二烯丙基二硫上调miR-22通过Wnt-1通路抑制人胃癌细胞增殖与迁移侵袭
2017-07-25唐云云
唐云云,唐 仪,刘 芳,苏 坚,4,夏 红,苏 波,曾 希,苏 琦
(1.南华大学肿瘤研究所, 湖南省胃癌研究中心,湖南省高校肿瘤细胞与分子病理学重点实验室,湖南 衡阳 421001;2.永州职业技术学院基础医学部,湖南 永州 425100;3.南华大学附属湘潭医院病理科,湖南 湘潭 411101;4南华大学附属第二医院病理科,湖南 衡阳 421001)
二烯丙基二硫上调miR-22通过Wnt-1通路抑制人胃癌细胞增殖与迁移侵袭
唐云云1,2,唐 仪1,3,刘 芳1,苏 坚1,4,夏 红1,苏 波1,曾 希1,苏 琦1
(1.南华大学肿瘤研究所, 湖南省胃癌研究中心,湖南省高校肿瘤细胞与分子病理学重点实验室,湖南 衡阳 421001;2.永州职业技术学院基础医学部,湖南 永州 425100;3.南华大学附属湘潭医院病理科,湖南 湘潭 411101;4南华大学附属第二医院病理科,湖南 衡阳 421001)
目的 探讨二烯丙基二硫(diallyl disulfide,DADS)上调miR-22是否通过Wnt-1通路抑制人胃癌MGC803细胞增殖与迁移侵袭。方法 MTT、细胞划痕实验、侵袭实验分别检测DADS与miR-22对MGC803细胞增殖与迁移侵袭的影响。在线预测软件寻找miR-22调控的靶基因,荧光素酶报告基因检测miR-22对Wnt-1 3′UTR荧光酶活性的影响。qRT-PCR检测Wnt-1mRNA表达变化。Western blot检测Wnt-1、β-catenin与TCF-4蛋白表达。结果 MTT显示,DADS 与miR-22可明显抑制MGC803细胞增殖(P<0.05)。划痕实验显示,DADS与miR-22可明显抑制MGC803细胞迁移,而miR-22+DADS更为明显(P<0.05)。侵袭实验显示,miR-22可抑制人胃癌MGC803细胞侵袭,而miR-22+DADS更为明显(P<0.05)。在线预测软件寻找miR-22调控的靶基因显示,Wnt-1可能是miR-22的靶基因,荧光素报告基因检测证实Wnt-1是miR-22直接调控的靶基因;qRT-PCR显示,DADS与miR-22能下调Wnt-1 mRNA表达,而miR-22+DADS更为明显(P<0.05)。Western blot显示,DADS 与miR-22能下调Wnt-1、β-catenin与TCF-4蛋白表达,而miR-22+DADS尤为明显(P<0.05)。结论 DADS可上调miR-22 通过Wnt-1通路明显抑制MGC803细胞增殖与迁移侵袭。
二烯丙基二硫;人胃癌细胞;miR-22;Wnt通路;迁移;侵袭
胃癌是最常见的恶性肿瘤之一,发生率与死亡率分别为全球第4位与第3位[1]。据最新统计,胃癌在我国每年约新发67.9万,死亡49.8万人,发生率与死亡率位于第2。由于患者就诊时大多已发生侵袭转移,常规外科手术和化学药物治疗效果较差,5年生存率低于10%[2-3]。因此,开发高效低毒药物与寻找靶点对防治胃癌具有重要意义。
二烯丙基二硫(diallyl disulfide,DADS)是大蒜中烯丙基硫化物的一种脂溶性的有效成分,对多种肿瘤均有明显的抑制作用[4]。近年来,miRNAs在肿瘤中的作用引起人们高度关注。miRNAs是一类含量丰富且高度保守的非编码内源性小RNA分子,通过与靶基因mRNA的3′非编码区完全或不完全结合,抑制靶基因的表达,从而调控细胞增殖、分化与凋亡等重要过程[5]。我们先前研究表明,miR-22在胃癌中表达下调与临床分期和淋巴结转移有关[6]。并且,DADS作用人胃癌MGC803细胞的差异miRNAs中,发现miR-22上调[7]。本文进一步探讨DADS上调miR-22是否通过Wnt-1通路抑制MGC803细胞迁移与侵袭。
1 材料与方法
1.1 试剂 质粒抽提试剂盒购自上海华瞬公司;逆转录试剂盒、荧光素酶活性检测试剂盒购自Promega公司;miR-22 mimics与Wnt-1基因3′UTR的结合位点序列分别由Exiqon公司与Invitrogen公司合成;qRT-PCR miRNA试剂盒购自上海吉玛公司;蛋白浓度测定试剂盒、ECL化学发光检测试剂盒购自Pierce公司;Wnt-1(sc-6280)、β-catenin (sc-7963)、TCF-4(sc-166699)、β-actin(sc-47778)抗体购自Santa Cruz公司;RPMI 1640培养液购自Hyclone公司,胎牛血清购自四季青生物公司;转染用Opti-MEM培养液购自Invtrogen公司。
1.2 细胞培养与分组 人胃癌MGC803细胞由本实验室保存,置于含10%小牛血清的RPMI 1640培养基中,37℃、5% CO2、饱和湿度的培养箱内传代培养。采用胰酶消化预先培养的MGC803细胞并接种于6孔板中,按每孔2 mL铺好,培养至细胞汇合度达30%~50%用于转染。用灭菌的无酶水稀释上述各种转染质粒干粉,按说明配制成20 μmol·L-1的溶液备用。用不含血清的Opti-MEM培养液分别稀释100 pmol转染质粒与5 μL的Lipofectamine 2000,混匀,室温孵育5 min。将MGC803细胞分为转染scramble对照组、scramble+DADS组、转染miR-22 mimics组与miR-22 mimics+DADS组,转染培养48 h,收获细胞。
1.3 MTT实验 用胰酶消化上述各组细胞,吹打成单细胞悬液并收集离心。取200 μL的5×103个细胞接种于96孔板中,设复孔6个。未接种细胞的孔中加入RPMI 1640培养液作调零孔。于37℃、5% CO2细胞培养箱中培养24~48 h,细胞汇合度达90%。接种细胞的孔中加入灭菌MTT液20 μL,继续孵育4 h。取出培养板,吸弃上清液,加入150 μL DMSO溶液,低速振荡10 min,使结晶物溶解。选择570 nm波长,酶标仪测定各孔吸光度值,记录结果,实验重复3次。
1.4 划痕实验 将上述细胞接种于6孔板中,RPMI 1640培养基37℃、5% CO2培养,直至形成单层细胞,每组3个平行样本。吸弃上层培养液,PBS缓冲液洗涤2次,用无菌10 μL Eppendorf Tip在细胞板上划痕,无血清培养液洗3次,加入新鲜无血清培养基。倒置显微镜下观察、测量划痕区相对距离。实验重复3次。
1.5 侵袭实验 将基质胶稀释液铺置在transwell小室中制成膜。加无血清RPMI 1640培养液100 μL至transwell小室的上腔,水化基质胶20 min。取100 μL上述各组细胞稀释液接种至小室上腔,下腔中加入含10%胎牛血清的RPMI 1640培养液500 μL。37℃、5% CO2的培养箱中培养48 h,取出transwell小室,用棉签擦弃小室上层细胞,PBS液洗3遍。4%多聚甲醛固定10 min,0.1%结晶紫染色20 min,PBS液清洗,晾干。光镜下观察并随机选取4个高倍视野进行细胞计数,取平均值。实验重复3次。
1.6 miR-22的靶基因验证
1.6.1 通过在线预测软件(Targetscan and Miranda)寻找miR-22调控的靶基因
1.6.2 荧光素酶报告基因检测 根据Wnt-1基因3′-UTR序列与miR-22结合位点,设计合成引物序列, Wnt-1-wt:F: 5′-CCGCTCGAGCCCTCCCCCAAAC-3′, R: 5′-GAATGCGGCCGCCTGGGAGTGGTAAAAGG GGAGGAT-3′;Wnt-1-mut:F: 5′-CCGCTCCTCCAAGCC ATTC-3′, R: 5′-ATGCCGACTTGGCCGAAT-3′。相应的上、下引物一起退火完成后,将其连接到含有荧光素酶报告基因的载体质粒上。将MGC-803细胞培养于12 孔培养板,细胞分为转染miR-22 mimics与Wnt-1-wt组、转染scramble与Wnt-1-wt组、转染miR-22 mimics与Wnt-1-mut组和转染scramble与Wnt-1-mut组,48 h后收获细胞。按照Promega公司双荧光素酶活性检测试剂盒操作说明书,在单光子检测仪检测细胞荧光素酶的活性,计算相对荧光素酶活性=荧光素酶活性值/海肾荧光素酶活性。实验独立重复3次。1.7 qRT-PCR检测 收集上述各组细胞,采用RNA抽提试剂盒抽提细胞总RNA,逆转录合成cDNA。设计合成PCR引物序列:Wnt-1:F: 5′-TGGCTGGGTTTCTGCTACG-3′,R: 5′-CCCGGATTTTGGCGTATC-3′;GAPDH:F: 5′-GCTGAGAACGGGAAGCT TGT-3′, R: 5′-GCCAGGGGTGCTAAGCAG-3′。PCR扩增反应体系为20 μL,包括PCR primers(5 mmol·L-1)0.4 μL、RT product 2.0 μL、Taq DNA polymerase(5 U·μL-1)0.2 μL、2×SYBR Mix 10 μL、灭菌蒸馏水7.4 μL。反应条件:95 ℃ 3 min、95 ℃ 12 s、62 ℃ 35 s、72 ℃ 30 s,共40个循环。以GAPDH为内参,采用2-ΔΔCT法计算Wnt-1 mRNA相对表达量。
1.8 Western blot检测 收集上述各组细胞,提取细胞总蛋白,BCA法测定蛋白浓度,每组取等量样本进行SDS-PAGE凝胶电泳,电泳后转膜,封闭1 h,加一抗,4 ℃过夜,TBST洗膜,加二抗,孵育1 h,洗膜,ECL发光,X线曝光、显影、定影。
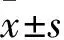
2 结果
2.1 DADS和miR-22对MGC803细胞增殖的影响 将miR-22 mimics转染于MGC803细胞中,转染miRNA的无关序列scramble作为对照,并用30 mg·L-1DADS分别处理。Tab 1 MTT结果显示,48、72、96 h后,DADS与miR-22高表达均可明显抑制MGC803细胞增殖(P<0.05)。表明DADS与miR-22能明显抑制MGC803细胞增殖,且miR-22可增强DADS的作用。
2.2 DADS和miR-22对MGC803细胞迁移的影响 Fig 1显示,48 h后,DADS处理组、miR-22组与miR-22+DADS组伤口愈合率分别为(60.17±2.22)%、(61.57±1.54)%与(49.85±1.98)%,较对照组(91.94±2.01)%明显减缓(P<0.01)。表明DADS与miR-22能明显抑制MGC803细胞的迁移能力,miR-22可增强DADS的抑制作用。

Fig 1 Effect of DADS and miR-22 on migration of MGC803 cells(×20)
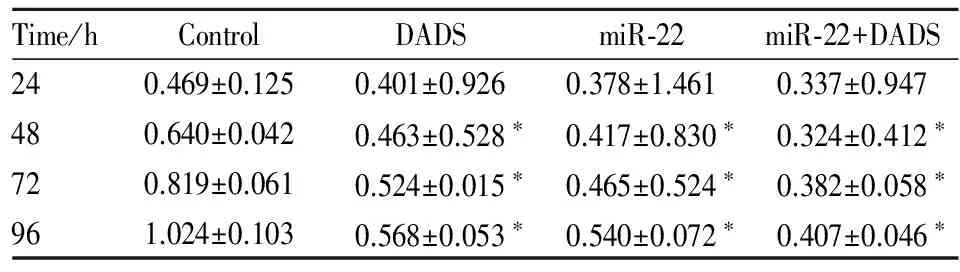
Tab 1 Effect of DADS and miR-22 on proliferation in MGC803 cells
*P<0.05vscontrol group
2.3 DADS和miR-22对MGC803细胞侵袭的影响 Fig 2显示,48 h后,DADS处理组、miR-22组与miR-2+DADS组穿过基质胶的细胞数分别为(137±11)、(120±9)与(70±10),较对照组(253±16)明显减少(P<0.01)。表明DADS与miR-22高表达能明显抑制MGC-803细胞的侵袭能力,且miR-22可增强DADS的抑制作用。

Fig 2 Effect of DADS and miR-22 on invasion in MGC803 cells(×10)
A:Control;B:DADS;C:miR-22;D:miR-22+DADS
2.4 Wnt-1是miR-22的靶基因
2.4.1 miR-22候选靶基因的筛选 为进一步明确miR-22调控胃癌生物学行为的作用机制,运用生物信息学方法,通过在线预测软件(Targetscan and Miranda)寻找miR-22相关蛋白编码的靶基因。经在线软件交叉预测后,发现miR-22有500多个靶基因,miR-22具有Wnt-1的3′UTR的结合位点,Wnt-1可能是miR-22的靶基因(Fig 3)。

Fig 3 Putative binding sites of miR-22 and 3′UTRs of Wnt-1
2.4.2 荧光素酶报告基因验证Wnt-1是miR-22直接调控的靶基因 为证实miR-22作用预测的结合位点导致荧光素酶活性发生改变,设计Wnt-1 3′UTR缺失的miR-22结合位点的突变序列和野生序列插入报告质粒中。胃癌MGC803细胞分别转染miR-22 mimics、Wt-miR-22/Wnt-1和Mut-miR-22/Wnt-1重组质粒。荧光素酶报告基因检测发现(Tab 2),Mut-miR-22/Wnt-1荧光素酶活性为(1.033±0.128),较对照组(1.075±0.062)差异无统计学意义(P>0.05)。但Wt-miR-22/Wnt-1荧光素酶活性为(0.522±0.318),较对照组(1.081±0.984)明显下降(P<0.05)。表明Wnt-1是miR-22直接的靶基因。
2.5 DADS与miR-22对MGC803细胞Wnt-1表达的影响 Fig 4 qRT-PCR结果显示,DADS组和miR-22组Wnt-1 mRNA的表达水平2-△△CT分别为(0.721±0.034)与(0.745±0.038)较对照组2-△△CT(1.066±0.714)明显下降,并以miR-22+DADS组2-△△CT(0.495±0.029)下降尤为明显(P<0.05)。Fig 5 Western blot结果显示,DADS处理与miR-22高表达的MGC803细胞 Wnt-1蛋白表达水平较对照组分别下调31.84%与28.77%,并且,miR-22+DADS下调尤为明显(48.9%)(P<0.05)。

Tab 2 Effect of miR-22 on luciferase activity
*P<0.05vsscramble group

Fig 4 Effect of DADS and miR-22 on Wnt-1 mRNA expression in MGC803 cells
1:Scramble;2:Scramble+DADS;3:miR-22;4:miR-22+DADS.*P<0.05vsscramble;#P<0.05vsmiR-22.
2.6 DADS与miR-22对β-catenin与TCF-4表达的影响 Western blot结果显示(Fig 6、7),DADS处理与miR-22高表达的MGC803细胞β-catenin与TCF-4蛋白表达水平较对照组分别下调,且miR-22+DADS下调尤为明显(P<0.05)。表明DADS上调miR-22通过Wnt-1信号下调β-catenin与TCF-4。
3 讨论
研究证实,miRNA在肿瘤中通过调控抑癌基因或癌基因的表达,表现为类似癌基因或抑癌基因样作用[8]。大量研究表明,miR-22在多种肿瘤中表达下调,可抑制肿瘤细胞迁移侵袭,表现为抑癌基因,其作用机制与靶向调控多种靶基因有关[9-19]。研究显示,miR-22通过靶向TIAM1抑制结肠癌HCT-116细胞增殖与迁移侵袭[9]。ATP citrate lyase(ACLY)在肺癌、前列腺癌、子宫颈癌、骨肉瘤等中表达上调,而miR-22表达下调,ACLY与miR-22表达呈负相关,且miR-22可直接靶向转录后调控ACLY抑制肿瘤生存与转移[10]。miR-22在胃癌中表达较正常组织明显下调,与淋巴结转移、远距离转移、临床分期和患者生存率下降有关。而miR-22高表达可通过靶向Sp1或MMP-14、Snail,抑制胃癌细胞迁移与侵袭[11-13]。Xu等[14]研究显示,miR-22通过靶向CDK6、MDM2、LEF1、MYB、FOS等基因在卵巢癌细胞迁移与侵袭中起着重要作用。miR-22在肾细胞癌表达下调与组织学类型、肿瘤分期和淋巴结转移有关,其通过直接靶向SIRT1,抑制肾细胞癌细胞增殖与迁移侵袭。miR-22高表达体外可抑制肾细胞癌细胞增殖、迁移侵袭与诱导凋亡,体内可抑制移植瘤生长。可活化p53及其下游靶点p21与PUMA,裂解CASP3与PARP,抑制EMT[15]。
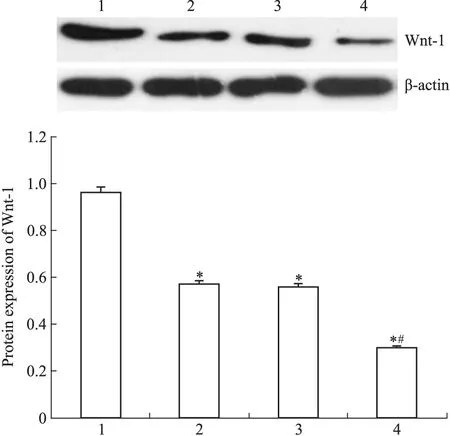
Fig 5 Effect of DADS and miR-22 on Wnt-1 protein expression in MGC803 cells
1: Scramble; 2: Scramble+DADS; 3: miR-22; 4: miR-22+DADS.*P<0.05vsscramble;#P<0.05vsmiR-22.

Fig 6 Effect of DADS and miR-22 on β-catenin protein expression in MGC803 cells
1: Scramble; 2: Scramble+DADS; 3: miR-22; 4: miR-22+DADS.*P<0.05vsscramble;#P<0.05vsmiR-22;△P<0.05vsDADS.
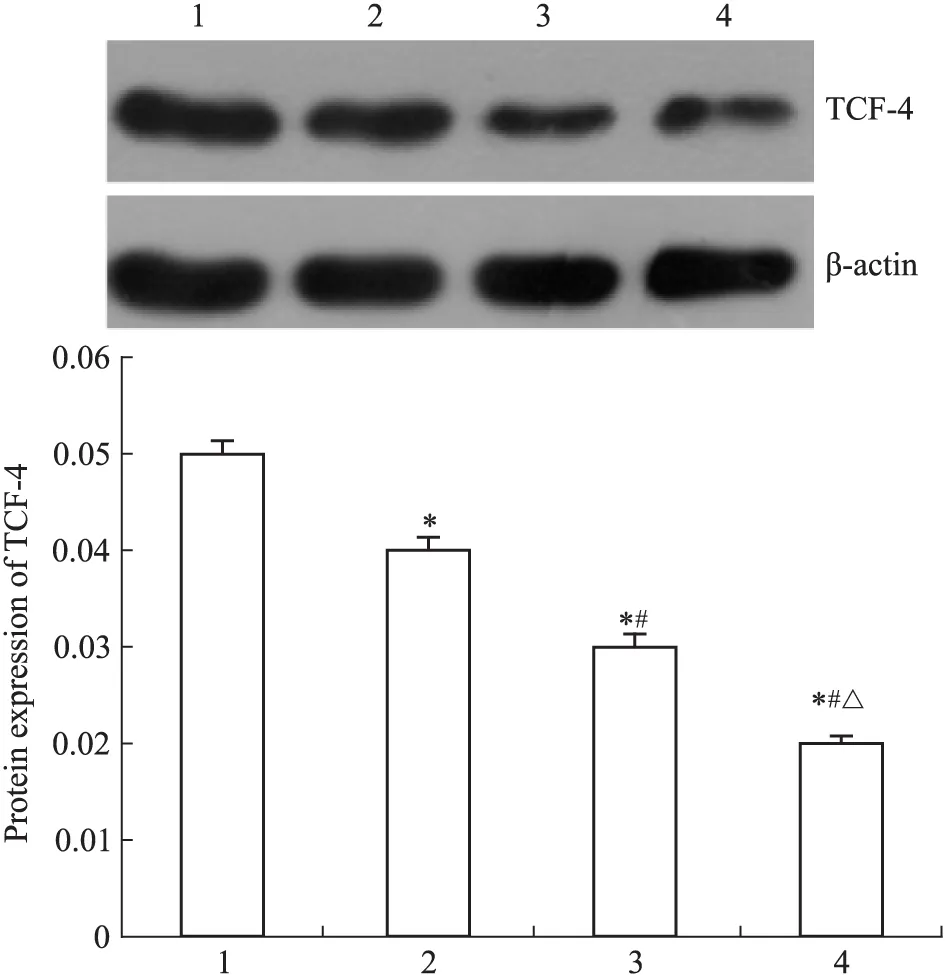
Fig 7 Effect of DADS and miR-22 on TCF-4 protein expression in MGC803 cells
1: Scramble; 2: Scramble+DADS; 3: miR-22; 4: miR-22+DADS.*P<0.05vsscramble;#P<0.05vsmiR-22;△P<0.05vsDADS.
众所周知,Wnt信号通路在肿瘤发生与侵袭转移中起着重要作用。当胞外Wnt蛋白增加时,Wnt与Frizzled结合,激活Dsh/Dvl蛋白,抑制GSK3β/APC/Axin复合物对β-catenin的降解,而促进β-catenin入核,与转录因子Tcf/Lef结合,引起靶基因的转录,导致肿瘤发生。研究发现,Wnt信号通路与miRNAs之间存在交叉对话,miRNA可调控Wnt信号通路,如miR-122、miR-148a、miR-22、miR-200b、miR-185-3p、miR-324-3p、miR-26a、 miR-487b、miR-329、miR-410、miR-374b等可靶向抑制Wnt通路[16]。Liang等[17]研究显示,miR-22可负调控Wnt/β-catenin信号通路,抑制β-catenin表达。近年来,研发天然植物有效成分防治肿瘤已成为新策略。研究表明,miR-22在肿瘤发生发展中可抑制肿瘤干细胞(CSC)表型与功能。姜黄素、大豆异黄酮、茶多酚、白藜芦醇、维生素D等天然植物有效因子通过靶向CSC相关基因,上调miR-22,抑制肿瘤增殖、迁移、侵袭与转移[18]。我们研究显示,DADS可上调miR-22靶向Wnt-1通路,抑制人胃癌细胞增殖与诱导凋亡[7]。
我们前期工作证明,DADS可体内外抑制MGC803细胞的增殖,其机制与激活p38与抑制ERK通路,调节ATR/Chk1/Cdc25C/cyclin B1,上调组蛋白乙酰化与p21,G2/M阻滞等有关。并且,DADS可通过Rac1-Pak1/Rock1通路下调LIMK1、MMP-9,上调TIMP-3,抑制人胃癌细胞EMT与侵袭[19-22]。
本研究在前期研究的基础上,进一步确定DADS上调miR-22是否通过Wnt-1通路抑制MGC803细胞迁移与侵袭。结果表明,DADS与miR-22均可明显抑制MGC803细胞增殖。DADS处理组、miR-22组与miR-2+DADS组迁移能力较对照组明显降低,并且穿过基质胶的细胞数较对照组明显减少,表明DADS与miR-22能明显抑制MGC-803细胞的迁移侵袭能力,且miR-22可增强DADS的抑制作用。靶基因预测发现,miR-22具有Wnt-1的3′UTR的结合位点,Wnt-1可能是miR-22的靶基因。荧光素酶报告基因检测显示,Mut-miR-22/Wnt-1荧光素酶活性较对照组差异无显著性,而Wt-miR-22/Wnt-1荧光素酶活性较对照组明显下降,确定Wnt-1是miR-22直接的靶基因。qRT-PCR验证显示,DADS组和miR-22组Wnt-1 mRNA表达较对照组明显下降,并以miR-22+DADS组更为明显。Western blot显示,DADS组与miR-22组Wnt-1蛋白表达较对照组明显下调,并且miR-22+DADS下调尤为明显。另外,DADS组与miR-22组β-catenin与TCF-4蛋白表达较对照组明显下调,且miR-22+ DADS下调尤为明显。上述结果证明,DADS可上调miR-22,通过Wnt-1通路下调β-catenin与TCF-4,抑制人胃癌细胞增殖与迁移侵袭。
[1] Torre L A,Bray F,Siegel R L,et al. Global cancer statistics, 2012[J].CACancerJClin,2015, 65(2):87-108.
[2] Chen W,Zheng R,Baade P D,et al.Cancer statistics in China, 2015[J].CACancerJClin, 2016,66(2):115-32.
[3] Orditura M,Galizia G,Sforza V,et al. Treatment of gastric cancer[J].WorldJGastroenterol,2014,20(7):1635-49.
[4] 唐云云,唐海林,苏 琦.MicroRNAs在胃癌中的生物学作用[J].中国肿瘤临床, 2014,41(2): 131-3.
[4] Tang Y Y, Tang H L, Su Q. Biological role of microRNAs in gastric cancer[J].ChinJClinOncol, 2014,41(2): 131-3.
[5] Yi L, Su Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide[J].FoodChemToxicol,2013, 57:362-70.
[6] Tang Y Y, Liu X P, Su B, et al. microRNA-22 acts as a metastasis suppressor by targeting metadherin in gastric cancer[J].MolMedRep,2015,11(1):454-60.
[7] Tang H,Kong Y,Guo J,et al. Diallyl disulfide suppresses proliferation and induces apoptosis in human gastric cancer through Wnt-1 signaling pathway by up-regulation of miR-200b and miR-22[J].CancerLett,2013,340(1):72-81.
[8] Cheng C J, Slack F J. The duality of oncomiR addiction in the maintenance and treatment of cancer[J].CancerJ,2012,18(3):232-7.
[9] Li B, Song Y, Liu T J, et al. miRNA-22 suppresses colon cancer cell migration and invasion by inhibiting the expression of T-cell lymphoma invasion and metastasis 1 and matrix metalloproteinases 2 and 9[J].OncolRep, 2013,29(5):1932-8.
[10]Xin M,Qiao Z,Li J,et al. miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer[J].Oncotarget,2016, 7(28):44252-65.
[11]Wang W,Li F,Zhang Y,et al. Reduced expression of miR-22 in gastric cancer is related to clinicopathologic characteristics or patient prognosis[J].DiagnPathol,2013,8:102.
[12]Guo M M,Hu L H,Wang Y Q,et al. miR-22 is down-regulated in gastric cancer, and its overexpression inhibits cell migration and invasion via targeting transcription factor Sp1[J].MedOncol,2013,30(2):542.
[13]Zuo Q F,Cao L Y,Yu T,et al. MicroRNA-22 inhibits tumor growth and metastasis in gastric cancer by directly targeting MMP14 and Snail[J].CellDeathDis,2015,6:e2000.
[14]Xu B,Zhao H,Xu C. Gene expression profiling analysis of the role of miR-22 in clear cell ovarian cancer[J].Neoplasma,2016,63(6):856-64.
[15]Zhang S,Zhang D,Yi C,et al. MicroRNA-22 functions as a tumor suppressor by targeting SIRT1 in renal cell carcinoma[J].OncolRep,2016,35(1):559-67.
[16]Peng Y,Zhang X,Feng X,et al. The crosstalk between microRNAs and the Wnt/β-catenin signaling pathway in cancer[J].Oncotarget,2017,8(8):14089-106.
[17]Liang W C,Fu W M,Wang Y B,et al. H19 activates Wnt signaling and promotes osteoblast differentiation by functioning as a competing endogenous RNA[J].SciRep,2016,6:20121.
[18]Bao B,Li Y,Ahmad A,et al. Targeting CSC-related miRNAs for cancer therapy by natural agents[J].CurrDrugTargets,2012,13(14):1858-68.
[19]Su B, Xiang S L, Su J, et al. Diallyl disulfide increased histone acetylation and p21WAF1 expression in human gastric cancer cellsinvivoandinvitro[J].BiochemPharmacol,2012,1(7):1-10.
[20]Su B, He H, Wang L, et al. Chk1, but not Chk2, is responsible for G2/M phase arrest induced by diallyl disulfide in human gastric cancer BGC823 cells[J].FoodChemToxicol, 2014,68: 61-70.
[21]夏 红,向姝霖,曾 颖, 等. DADS对Chk1/2基因高表达人胃癌MGC803细胞G2/M期的影响[J].中国药理学通报, 2016, 32(2):199-204.
[21]Xia H, Xiang S L, Zeng Y,et al. Overexpression of Chk1/2 gene to affect the G2/M arrest in MGC803 Cells induced by diallyl disulfide[J].ChinPharmacolBul,2016, 32(2):199-204.
[22]Su B, Su J, Zeng Y, et al. Diallyl disulfide suppresses epithelial-mesenchymal transition, invasion and proliferation by downregulation of LIMK1 in gastric cancer[J].Oncotarget, 2016, 7(9):10498-512.
Up-regulation of miR-22 through Wnt pathway suppresses proliferation, migration and invasion in human gastric MGC803 cells by DADS
TANG Yun-yun1,2, TANG Yi1,3, LIU Fang1, SU Jian1,4, XIA Hong1, SU Bo1, ZENG Xi1, SU Qi1
(1.CancerResearchInstitute,CenterforGastricCancerResearchofHunanProvince,KeyLabofCancerCellularandMolecularPathologyofHunanProvincialUniversity,UniversityofSouthChina,HengyangHunan421001,China;2.DeptofBasicMedicine,YongzhouVocationalTechnicalCollege,YongzhouHunan425100,China; 3.DeptofPathology,theAffiliatedXiangtanHospital,UniversityofSouthChina,XiangtanHunan411101,China;4.DeptofPathology,theSecondAffiliatedHospital,UniversityofSouthChina,HengyangHunan421001,China)
Aim To investigate the up-regulation of miR-22 through Wnt pathway inhibits the proliferation, migration and invasion in human gastric MGC803 cells induced by diallyl disulfide(DADS).Methods The effects of proliferation, migration, and invasion of gastric cancer cells were evaluated by MTT, wound-healing and invasion assays. Online prediction software was applied to search the target gene of miR-22. Luciferase report gene assay was used to assess the target genes Wnt-1 of miR-22. The expressions of Wnt-1, β-catenin and TCF-4 were tested by qRT-PCR and Western blot, respectively.Results MTT showed that DADS and miR-22 notably decreased the proliferation compared with control group(P<0.05). Wound-healing assay showed that DADS and miR-22 could significantly inhibit the migration of MGC803 cells compared with the control group, especially in miR-22+DADS(P<0.05). Invasion assay showed that DADS and miR-22 could markedly inhibit the invasion of MGC803 cells compared with the control group, especially in miR-22+DADS(P<0.05). Online prediction software to search the target gene exhibited that Wnt-1 may be a target gene of miR-22. Luciferase report gene assay disclosed that Wnt-1 was identified as a direct target of miR-22. qRT-PCR showed that the expression of Wnt-1 mRNA was respectively down-regulated by DADS and miR-22 compared with control group, especially in miR-22+DADS(P<0.05). Western blot exhibited that DADS and miR-22 obviously suppressed the expressions of Wnt-1, β-catenin and TCF-4 proteins, especially in miR-22+DADS(P<0.05).Conclusion Up-regulation of miR-22 through Wnt pathway can remarkably suppress the proliferation, migration and invasion in MGC803 cells by DADS.
diallyl disulfide; human gastric cancer cells; miR-22; Wnt pathway; migration; invasion
2017-04-15,
2017-05-12
国家自然科学基金资助项目(No 81374013, 81102854, 31100935)
唐云云(1984-),女,硕士,助教,研究方向:胃癌发生与防治的分子机制,E-mail: 176024235@qq.com; 唐 仪(1983-),女,硕士,主治医师,研究方向:胃癌发生与防治的分子机制,并列第一作者,E-mail: tangyi9921@126.com; 苏 琦(1945-),男,教授,博士生导师,研究方向:胃癌发生与防治的分子机制,通讯作者,E-mail: suqi1945@163.com
时间:2017-7-7 11:05 网络出版地址:http://kns.cnki.net/kcms/detail/34.1086.R.20170707.1104.040.html
10.3969/j.issn.1001-1978.2017.08.020
A
1001-1978(2017)08-1141-07
R329.24;R394.2;R735.2;R977.3;R977.6