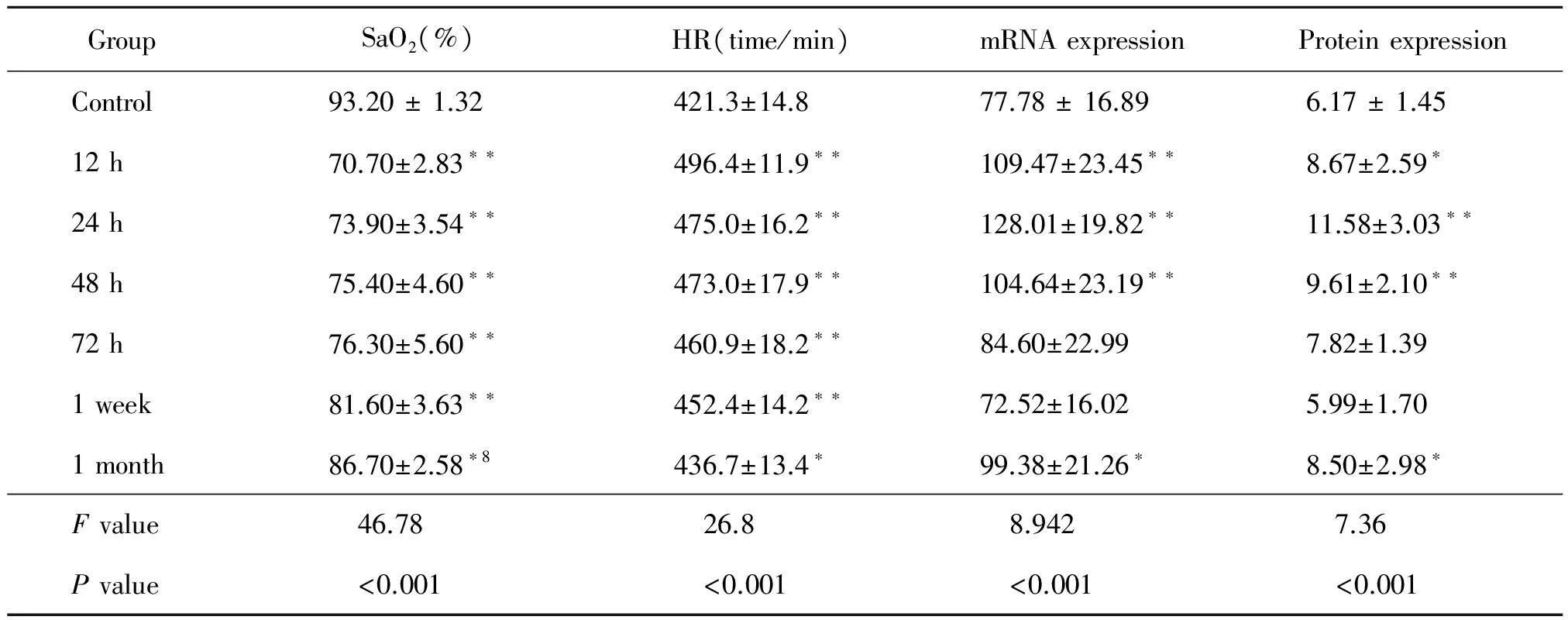
Table 1 Relationship between high-altitude hypobaric hypoxic exposure of SD rats and SaO2,HR,NGB mRNA,protein expression(means±SD n=10)
Notes:SaO2,Arterial Oxygen Saturation;HR,heart rate;hypoxia groups versus control for F and P values by ANOVA analyses;**P<0.01;*P<0.05.
Rat NGB gene cloning
The nucleotide sequence of SD rat cDNA has been determined previously.We designed multiple primers that allowed amplifying overlapping fragments of the SD rat gene by PCRs.The sequences of the PCR fragments were essentially identical(>98%)to that previously cloned as shown by BLAST analyses.In addition,the PCR products were resolved by 1.5% agarose electrophoresis and a 245 bp DNA segment was seen as expected(Figure 1).

A 245bp DNA segment was seen,which was expected.Lane 1-12 shows the results under different annealing temperatures from 55 ℃ to 62 ℃.The optimal temperature of PCR reaction was deemed to 58 ℃ with a believable result.
Figure 1 Gradient PCR product of the cDNA of NGB in 1.5% agarose electrophoresis Changes of NGB gene expression in high-altitude hypoxic SD rats
The amplified PCR product was consistent with the expected size of the fragment based on the NGB primers of the SD rat NGB sequence,and therefore,the primers were reliable.The RT-PCR results showed that NGB mRNA expression increased rapidly and reached the peak level after an exposure of 24 h(128.01±19.82)at the high-altitude hypobaric hypoxia condition,which was significantly different from the control group(77.78±16.89)(P<0.01).NGB mRNA expression declined over time and returned to the normal level after one week of exposure to hypoxia(72.52±16.02),leading to no difference from the control group(P=0.572).After exposure for one month(99.38±21.26),NGB mRNA expression increased slowly once more and stayed at a higher level(P=0.023)(Table 1 and Figure 2).
Hypoxia.NGB mRNA levels were determined by semi-quantitative RT-PCR.Control group was housed in Xining and
hypoxic groups were housed in Kekexili National Natural Reserve Areas.
Figure 2 Expression of NGB mRNA in SD rats′ cerebral cortices exposed to high-altitude hypobaric Quantitative western blotting
Proteins were extracted from the cerebral cortices of SD rats that had been housed for 12 h,24 h,48 h,72 h,1week,and 1 month at high-altitude hypobaric hypoxia(altitude 4600m).Equal amounts of total protein extracts(20μg per lane,Figure.3)were analyzed by quantitative western blotting.The results showed that there were two specific bands,NGB protein at 17 kDa and GAPDH at 35 kDa.The changes in NGB protein level were consistent with its gene expression.NGB protein level peaked after an exposure of 24 h(11.58±3.03)and then began to decrease.NGB recovered to the baseline level after one week of hypoxia(5.99±1.70)and then increased slowly.There were significant differences between the control group(6.17±1.45)and the hypoxia groups(Table 1 and Figure 3).

Hypobaric hypoxia.NGB protein levels were determined by swestern blot.Control group was housed in Xining and hypoxic groups were housed in Kekexili National Natural Reserve Areas.
Figure 3 Expression of NGB protein expression in SD rats′ cerebral cortices exposed to high-altitude
Discussion
NGB is a recently identified member of the vertebrate globin superfamily[7]and is probably present in all vertebrate taxa including fish[27,28],amphibians and birds.NGB is an endogenous neuroprotectant[29]Although most studies support the idea of an important role of NGB in oxygen homeostasis of nerve tissues[7,9,30-32],other roles or additional functions of NGB are also conceivable[12,30]and many open questions remain to be answered.Peroni et al.[33]suggest a way of treating cerebrovascular disease and brain injury at high altitudes using NGB protein.Hypoxia inevitably results in the death of neurons and the partial loss of nerve function owing to insufficient supply of oxygen.It is inferred that delivery of NGB-stored oxygen could partially buffer the changes of oxygen pressure,delay the death of cells,and maintain normal nerve functions.
Our study showed that SaO2was the lowest and HR was the highest after animals were exposed for 12 h at an elevation of 4600 m,and then SaO2increased gradually and HR decreased slowly as the exposure time prolonged,which may be due to high-altitude acclimatization.The changes in NGB mRNA level and protein level in rat cerebral cortices showed the same trends after acute and chronic high-altitude hypobaric hypoxia.NGB mRNA and protein significantly increased after the exposure to an elevation of 4600 m,suggesting that NGB protein may play protective roles in the adaptation of high-altitude hypoxia.The expression of NGB increased gradually as the exposure time prolonged and reached a peak after 24 h,suggesting that NGB protein was very sensitive to high-altitude hypoxia.In future investigations of the response mechanism,we may consider the following aspects.Zhu et al.[34]demonstrated that high-altitude hypoxia induced to extensive damages of cerebral microvasculature,disorder of brain cell metabolism,inactivation of the Na+-K+-ATP enzyme,increase in intracellular osmotic pressure,and neuronal oxidative stress.The compensative up-regulation of NGB expression may serve to increase the ventilation of neurons in order to maintain the stability of cellular membrane.We speculate that acute hypoxia may cause fluid retention andbrain blood redistribution,which may induce the up-regulation of NGB.
There was a significant difference in SaO2levels between the control group and one-month hypoxia group.It was possible that the production and clearance of active oxygen was imbalanced after long-term hypobaric hypoxia.The increase of NO and ROS from cerebral vascular endothelial cells and glia can destroy the normal structure of phospholipid membrane,leading to cytolysis and increase of permeability of the blood brain barrier(BBB),and eventually leading to vasogenic brain edema. However,the neuroprotective effect might be due to radical scavenging or activation of protective mechanisms[30].NGB may scavenge endosomatic ROS such as H2O2and NO2. Herold et al[17]and Fordel et al[35]also demonstrated that NGB play an important role in resisting oxidative damage.NGB is possible sensor of oxidative stress,which prolongs the survival of neurons[36,37].
A myoglobin-like role of NGB is also corroborated by the correlation of NGB expression with oxygen consumption levels[38]and its capability to enhance neuronal survival[20].In addition,Khan et al[39,40]has showed that NGB may protect neurons by inhibiting the formation of a death-signaling membrane complex,and interventions that increase NGB expression could have therapeutic application in AD and other neurodegenerative disorders.The physiological role of NGB is conceivable;therefore,the study of NGB may open new avenues for the prevention of hypoxic-ischemic injury that causes multiple-organ dysfunction syndrome in high-altitude environments.High altitude cerebral edema(HACE)is the same as vasogenic brain edema[41].Dysbolism of brain cells and an increase of oxygen free radicals caused by high-altitude hypoxia play a key role in the pathogenesis of HACE.Our study suggests that an increase of NGB after hypoxic exposure is an endogenous neuroprotective response to cerebral hypoxia at high altitude.
[1]Dickerson RE,Geis I.Hemoglobin:structure,function,evolution,and pathology.Menlo Park,CA:Benjamin/Cummings,1983.
[2]Vogt M,Puntschart A,Geiser J ,et al.Molecular adaptation in human skeletal muscle to endurance training under simulated high-altitude condition.J Appl Physiol,2001,91(1):173-182.
[3]Antonini E,Brunori M.Hemoglobin and myoglobin in their reactions with ligands.North-Holland,Amsterdam,1971.
[4]Bunn HF.Evolution of mammalian hemoglobin function.Blood 1981,58(2):189-197.
[5]Brunori M.Nitric oxide,cytochrome-c oxidase and myglobin.Trends Biochem Sci,2001,26(1):21-23.
[6]Burmester T,Ebner B,Weich B,et al.Cytoglobin:a novel globin type ubiquitously expressed in vertebrate tissue.Mol Biol Evol,2002,19(4):416-421.
[7]Burmester T,Weich B,Reinhardt S,et al.A vertebrate globin expressed in the brain.Nature,2000,407(28):520-523.
[8]Reuss S,Saaler-Reinhart S,Weich B,et al.Expression analysis of neuroglobin mRNA in rodent tissues.Neuroscience,2002,115(3):645-656.
[9]Geuens E,Brouns I,Flamez D,et al.A globin in the nucleus.J Biol Chem 2003,278(33):30417-30420.
[10]Pesce A,Dewild S,Burmester T ,et al.The human brain hexacoordinated neuroglobin three-dimensional structure.Micron,2004,35:63-65.
[11]Alessandra P,Sylvia D,Marco N,et al.Human brain neuroglobin structure reveals a distinct mode of controlling oxygen affinity.Structure,2003,11:1087-1096.
[12]Counture M,Weich B,Reinhardt S,et al.The heme environment of mouse neuroglobin.J Biol Chem 2001,276:36377-36382.
[13]Trent JT,Watts RA,Hargrove MS.Human neuroglobin,a hexacoordinate hemoglobin that reversibly binds oxygen.J Biol Chem,2001,276:30106-30110.
[14]Burmester T,Hankeln T.Neuroglobin,a respiratory protein of the nervous system.News Physiol Sci,2004,19:110-113.
[15]Dewilde S,Kinger I,Burmester T,et al.Biochemical characterization and ligand binding properties of neuroglobin,a novel member of the globin family.J Biol Chem,2001,276(42):38949-38955.
[16]Doorsaler SV,Dewilde S,Kiger L,et al.Nitric oxide binding properties of neuroglobin,J Biol Chem,2003,278(7):4919-4925.
[17]Herold S,Fago A,Weber R E,et al.Reactivity studies of the Fe(Ⅲ)and Fe(Ⅱ)NO forms of human neuroglobin reveal a potential role against oxidative stress.J Biol Chem 2004,279(22):22841-22847.
[18]Bligh J,Johnson K.G.Glossary of terms for thermal physiology.J Appl Physiol,1973,35:941-961.
[19]Sun Y J,Jin K L,Greenberg D A,et al.Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury.PNAS,2001,98(26):15306-15311.
[20]Sun Y,Jin K,Peel A,et al.Neuroglobin protects the brain from experimental stroke in vivo.PNAS,2003,100(6):3497-3500.
[21]Shang A,Zhou D,Wang L,et al.Increased neuroglobin levels in the cerebral cortex and serum after ischemia-reperfusion insults.Brain Res,2006,1078(1):219-226.
[22]Milton S L,Nayak G,Lutz P L,et al.Gene transcription of neuroglobin is upregulated by hypoxia and anoxia in the brain of the anoxia-tolerant turtle Trachemys scripta.J Biol Sci,2006,13:509-514.
[23]Wang X.L,Liu X,Zhu H.H,et al.Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia.Stroke,2008,(39):1869-1874.
[24]Schmidt-Kastner R,Habrtkamp M,Schmitz C,et al.Neuroglobin mRNA expression after transient global brain ischemia and prolonged hypoxia in cell culture.Brain Res,2006,1103(1):173-180.
[25]Hundahl C,Stoltenberg M,Fago A,et al.Effects of short-term hypoxia on neuroglobin levels and localization in mouse brain tissues.Neuropathol ApplNeurobiol,2005,31(6):610-617.
[26]Hundahl C,Kelsen J,Kjaer K,et al.Does neuroglobin protect neurons from ischemic insult?A quantitative investigation of neuroglobin expression following transient MCAo in spontaneously hypertensive rats.Brain Res,2006,1085(1):19-27.
[27]Awenius C,Hankeln T,Burmester T.Neuroglobins from the Zebrafish Danil mrerio and the Pufferfish Tetraodon nigrovirdis.Biochem Biophys Res Commun,2001,287(2):418-421.
[28]Fuchs C,Heib V,Kiger L,et al.Zebrafish reveals different and conserved features of vertebrate neuroglobin gene structure,expression pattern and ligand bingding.J Biol Chem,2004,279(23):24116-24122.
[29]Greenberg DA,Jin KN,Khan AA.Neuroglobin:an endogenous neuroprotectant.Neuroscience,2008,8:20-24.
[30]Brunori M,Vallone B.Neuroglobin,seven years after.Cell Mol Life Sci,2007,64:1259-1268.
[31]Hackett P,Roach R.High altitude cerebral edema.High Alt Med Biol,2004,5(2):136-146.
[32]Khan A A,Mao X,Banwait S,et al.Neuroglobin attenuates β-amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo.PNAS,2007,104(48):19114-19119.
[33]Peroni D,Negro A,Bahr M,et al.Intracellular delivery of neuroglobin using HIV-1 TAT protein transduction domain fails to protect against oxygen and glucose deprivation.Neurosci Lett,2007,421(2):110-114.
[34]Zhu Qi-quan,Wang Jing,Wang Yun-li,et al.Effects of oxygen free radical on the blood brain barrier after high-altitude exposure.Journal of Chinese Circulation,2007,11:149-153.
[35]Fordel E,Thijs L,Martinet W,et al.Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death.Neurosci Lett,2006,410(2):146-151.
[36]Wakasugi K,Nakano T Morishima I.Oxidized-human neuroglobin acts as a heterotrimeric G-alpha protein guanine nucleotide dissociation inhibitor.J Biol Chem,2003,278(38):36505-36512.
[37]Wilson M T,Reeder B J.Oxygen-binding haem proteins.Exp Physiol,2008,93(1):128-132.
[38]Schmidt M,Giessl A,Laufs T,et al.How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply of the mammalian retina.J Biol Chem,2003,278:1932-1935.
[39]Khan A,Sun Y,W ang Y,et a1.Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia.PNAS,2006,103(47):17944-17948.
[40]Khan A A,Mao X,Banwait S,et al.Regulation of hypoxic neuronal death signaling by neuroglobin.PNAS,2008,28(50):13511-13521.
[41]Hankeln T,Ebner B,Fuehs C,et a1.Neuroglobin and cytoglobin in search of their role in the vertebrate globin family.J Inorg Bio,2005,99(1):110-119.
[编辑 马爽]
高原低氧增加大鼠大脑皮层脑红蛋白的表达*
韩淑芬1,2,白振忠1,曹越1,罗朋立3,靳国恩1,杨应忠1,格日力1#
(1.青海大学医学院高原医学研究中心,青海 西宁 810001; 2.苏州大学公共卫生学院,江苏 苏州 215123;3.青海大学附属医院,青海 西宁 810001)
目的 大量研究显示脑红蛋白(neuroglobin,NGB)对缺氧缺血性脑损伤具有保护作用。本研究探讨了SD大鼠暴露于高原低压低氧后NGB的表达情况,为阐明高原低氧性脑损伤的发生机制提供了新的思路。方法 70只雄性SD大鼠按体重随机分为两组,正常对照组(N=10,西宁,海拔2260米)和实验组(N=60,可可西里,海拔4600米),实验组是经西宁陆运至可可西里(海拔4600米)制作的急慢性高原缺氧模型组;依据高原暴露时间不同,将实验组又随机分为缺氧12 h组、24 h组、48 h组、72 h组、168 h组、720 h组(慢性组),每组10只。采用RT-PCR和Western blot检测高原低氧暴露不同时间脑皮质神经元中NGB mRNA和蛋白的表达含量。结果 大鼠初入特高海拔地区后,SaO2较对照组明显下降(P<0.01),适应一段时间后SaO2开始回升,但168 h组和720 h组仍明显低于对照组(P<0.05);HR的变化则相反。与对照组相比,高原低压低氧暴露24 h(mRNA表达128.01±19.82;蛋白表达11.58±3.03)脑皮质神经元中NGB的表达出现首个高峰,此后逐渐下降,至168 h(mRNA表达72.52±16.02;蛋白表达5.99±1.70)恢复正常(P>0.05),而后有缓慢升高趋势,至720 h(mRNA表达99.38±21.26;蛋白表达8.50±2.98)后保持一个较高水平。结论 急慢性高原低氧环境下,脑红蛋白表达的增高,增强了大鼠对高原低氧的耐受性,提示NGB表达变化可能是脑组织对脑缺氧的一种内源性神经保护反应,增加了脑组织的供氧,维持了神经元的正常功能。
脑红蛋白 低氧 脑损伤 活性氧
R363.1
A
10.13452/j.cnki.jqmc.2017.02.001
※This study was funded by a grant from 973 national project of China(2006CB504100)and National Natural Science Foundation of China(No.30260034).We are grateful to Qinghai University and staffs of Kekexili Natural Reservation. Han Shufen(1981~),female,Associate Professor
#:Corresponding author:Ph.D.& Professor,Tel:+86-971-6142063,E-mail:geriligao@hotmail.com
