过氧化物酶体增殖物激活受体—γ激动剂对急性胰腺炎大鼠肺损伤的保护机制研究
2017-07-25陆贝于源泉殷俊杰蔡阳
陆贝 于源泉 殷俊杰 蔡阳
[摘要] 目的 探讨过氧化物酶体增殖物激活受体-γ激动剂对胰腺炎大鼠肺损伤的保护作用及调节机制。 方法 72只大鼠随机分为假手术组、模型组和罗格列酮组,各组再分为术后6 h、12 h、24 h组。模型组采用两次腹腔注射L-精氨酸制备SAP模型,罗格列酮组术后30 min静脉注射10%罗格列酮6 mg/kg,假手术组腹腔注射等体积生理盐水。观察大鼠肺病理改变,测定肺TLR4、NF-κB、肺髓过氧化物酶活性、肺干/湿重比、血清TNF-α、IL-1β、IL-6含量。使用重复测量方差分析,SNK-q检验比较差异。 结果 ROSI组大鼠肺组织病理损害较SAP组减轻;与SAP组比较,肺内NF-κB、TLR4蛋白下降(P<0.05),髓过氧化物酶活性及肺干/湿重比降低(P<0.05);ROSI组外周血IL-1β、IL-6、TNF-α含量与SAP组比较明显下降(P<0.05)。 结论 PPAR-γ激动剂能减轻急性胰腺炎大鼠肺组织的损伤,抑制NF-κB、TLR4表达,降低促炎细胞因子水平,推测PPAR-γ激动剂对NF-κB/TLR4通路的调节可能是胰腺炎的保护机制之一。
[关键词] 急性胰腺炎;过氧化物酶体增殖物激活受体;核转录因子-κB;Toll样受体
[中图分类号] R576 [文献标识码] A [文章编号] 1673-9701(2017)18-0038-04
Protection of PPAR-γ agonists on lung injury in rats with acute pancreatitis
LU Bei1 YU Yuanquan2 YIN Junjie1 CAI Yang1
1.Department of HPB Surgery, Hangzhou First Peoples Hospital, Hangzhou 310006, China; 2.Department of HPB Surgery, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou 310009, China
[Abstract] Objective To find the protection of PPAR-γ agonists on injured lung cells in rats and mechanism during acute pancreatitis. Methods Rats were divided randomly into three groups: sham operation group, SAP model group, rosiglitazone group. Each group was divided into 6 h, 12 h, and 24 h group after operation. SAP rats model were made via twice peritoneal injection of L-arginine. Rats in ROSI group were injected with 6 mg/kg of 10% rosiglitazone via femoral vein 30 min after operation. Sham operation group rats were peritoneally injected of same volume NS as SAP group. Pathological changes of lung tissue, TLR4, NF-κB, MPO, dry and wet ratio in lung tissue were tested. Serum TNF-α, interieukin-1β, interieukin-6 were also determined. Results The pathological injuries of lung cell were relieved in ROSI group compared with SAP group. There were statistic differences in TLR4, NF-κB, MPO, dry and wet ratio in lung between ROSI and SAP group, which were decreased in ROSI group compared with SAP group(P<0.05). TNF-α, IL-1β, IL-6 were decreased in ROSI group compared with those in SAP group(P<0.05). Conclusion The lung tissue injury during SAP may relived by PPAR-γ agonists, and also decrease NF-kappa B, TLR4 and inflammatory cytokines. The probable reason is that PPAR-γ agonists protect pancreas by adjusting NF-κB/TLR4 signal pathway.
[Key words] Severe acute pancreatitis; Peroxisome proliferator activating receptor; Nuclear factor-kappa B; Toll-like receptor
由重癥急性胰腺炎引发的胰外损害中急性肺损伤较为常见,严重者可发生呼吸衰竭甚至死亡。胰腺连锁炎症反应与核转录因子-κB(nuclear transcription factor-κB,NF-κB)关系密切,其中过氧化物酶体增殖物激活受体-γ(peroxisome proliferators-activated receptor-γ,PPAR-γ)激动剂如罗格列酮可能与NF-κB活化抑制以及AP进展有关。本研究证明罗格列酮能减轻SAP大鼠肺损害,其机制可能与NF-κB/TLR4相关。
1 材料与方法
1.1实验材料
选择72只SD大鼠,体质量269.7~320.4 g(浙江省中医药大学实验动物中心提供)。戊巴比妥钠、L-精氨酸针(Sigma,USA),罗格列酮(Cayman,USA),NF-κB、TLR4抗体(Santa Cruz,USA)、MPO等ELISA试剂盒购自R&D公司(R&D,USA)。
1.2 方法
1.2.1 建模与分组 大鼠随机平均分为三组:模型组(SAP组)、罗格列酮组(ROSI组)、假手术组(SO组)(n=24),术后每组再随机平均分为三组:6 h、12 h、24 h组(n=8)。2.5%戊巴比妥钠0.2 mL/100 g腹腔内注射麻醉成功。SAP组:腹腔内注射浓度为20 g/L的L-精氨酸两次,间隔1 h,每次2.0 g/kg,制模成功。SO组:腹腔内注射等体积生理盐水两次,间隔1 h。ROSI组:术后30 min股静脉注射10%罗格列酮溶液6 mg/kg。
1.2.2 肺组织病理 取右肺前叶HE染色制备组织切片。
1.2.3 肺TLR4、NF-κB检测 取右肺中叶参照试剂盒说明书检测TLR4、NF-κB。结果判定:光镜下随机取5个视野,阳性单位(positive unit)=阳性细胞平均光密度×阳性细胞面积比[3]。
1.2.4 肺髓过氧化物酶活性 取右肺后叶组织,制成组织匀浆后用酶联免疫吸附试验检测MPO活性,具体操作参照MPO ELISA试剂盒说明书。
1.2.5 肺含水量(干/湿重比) 取左肺叶组织,去除脂肪,滤纸吸干表面液体,置电子天平称量左肺湿重。置80℃烤箱干燥48 h,称量干重,计算肺组织干/湿重比。
1.2.6 细胞因子检测 心脏采血后,用ELISA试剂盒测定血清肿瘤坏死因子及白细胞介素1β、白介素6的含量。
1.3 統计学方法
使用SPSS16.0统计学软件分析,用SNK-q检验及重复测量方差分析比较结果。P<0.05为差异有统计学意义。
2 结果
2.1 肺组织病理变化
SO组肺组织病理无明显改变,见封三图3;SAP组肺组织肉眼可见明显点状出血斑,光镜下肺间质、肺泡、气管出血,间质及血管内中性粒细胞浸润明显,肺泡隔增宽,肺泡破裂、塌陷,见封三图4。与SAP组相比,ROSI组给药12 h后病理改变程度减轻,见封三图5。
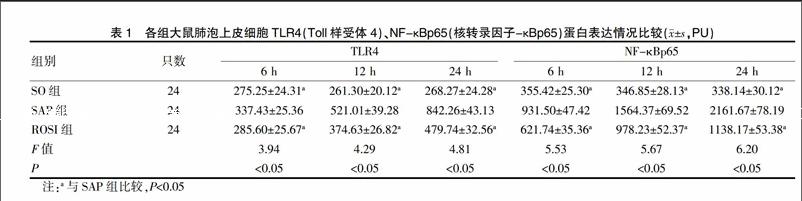
2.2 肺组织TLR4、NF-κB比较
SAP组肺泡上皮细胞TLR4、NF-κBp65比SO组升高明显(P<0.05)。ROSI组比SAP组降低明显(P<0.05),见表1。
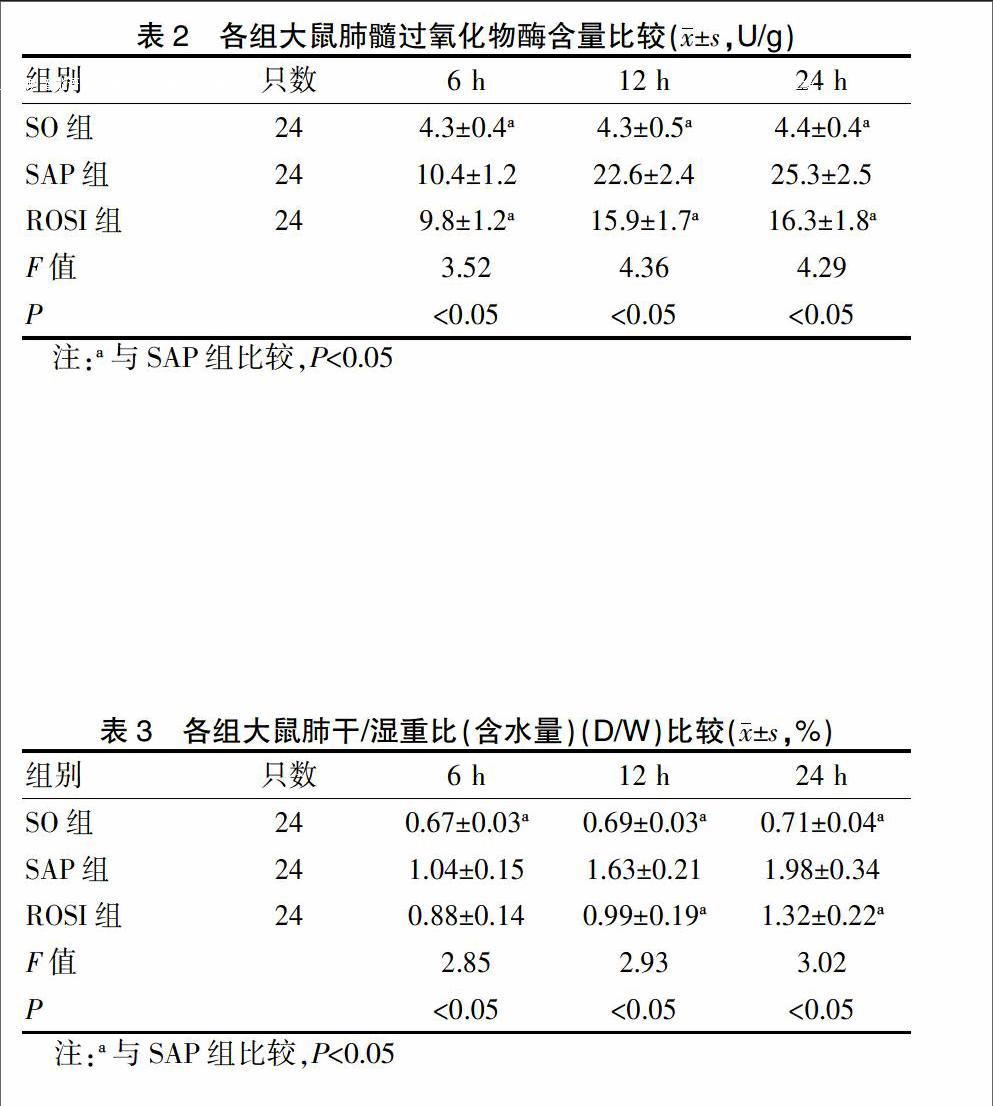
2.3 肺髓过氧化物酶活性
SAP组肺组织MPO活性较SO组明显升高(P<0.05),ROSI组MPO活性不同程度低于SAP组(P<0.05),差异有统计学意义,见表2。
2.4 各组肺干/湿重比(含水量)比较
SAP组肺脏干/湿重比较SO组增高(P<0.05),并随时间延长逐渐递增,ROSI组干/湿重比与SAP组比较有统计学差异(P<0.05)(除6 h),见表3。
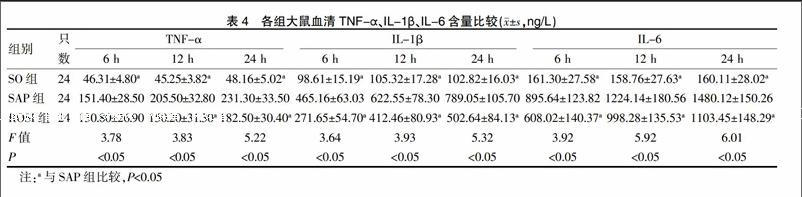
2.5 各组血清炎症细胞因子比较
SAP组、ROSI组TNF-α、IL-1β、IL-6等细胞因子均有不同程度升高,但两组比较差异有统计学意义(P<0.05)(除6 h),见表4。
3讨论
重症急性胰腺炎除自身的出血坏死以外,早期胰外器官损伤中急性肺损伤(acute lung injury,ALI)的比例超过50%,早期因呼吸衰竭死亡患者比例高达60%[1-2]。但SAP引起ALI的具体机制比较复杂,可能与中性粒细胞、巨噬细胞、肥大细胞等炎症反应细胞有关,或者与TNF-α、IL等炎症因子和趋化因子的作用有关,也可能与血小板活化因子、核因子-κB、基质金属蛋白酶等有关。核转录因子-κB控制多种炎症因子,如TNF-α、IL-6、IL-8的表达,介导多种组织细胞凋亡,过度表达可对胰腺及胰外组织细胞造成不可逆损伤,因此被认为是SAP炎症级联瀑布反应的上游靶点。抑制NF-κB活化后,胰腺及胰外器官的损害减轻,炎症细胞因子含量降低[3-9]。Jiang C等[10]在上世纪末第一次证实PPAR-γ激动剂对炎症细胞因子有调节作用,具体与NF-κB基因表达抑制和细胞因子降低密切相关。Hashimoto等[11]通过对AP大鼠注射PPAR-γ激动剂,证实能降低NF-κB活性,首次在动物层面证明PPAR-γ激动剂对AP治疗有效。大多数实验研究表明抑制NF-κB对AP有利,但同时也可能加重AP,并且该双重作用可能与抑制剂的剂量相关,当大剂量使用NF-κB抑制剂时,细胞凋亡过程被同时阻断,导致组织损伤加重[12]。
本实验中SAP组大鼠肺组织有明显的急性损伤病理改变,ROSI组大鼠在SAP造模后30 min通过股静脉注射10%罗格列酮,大体及镜下肺病理损伤有减轻,肺组织NF-κB、TLR4活性下降,外周血IL-1β、IL-6、TNF-α下调,肺组织MPO活性及肺含水量(干/湿重比)下降,对比差异有统计学意义,罗格列酮的抑制作用与其他学者研究结果一致,并且罗格列酮的作用机制与NF-κB/TLR4通路相关,降低外周血细胞因子水平,减轻胰腺炎造成的肺损害。
罗格列酮是高选择性PPAR-γ激动剂,已广泛投入临床用于治疗糖尿病等疾病,同时它也发挥着独特的抗炎作用[13-22]。裴红红等[23]发现罗格列酮能抑制NF-κB表达,减轻AP大鼠胰腺损伤,罗格列酮抑制剂可加重胰腺损伤,NF-κB表达增加。Ivashchenko等[24]对去除PPAR-γ表达的AP大鼠注射罗格列酮,发现其保护作用减弱明显,间接证实PPAR-γ在AP过程中的重要作用。本研究进一步证实罗格列酮对AP大鼠的肺损伤有保护作用,NF-κB/TLR4通路与此过程密切相关,抑制NF-κB/TLR4通路能调控炎症反应,减轻组织器官损伤,希望不久的将来能为SAP肺损伤患者提供确切的治疗。
[參考文献]
[1] 中华医学会消化病学分会胰腺疾病学组,《中华胰腺病杂志》编辑委员会,《中华消化杂志》编辑委员会.中国急性胰腺炎诊治指南(2013,上海)[J].中华胰腺病杂志,2013,13(2):73-78.
[2] Shields CJ,Winter DC,Redmond HP. Lung injury in acute pancreatitis:Mechanisms,prevention,and therapy[J].Curr Opin Crit Care,2002,8(2):158-163.
[3] Kan SH,Huang F,Tang J,et al.Role of intrapulmonary expression of inducible nitric oxide synthase gene ang nuclear factor kappa B activation in severe pancreatitis associated lung injury[J]. Inflammation,2010,33(5):287-294.
[4] Yang R,Uchiyama T,Alber SM,et al. Ethyl pyruvate ameliorates distant organ injury in a urine model of acute necrotizing pancreatitis[J]. Crit Care Med,2004,32(7):1453-1459.
[5] Letoha T,Somlai C,Takacs T,et al. A nuclear import inhibitory peptide amcliorates the severity of cholecystokinin indeced acute pancreatitis[J]. World J Gastroenterol,2005,11(7):990-999.
[6] Masamune A,Shimosegawa T,Satoh A,et al.Nitric oxide decreases endothelial activation by rat experimental severe pancreatiti associated ascitic fluids[J]. Pancreas,2000,20(3):297-304.
[7] Mia ZH,Mia QY,Wang LC,et al.Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis[J]. Inflamm Res,2005,54(12):522-527.
[8] Letoba T,Kusz E,Papai G,et al. In vitro and in vivo nuclear factor-kappa B inhibitory effects of the cell-penetrating penetratin peptide[J]. Mol Pharmacol,2006,69(6):2027-2036.
[9] Ceyhan GO,Timm AK,Bergmann F,et al. Prophymlamctic glycine administration attenuates pancreatic damage and inflammation in experimental acute pancreatitis[J]. Pancreatology,2011,11(1):57-67.
[10] Jiang C,Ting AT,Seed B.PPAR gamma agonists inhibit production of monocyte inflammatory cytokines[J].Nature,1998,391(6662):82-86.
[11] Hashimoto K,Ethridge RT,Saito H,et al. The PPAR gamma ligand,15d-PGJ2,attenuates the severity of cerulean-induced acute pancreatitis[J]. Pancreas,2003, 27(1):58-66.
[12] Rakonczay Z,Jdrmay K,Kaszaki J,et al.NF-kappa B activation is detrim ental in arginine-induced acute pancreatitis[J].Free Radic Biol Med,2003,34(6):696-709.
[13] Ewald N,Hardt PD,Kloer HU. Severe hypertglyceridemia and pancreatitis:Presentation and management[J]. Curt Opin Lipidol,2009,20(6):497-504.
[14] Czako L,Szabolcs A,Vajda A,et al.Hyperlipidemia induced by acholesterol-rich diet aggravates necrotizing pancreatitis in rats[J]. EurJ Pharmacol,2007,572(1):74-81.
[15] Zhang H,Cai CZ,Zhang XQ,et al.Breviscapine attenuates acute pancreatitis by inhibitin g expression of PKC alpha and NF-kappa B in pancreas[J].World J Gastroenterol,2011,17(14):1825-1830.
[16] Wang YZ,Wang SW,Zhang YC,et al.Protective effect of exogenous IGF-I on the intestinal mucosal barrier in rats with severe acute pancreatitis[J]. World J Emerg Med,2012,3(3):213-220.
[17] Zhou M,Chen B,Sun H,et al.The protective effects of Lipoxin A(4) during the early phase of severe acute pancreatitis in rats[J]. Scand J Gastroenterol,2011,46(2):211-219.
[18] Luan ZG,Zhang J,Yin XH,et al.Ethyl pyruvate significantly inhibits tumour necrosis factor-d,interleukin-1B and high mobility group box 1 releasin g and attenuates sodium taurocholate-induced severe acute pancreatitis associated with acute lung injury[J]. Clin Exp Immunol,2013,172(3):417-426.
[19] Kimr IH,Bae GS,Oh HJ,et al. 2,4,6 Tris (methoxy-methoxy)chalcone(TM MC) attenuates the severity of cerulean-induced acute panereatitis and associated lung injury[J]. Am J Physiol Gastreintest Liver Physiol,2011, 301(4):G694-G706.
[20] Yin K,Dang SC,Zhang JZ.Relationship between expression of triggring receptor-1 on myeloid cells in intestinal tissue and intestinal barrier dysfunction in severe acute pancreatitis[J]. World J Emerg Med,2011,2(3):216-221.
[21] Bemot D,Peiretti F,Canauh M,et al.Upregulation of TNF-alpha induced ICAM-1 surface expression by adenylate cyclasedependent pathway in human endothelial cells[J]. J Cell Physiol,2005,202(2):434-441.
[22] Hsu WY,Chao YW,Tsai YL,et al.Resistin induces monocyte-endoth elial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38 MAPK-dependent pathway[J]. J Cell Physiol,2011, 226(8):2181-2188.
[23] 裴紅红,乔万海,柏玲.过氧化物酶体增殖剂激活受体-γ对实验性胰腺炎大鼠核转录因子-κB表达的影响[J].中国危重病急救医学,2008,20(5):297-298.
[24] Ivashchenko CY,Duan SZ,Usher MG,et al.PPAR-γ knockout in pancreatic epithelial cells abolishes the inhibitory effect of rosiglitazone on caerulein-induced acute pancreatitis[J]. Am J Physiol Gastrointest Liver Physiol,2007,293(4):G319-G326.
(收稿日期:2017-02-24)
