黄连素调控胰岛素抵抗相关2型糖尿病的研究进展
2017-07-13李骋何金枝周学东徐欣
李骋+何金枝+周学东+徐欣
[摘要] 胰岛素抵抗是肝脏、肌肉和脂肪组织等周围靶组织细胞对胰岛素的敏感性降低导致葡萄糖摄取和利用效率下降而产生的一系列临床表现,是2型糖尿病的重要发病机制之一。黄连素提取自天然植物,安全性高、毒副反应小,具有显著的降血糖、改善胰岛素抵抗的作用,对2型糖尿病及其并发症有较好疗效。该文对黄连素调控胰岛素抵抗相关2型糖尿病研究进展进行综述,探讨黄连素对胰岛素抵抗及2型糖尿病防治的相关机制。黄连素的生物利用度极低,提示其可能通过调节肠道菌群来发挥降脂、降糖的作用。肠道微生物可能成为黄连素治疗胰岛素抵抗相关2型糖尿病的新靶点。
[关键词] 黄连素; 胰岛素抵抗; 2型糖尿病; 肠道菌群
[Abstract] Insulin resistance (IR) is defined as a series of clinical manifestations for diminished effectiveness of insulin in lowering blood sugar levels caused by decreased sensitivity to insulin of liver, muscle and adipose tissue. IR is the major contributor to the etiology and pathogenesis of type 2 diabetes mellitus (T2DM). Berberine, a traditional Chinese herb extract, has been shown to be safe and effective in lowering blood sugar, alleviating insulin resistance and moderating type 2 diabetes mellitus and its complications. The bioavailability of berberine is extremely low, suggesting that it may play a role in lowering blood sugar and lipid by regulating intestinal flora. Intestinal microbiota may serve as a new potential target for berberine treatment of type 2 diabetes mellitus.
[Key words] berberine; insulin resistance; type 2 diabetes mellitus; intestinal microbiota
糖尿病是一組以高血糖为特征的代谢性疾病,由胰岛素分泌缺陷和/或其生物作用受损引起。高血糖可导致机体各种组织,特别是眼、肾、心脏、血管、神经的慢性损害、功能障碍。世界卫生组织(world health organization,WHO)2014年统计结果显示,全世界糖尿病患者已达34.7亿[1],严重危害人类健康。根据其发病机制,糖尿病主要分为1型糖尿病(type 1 diabetes mellitus,T1DM)和2型糖尿病(type 2 diabetes mellitus,T2DM)。T2DM特指因胰岛素抵抗和胰岛素分泌不足而产生的葡萄糖和脂肪代谢紊乱综合征。胰岛素抵抗(insulin resistance,IR)指肝脏、肌肉和脂肪组织等周围靶组织细胞对胰岛素的敏感性降低、胰岛素促进的葡萄糖摄取和利用效率下降,从而产生高血糖症、高胰岛素血症、血脂紊乱等一系列临床表现。T2DM多在35~40岁之后发病,占糖尿病患者90%以上。研发有效、低毒的T2DM治疗药物对于糖尿病的控制具有重要意义。
黄连素又名盐酸小檗碱(berberine),是黄连的主要成分,毒副反应小,安全价廉。目前,黄连素调节、改善糖脂代谢的作用已被证实[2-3]。大量研究结果表明黄连素具有显著的降血糖、降血脂、改善IR及抗氧化作用,对T2DM及其各种并发症具有较好疗效,具有很大的开发价值。近期研究表明,肠道微生物与机体胰岛素抵抗及2型糖尿病的调控密切相关,提示肠道微生物可能成为黄连素治疗胰岛素抵抗相关2型糖尿病的新靶点,但目前对此方面研究的综述十分缺乏。本文对黄连素调节肠道菌群参与调控胰岛素抵抗相关2型糖尿病研究进展进行综述,为进一步研究提供思路。
1 黄连素抗T2DM的体内研究
1.1 黄连素抗T2DM的临床研究
迄今为止,已有数个临床试验证实黄连素对T2DM的治疗效果。Yin等[4]对36例T2DM患者给予黄连素治疗(每日3次,每次0.5 g),发现黄连素可明显降低患者空腹血糖、餐后血糖、糖化血红蛋白和甘油三酯。Cannillo等[5]给予112例糖尿病患者黄连素每日3次,每次1.5~3.0 g,观察到黄连素具有显著的降血糖作用,总有效率高达90%。Xia等[6]用黄连素治疗肝源性糖尿病患者,研究发现黄连素可降低患者空腹血糖、改善葡萄糖受损耐量和肝功能。其他临床研究均显示黄连素可有效调节血糖,体现在治疗组空腹血糖(FBG)、空腹血清胰岛素(FINS)、稳态模型评估胰岛素抵抗指数(HOMA-IR)水平较治疗前及对照组均降低[7-8]。
1.2 黄连素抗T2DM的动物实验研究
Wu等[9]学者通过饮食诱导肥胖大鼠模型研究,发现黄连素可改善IR,与二甲双胍具有相似疗效。与二甲双胍相比,经黄连素治疗的糖耐量受损大鼠其游离脂肪、载脂蛋白B、血浆甘油三酯和总胆固醇显著降低,并且黄连素可导致胰岛素抵抗大鼠的胰岛素敏感指数(ISI)、体重及内脏脂肪细胞面积数值显著降低,提示黄连素有增加胰岛素敏感性、改善IR作用[10]。Wang等[11]通过高脂饮食与注射STZ建立糖尿病大鼠模型,研究结果显示黄连素可降低模型大鼠空腹血糖。代谢组学分析表明,高脂饮食诱导的Wistar大鼠经黄连素治疗可增加血清中丙酮酸、生酮氨基酸和糖原水平[12]。黄连素还可降低高脂诱导肥胖小鼠的体重、减少能量摄入和血糖血脂水平[13]、降低血清脂多糖结合蛋白(lipopolysaccharide-binding protein,LBP)水平、核细胞趋化蛋白-1、脂联素,升高瘦素水平[14]。Lee等[15]研究发现,黄连素可减轻胰岛素抵抗模型大鼠体重,改善IR;Wu等[16]也观察到胰岛素抵抗模型大鼠ISI降低。
2 黄连素抗糖尿病作用机制的研究
2.1 保护胰岛细胞
黄连素对胰岛细胞具有保护作用。黄连素可修复糖尿病大鼠受损的胰腺组织[17]。黄连素还可通过增加胰岛细胞内肝细胞核因子4α(hepatocyte nuclear factor 4α,HNF4α)的转录及表达水平,保护胰岛细胞[18]。此外,黄连素可下调 Bax/Bcl-2比值,从而抑制小鼠胰岛细胞的凋亡[19]。
2.2 促胰岛素分泌
Zhou [20]通过STZ诱导的糖尿病大鼠模型研究,发现黄连素能增加胰岛素分泌、减少胰岛β细胞氧化应激、增加抗氧化酶的活性、降低脂类的氧化、促进 β 细胞的再生。Ko等[21]研究显示黄连素可引起MIN6胰岛β细胞株葡萄糖刺激后的胰岛素分泌,同时促进胰岛B细胞增生。Lee等[15]研究表明,黄连素能够改善高脂饮食Wistar大鼠的胰岛素分泌,恢复胰岛功能。Shen 等[22]同样证实黄连素可促进胰岛β细胞释放胰岛素。Zhou等[23]研究发现黄连素可显著增加胰岛细胞AMPK活性,通过环腺苷酸(cAMP)信号通路调节胰岛素分泌。值得注意的是,黄连素促胰岛素分泌作用尚具有争议。Yin等[4]体外实验发现,黄连素对β-TC3细胞胰岛素分泌无显著促进作用。
2.3 降低炎症反应
机体炎症反应与IR以及胰岛β细胞损伤密切相关。炎症因子可导致细胞凋亡并影响胰腺β细胞功能。脂多糖结合蛋白LBP是外源性抗原负荷的生物标志,单核细胞趋化蛋白-1(MCP-1)可作为系统性炎症水平的评价指标,研究发现黄连素能降低高脂喂养大鼠血浆中增加的LBP和MCP-1水平,改善系统性炎症[24-25]。肿瘤坏死因子(TNF-α)可干扰外周组织胰岛素功能,导致IR。动物研究发现,黄连素具有明显的抗炎作用,可降低胰岛素抵抗大鼠模型血清TNF-α,IL-6水平,上调抗炎症因子如IL-10的表达,增加胰岛素敏感性[26-27]。
2.4 改善血脂紊乱
脂毒性是肥胖导致胰岛β细胞功能受损的病理机制之一。黄连素可显著降低血清总胆固醇TC、甘油三酯TG和低密度脂蛋白LDL-c水平,增加高密度脂蛋白HDL-c及一氧化氮NO浓度[17]。Brusq等[28]临床研究表明,黄连素可显著降低血清TG,可能与黄连素通过激活AMPK信号通路抑制人肝细胞内脂质合成有关。Lee等[15]的研究表明黄连素可减轻高脂饲养大鼠血浆中甘油三酯含量,改善胰岛功能。Zhou等[29]研究结果显示,黄连素能降低糖尿病大鼠肝脏质量及肝脏指数,促进血糖、TC和TG恢复正常,并降低LDL-c和载脂蛋白Al水平。
2.5 促外周组织葡萄糖吸收利用
葡萄糖吸收入血后,依赖葡萄糖转运体(GLUTs)进入细胞。GLUT1在人类所有组织中均存在表达,GLUT4主要表达于胰岛素敏感的骨骼肌、脂肪细胞和心肌中。黄连素可激活GLUT1[30]并上调GLUT1表达水平[23]、增加GLUT4的表達和转位活性[31],从而增加机体组织对葡萄糖的摄取。Xu[32]和Ko[21]等学者的研究均证实在3T3-L1脂肪细胞上,黄连素通过激活IRS-1-PI3K-Akt-GLUT-4通路,增加脂肪细胞葡萄糖摄取及对胰岛素的敏感性,改善游离脂肪酸诱导的IR。此外,蛋白激酶AMPK信号通路被激活,可抑制合成代谢,促进分解代谢,与IR的改善密切相关。黄连素可明显增加3T3-L1脂肪细胞中的AMP水平,增加AMPK磷酸化程度[15,23],上调线粒体解偶联蛋白 UCP2,增加能量消耗,促进葡萄糖分解代谢。
2.6 其他功能
Kong等[33]研究显示,黄连素可上调Hep G2肝细胞胰岛素受体水平,增加胰岛素敏感性。在高脂饮食加STZ注射诱导的糖尿病仓鼠模型中,黄连素可提高肝脏X受体α(LXRα)、过氧化物酶体增殖因子活化受体α/δ(PPARα/δ)以及PPARα的mRNA水平,减少PPARγ表达,降低SREBPs的mRNA水平,进而降低肝脏质量以及肝脏与血浆的甘油三酯和胆固醇水平[34-35]。黄连素还可提高大鼠HNF4α表达,改善高胰岛素血症,降低胰岛素抵抗指数[36]。此外,Xia等[6]提出黄连素在肝脏中对胰岛素信号通路没有影响,黄连素可下调糖元合成基因PEPCK和G6Pase,以及转录因子FoxO1,SREBP1,ChREBP的表达,从而发挥降糖作用。
3 黄连素治疗T2DM的新靶点——肠道微生物
肠道是人体巨大的微生态系统,定植于肠道的微生物集合统称为肠道菌群[37]。Eckburg等[38]通过宏基因组学研究发现,人体肠道菌群主要由厚壁菌门Firmicutes、拟杆菌门Bacteroides、放线菌门Actinobacteria、变形菌门Proteobacteria、梭杆菌门Fusobacteria及疣微菌门Verrucomicrobia构成。其中,硬壁菌门、拟杆菌门比例占90%以上。肠道菌群具有多样性和特异性,受宿主遗传基因、年龄、病理、饮食等多种因素影响[39-42]。目前认为,肠道菌群直接参与营养吸收、生物屏障、免疫调节、脂肪代谢、抗肿瘤等诸多生理过程,对维持宿主正常生理功能不可或缺[43];与肥胖、脂肪肝、糖尿病等疾病关系密切[44]。
3.1 肠道菌群与T2DM关系密切
Yazigi和Turnbaugh等[45-46]发现消瘦无菌小鼠肠道内移植肥胖小鼠的肠道菌群后,消瘦小鼠的体重和脂肪含量均有增加,提示肠道菌群与肥胖之间的潜在关系。代谢综合征患者十二指肠移植正常人肠道菌群6周后,胰岛素敏感性增加、IR症状改善;进一步研究发现,接受菌群移植后,代谢综合征患者十二指肠定植菌群中产丁酸盐菌-霍氏真杆菌Eubacterium hallii丰度增加,提示改变肠道菌群可改善IR,其机制可能与十二指肠菌群产丁酸盐能力上调有关[47]。
现有研究表明,促糖尿病发生相关肠道细菌主要包括: 粪拟杆菌、变异梭状芽胞杆菌、大肠埃希菌、脱硫弧菌属、加氏乳杆菌、变形链球菌和副流感嗜血杆菌等;具有抗糖尿病作用的细菌主要有:梭状芽胞杆菌、直肠真杆菌、罗斯氏菌、疣微菌科、Akkermansia muciniphila菌和普氏粪杆菌等[48-49]。Zhang等[50]通过对肠道细菌16S rRNA的高通量测序,发现菌群多样性与机体代谢参数明显相关。肠道内低浓度的柔嫩梭菌属Faecalibacterium prausnitzii,罗氏菌属Roseburia,拟杆菌属与肥胖及糖尿病密切相关[51]。研究显示,2型糖尿病患者肠道艰难梭菌Clostridium difficile的丰度增高,拟杆菌丰度降低,但产气菌Dore属、普氏菌Prevotella,柯林斯氏菌Collinsella豐度更高[14]。对十二指肠菌群的测序研究发现,肥胖组较健康组十二指肠厌氧菌比例、乙酰辅酶A脱氢酶基因增加,而需氧菌和蔗糖磷酸酶和1-4α葡萄糖支链酶减少[52]。另有研究发现,糖尿病患者肠道的有益菌-双歧杆菌Bifidobacterium数量降低,而粪肠球菌Enterococcus faecalis数目增高[53];在糖耐量损伤的小鼠中,双歧杆菌减少,产硫酸盐、内毒素的细菌增多[54]。目前认为肠道乳酸菌Lactobacillus,双歧杆菌等益生菌的减少与糖耐量异常密切相关。将含有嗜酸乳杆菌Lactobacillus acidophilus和干酪乳杆菌L. casei的酸奶喂养高果糖饮食大鼠,具有明显抗糖尿病效果[55]。有研究发现添加益生菌饮食可使肥胖小鼠小肠硬壁菌门和梭状芽孢杆菌群ⅩⅣab Clostridium clusterⅩⅣab丰度降低,硬壁菌门/拟杆菌门比值减低,减缓小鼠体重增长,降低肥胖相关代谢指标[56]。
近期研究发现,Akkermansia muciniphila菌与胰岛素、血糖、甘油三酯水平、肥胖、代谢性内毒素血症等糖尿病危险因子呈负相关,该细菌通过降解肠黏膜中的黏蛋白,产生游离脂肪酸,影响人体代谢平衡和免疫功能[54]。有研究通过联用绿茶和植物乳杆菌L. plantarum治疗高脂小鼠,发现处理后Akkermansia菌数量无显著改变,但小肠细菌群落多样性明显增加、乳酸菌生长增多,高脂饮食诱导的炎症反应得以缓解[57]。另有学者将蔓越莓提取物喂养髙脂饮食小鼠后,发现其可增加肠道中Akkermansia菌群数量、降低体重和内脏脂肪、循环血液中的LPS、减少胰岛素抵抗、增加胰岛素敏感性[58]。
3.2 黄连素通过调节肠道菌群缓解T2DM
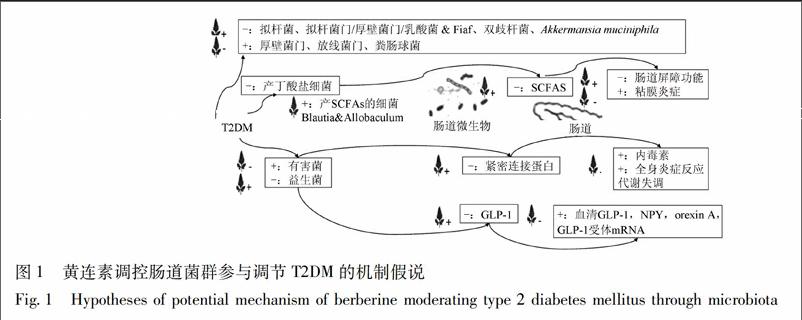
研究表明黄连素具有抗炎、抑制葡萄糖吸收、调节肠道菌群、保护肠黏膜屏障等作用[59]。黄连素的生物利用度极低,在肠道吸收率仅5%~10%[60]。Li等[61]研究发现,在STZ诱导的糖尿病小鼠中,黄连素几乎不被肠道吸收,但可显著降低肠道二糖酶和β-葡糖苷酸酶的活力,从而减少葡萄糖吸收,降低餐后高血糖。因此,目前认为黄连素并不主要经消化道吸收进入血液循环后发挥生理学作用,可能通过调节肠道菌群结构,间接起到降脂、降糖的作用[14,62]。黄连素可通过调控肠内菌群生长,影响肠内糖脂成分的吸收及体内糖脂代谢,起到间接的降血糖、调血脂作用[14]。Zhang等[63]研究发现,黄连素不仅能改变高脂饮食大鼠肠道菌群结构,抑制细菌生长,还能改善大鼠的肥胖和IR。肥胖小鼠或肥胖人群与非肥胖者相比,微生物多样性明显降低,被称为“胖菌”的厚壁菌门及放线菌门比例增高,而被称为“瘦菌”的拟杆菌门比例减少[64-65]。拟杆菌/厚壁菌门的比值增加可显著抑制粪肠球菌的生长,增加乳酸菌和双歧杆菌的生长[66]。Li等[12]通过16S rDNA测序发现黄连素治疗可导致包括拟杆菌,布劳特氏菌Blautia,埃希氏菌属Escherichia等在内的肠道益生菌丰度增加。Guo等学者研究显示,黄连素治疗组的小鼠回肠末端和大肠的拟杆菌属丰度增加[67]。Cao等[68]发现,高脂饮食小鼠经黄连素治疗后,双歧杆菌相对丰度及拟杆菌门/厚壁菌门的比值回升。Aronsson等[69]研究显示,黄连素上调髙脂饮食小鼠肠道乳酸菌属丰度,增加内脏和肠道脂肪组织的禁食诱导脂肪细胞因子(fasting-induced adipose factor,Fiaf)基因表达,减少脂肪储存。Fiaf是一种负性调节肠道微生物的关键蛋白,Xie等[70]的研究发现,黄连素可能通过上升Fiaf的表达,上升PGC1α,UCP2,CPT1α,Hadhb等线粒体能量代谢相关的mRNA的表达,进而调节机体的能量代谢。
3.3 黄连素调节肠道菌群参与T2DM的3种假说
3.3.1 内毒素学说 Cani[71]首次提出“代谢性内毒素血症”假说:高脂饮食诱导肠道菌群改变,有益菌数量下降,机会致病菌数量增加并产生内毒素破坏肠道黏膜,肠道通透性增强,入血内毒素量增加,导致机体长期处于低水平全身性炎症反应状态,进而产生肥胖等代谢失调。糖代谢异常的肥胖小鼠经抗生素处理后,血清LPS浓度及随机血糖水平均显著下降[72]。代谢性内毒素血症引起的慢性系统性炎症与肥胖、胰岛素抵抗及脂肪肝密切相关[73]。内毒素血症模型小鼠结肠紧密连接(tight junction,TJ)蛋白表达量减少,且从黏膜表面向隐窝移位,黄连素预先灌胃能改善这种不良改变及对紧密连接的损伤[74],影响 TJ 蛋白的表达、分布及结构,抑制肠道通透性增加,改善内毒素血症[75]。Zhang等[76]的研究表明,黄连素能通过促进回肠超氧化物歧化酶(superoxide dismutase,SOD)和谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)的表达,抑制TLR4(toll-like receptor 4)和核转录因子NF-κB的表达,减少肠道损伤,改善回肠和系统性炎症。
3.3.2 短鏈脂肪酸学说 短链脂肪酸(SCFAs)作为能量调节的信号分子,可通过免疫和神经内分泌机制调节宿主能量摄入和代谢[77]。SCFAs可作为能源被消耗,维护肠道上皮细胞的完整性和杯状细胞的分泌功能,增强肠屏障功能,还可促进小肠糖异生,增加固有层淋巴细胞(lamina propria lymphocytes,LPL)活性[78-79]。SCFAs还可提高结肠内的酸性环境,抑制有害菌的生长,减少炎症因子生成,有利于减轻黏膜炎症[80]。有临床研究通过在高脂饮食中适当补充丁酸盐 (SCFAs的主要成分),证明了SCFAs改善IR的作用[81]。Qin等[50]基于鸟枪法测序技术,通过比较受试者的肠道微生物DNA,发现2型糖尿病患者肠道微生物菌群失调、各种条件致病菌数增加、产丁酸盐细菌数下降、SCFAs合成减少,提示2型糖尿病、肠道微生物及SCFAs间存在密切联系。Zhang等[14]的研究显示,黄连素可富集肠道内产SCFAs细菌Blautia和Allobaculum,增加肠道SCFAs,进而改善IR。
3.3.3 生长因子学说 远端肠道的L细胞可分泌胰高血糖素样肽-1(glucagon like peptide-1,GLP-1)和胰高血糖素样肽-2(GLP-2)。GLP-1可刺激胰岛β细胞的增殖分化,抑制胰岛β细胞凋亡,促进胰岛素基因的转录、胰岛素的合成和分泌;作用于胰岛α细胞,抑制胰高血糖素的释放;延缓胃排空,延缓食物吸收,进而发挥降糖作用;GLP-2可调节肠道屏障功能[82]。肠道有益菌还可发酵膳食纤维,促进结肠L细胞分化,增加内分泌调节肽(PYY)、胰高血糖素样肽GLP-1和GLP-2的分泌[83]。Yu等[84]研究发现黄连素可提高正常小鼠糖负荷后肠道GLP-1分泌,抑制PKC及 MAPK途径。Zhang等[85]的研究也提示黄连素的降糖机制可能与肠道MAPK和GnRh-Glp-1通路有关。Sun等[86]的研究表明黄连素不仅可以调节肠道微生物的结构和多样性、促进肠道GLP-1的分泌,还能升高血清GLP-1和神经肽Y(neuropeptide Y,NPY)水平、降低的食欲素A(orexin A)水平、上调GLP-1受体mRNA水平、改善下丘脑的超微结构,进而提出黄连素可通过调节微生物-肠-脑轴(microbiota-gut-brain axis),提高高脂饮食喂养小鼠的代谢水平。
4 展望
黄连素作为一种抗感染、抗炎症的传统中药,常用于胃肠道疾病治疗。近期研究表明黄连素具有调控糖脂代谢、改善胰岛素抵抗的作用。黄连素可通过抑制肠道有害菌、促进肠道益生菌的生长,起到调控糖脂代谢,缓解T2DM症状的作用(图1)。黄连素通过调控肠道菌群,进而改善糖尿病患者代谢功能紊乱的机制有待进一步研究。另外,亟待大规模临床研究,确定黄连素用于T2DM治疗的安全剂量、最适剂量和剂型,研究特征人群间的差异及个性化治疗方案,最终为糖尿病的防治提供新的思路与模式。
[参考文献]
[1] Tai N, Wong F S, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity[J]. Rev Endocr Metab Disord,2015,16(1):55.
[2] Pang B, Zhao L H, Zhou Q, et al. Application of berberine on treating type 2 diabetes mellitus[J]. Int J Endocrinol,2015,2015:905749.
[3] Liu C, Wang Z, Song Y, et al. Effects of berberine on amelioration of hyperglycemia and oxidative stress in high glucose and high fat diet-induced diabetic hamsters in vivo[J]. Biomed Res Int,2015,2015:313808.
[4] Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus[J]. Metabolism,2008,57(5):712.
[5] Cannillo M, Frea S, Fornengo C, et al. Berberine behind the thriller of marked symptomatic bradycardia[J]. World J Cardiol,2013,5(7):261.
[6] Xia X, Yan J, Shen Y, et al. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis[J]. PLoS ONE,2011,6(2):e16556.
[7] Dai P, Wang J, Lin L, et al. Renoprotective effects of berberine as adjuvant therapy for hypertensive patients with type 2 diabetes mellitus: evaluation via biochemical markers and color Doppler ultrasonography[J]. Exp Ther Med,2015,10(3):869.
[8] Hu Y, Young A J, Ehli E A, et al. Metformin and berberine prevent olanzapine-induced weight gain in rats[J]. PLoS ONE,2014,9(3):e93310.
[9] Yin J, Gao Z, Liu D, et al. Berberine improves glucose metabolism through induction of glycolysis[J]. Am J Physiol Endocrinol Metab,2008,294(1):E148.
[10] Leng S H, Lu F E, Xu L J. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion[J]. Acta Pharmacol Sin,2004,25(4):496.
[11] Wang F L, Tang L Q, Yang F, et al. Renoprotective effects of berberine and its possible molecular mechanisms in combination of high-fat diet and low-dose streptozotocin-induced diabetic rats[J]. Mol Biol Rep,2013,40(3):2405.
[12] Li M, Shu X, Xu H, et al. Integrative analysis of metabolome and gut microbiota in diet-induced hyperlipidemic rats treated with berberine compounds[J]. J Transl Med,2016,14(1):237.
[13] Hu Y, Davies G E. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice[J]. Fitoterapia,2010,81(5):358.
[14] Zhang X, Zhao Y, Zhang M, et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats[J]. PLoS ONE,2012,7(8):e42529.
[15] Lee Y S, Kim W S, Kim K H, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states[J]. Diabetes,2006,55(8):2256.
[16] Wu S, Lu F E, Dong H. Effects of berberine on the pancreatic beta cell apoptosis in rats with insulin resistance[J]. Chin J Integr Med,2011,31(10):1383.
[17] Tang L Q, Wei W, Chen L M, et al. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats[J]. J Ethnopharmacol,2006,108(1):109.
[18] Wang Z Q, Lu F E, Leng S H, et al. Facilitating effects of berberine on rat pancreatic islets through modulating hepatic nuclear factor 4 alpha expression and glucokinase activity[J]. World J Gastroenterol,2008,14(39):6004.
[19] Chueh W H, Lin J Y. Berberine, an isoquinoline alkaloid in herbal plants, protects pancreatic islets and serum lipids in nonobese diabetic mice[J]. J Agric Food Chem,2011,59(14):8021.
[20] Zhou J, Zhou S, Tang J, et al. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats[J]. Eur J Pharmacol,2009,606(1/3):262.
[21] Ko B S, Choi S B, Park S K, et al. Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma[J]. Biol Pharm Bull,2005,28(8):1431.
[22] Shen N, Huan Y, Shen Z F. Berberine inhibits mouse insulin gene promoter through activation of AMP activated protein kinase and may exert beneficial effect on pancreatic beta-cell[J]. Eur J Pharmacol,2012,694(1/3):120.
[23] Zhou L, Yang Y, Wang X, et al. Berberine stimulates glucose transport through a mechanism distinct from insulin[J]. Metabolism,2007,56(3):405.
[24] Tesch G H. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy[J]. Am J Physiol Renal Physiol,2008,294(4):F697.
[25] Lepper P M, Schumann C, Triantafilou K, et al. Association of lipopolysaccharide-binding protein and coronary artery disease in men[J]. J Am Coll Cardiol,2007,50(1):25.
[26] Lou T, Zhang Z, Xi Z, et al. Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes[J]. Inflammation,2011,34(6):659.
[27] 尚文斌,劉佳,于希忠,等. 小檗碱对肥胖小鼠炎症因子分泌和炎症信号通路的作用 [J]. 中国中药杂志,2010,35(11):1474.
[28] Brusq J M, Ancellin N, Grondin P, et al. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine[J]. J Lipid Res,2006,47(6):1281.
[29] Yang Y T, Liu S Y, He Y L, et al. Effect of Longzhang gargle on biofilm formation and acidogenicity of Streptococcus mutans in vitro[J]. Biomed Res Int, 2016,2016:5829823.
[30] Cok A, Plaisier C, Salie M J, et al. Berberine acutely activates the glucose transport activity of GLUT1[J]. Biochimie,2011,93(7):1187.
[31] Kim S H, Shin E J, Kim E D, et al. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes[J]. Biol Pharm Bull,2007,30(11):2120.
[32] Xu L J, Lu F E, Yi P, et al. 8-Hydroxy-dihydroberberine ameliorated insulin resistance induced by high FFA and high glucose in 3T3-L1 adipocytes[J]. Acta Pharm Sin,2009,44(11):1304.
[33] Kong W J, Zhang H, Song D Q, et al. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression[J]. Metabolism,2009,58(1):109.
[34] Xie X, Li W, Lan T, et al. Berberine ameliorates hyperglycemia in alloxan-induced diabetic C57BL/6 mice through activation of Akt signaling pathway[J]. Endocr J,2011,58(9):761.
[35] Zhou J Y, Zhou S W, Zhang K B, et al. Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats[J]. Biol Pharm Bull,2008,31(6):1169.
[36] Gao Z, Leng S, Lu F, et al. Effect of berberine on expression of hepatocyte nuclear factor-4 alpha in rats with fructose-induced insulin resistance[J]. J Huazhong Univ Sci Technol,2008,28(3):261.
[37] Tomasello G, Bellavia M, Palumbo V D, et al. From gut microflora imbalance to mycobacteria infection: is there a relationship with chronic intestinal inflammatory diseases[J]. Ann Ital Chir,2011,82(5):361.
[38] Eckburg P B, Bik E M, Bernstein C N, et al. Diversity of the human intestinal microbial flora[J]. Science,2005,308(5728):1635.
[39] Khachatryan Z A, Ktsoyan Z A, Manukyan G P, et al. Predominant role of host genetics in controlling the composition of gut microbiota[J]. PLoS ONE,2008,3(8):e3064.
[40] De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa[J]. Proc Natl Acad Sci USA,2010,107(33):14691.
[41] Swann J R, Tuohy K M, Lindfors P, et al. Variation in antibiotic-induced microbial recolonization impacts on the host metabolic phenotypes of rats[J]. J Proteome Res,2011,10(8):3590.
[42] Wang Z, Klipfell E, Bennett B J, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease[J]. Nature,2011,472(7341):57.
[43] El A S, van den Bogert B, Kleerebezem M. The small intestine microbiota, nutritional modulation and relevance for health[J]. Curr Opin Biotechnol,2015,32:14.
[44] Carding S, Verbeke K, Vipond D T, et al. Dysbiosis of the gut microbiota in disease[J]. Microb Ecol Health Dis,2015,26:10.
[45] Yazigi A, Gaborit B, Nogueira J P, et al. Role of intestinal flora in insulin resistance and obesity[J]. Presse Med,2008,37(10):1427.
[46] Turnbaugh P J, Backhed F, Fulton L, et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome[J]. Cell Host Microbe,2008,3(4):213.
[47] Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome[J]. Gastroenterology,2012,143(4):913.
[48] Hartstra A V, Bouter K E, Backhed F, et al. Insights into the role of the microbiome in obesity and type 2 diabetes[J]. Diabetes Care,2015,38(1):159.
[49] Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes[J]. Nature,2012,490(7418):55.
[50] Zhang X, Shen D, Fang Z, et al. Human gut microbiota changes reveal the progression of glucose intolerance[J]. PLoS ONE,2013,8(8):e71108.
[51] Remely M, Aumueller E, Merold C, et al. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity[J]. Gene,2014,537(1):85.
[52] Angelakis E, Armougom F, Carriere F, et al. A metagenomic investigation of the duodenal microbiota reveals links with obesity[J]. PLoS ONE,2015,10(9):e137784.
[53] Vrieze A, Holleman F, Zoetendal E G, et al. The environment within: how gut microbiota may influence metabolism and body composition[J]. Diabetologia,2010,53(4):606.
[54] Hooper L V, Littman D R, Macpherson A J. Interactions between the microbiota and the immune system[J]. Science,2012,336(6086):1268.
[55] Yadav H, Jain S, Sinha P R. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats[J]. Nutrition,2007,23(1):62.
[56] Ji Y S, Kim H N, Park H J, et al. Modulation of the murine microbiome with a concomitant anti-obesity effect by Lactobacillus rhamnosus GG and Lactobacillus sakei NR28[J]. Benef Microbes,2012,3(1):13.
[57] Axling U, Olsson C, Xu J, et al. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice[J]. Nutr Metab (Lond),2012,9(1):105.
[58] Anhe F F, Roy D, Pilon G, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice[J]. Gut,2015,64(6):872.
[59] Chen C, Yu Z, Li Y, et al. Effects of berberine in the gastrointestinal tract-a review of actions and therapeutic implications[J]. Am J Chin Med,2014,42(5):1053.
[60] Chen W, Miao Y Q, Fan D J, et al. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats[J]. AAPS Pharm Sci Tech,2011,12(2):705.
[61] Liu L, Deng Y, Yu S, et al. Berberine attenuates intestinal disaccharidases in streptozotocin-induced diabetic rats[J]. Pharmazie,2008,63(5):384.
[62] Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine[J]. Med Sci Monit,2011,17(7):A164.
[63] Zhao L, Shen J. Whole-body systems approaches for gut microbiota-targeted, preventive healthcare[J]. J Biotechnol,2010,149(3):183.
[64] Furet J P, Kong L C, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers[J]. Diabetes,2010,59(12):3049.
[65] Shen J, Obin M S, Zhao L. The gut microbiota, obesity and insulin resistance[J]. Mol Aspects Med,2013,34(1):39.
[66] Qiao Y, Sun J, Xia S, et al. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity[J]. Food Funct,2014,5(6):1241.
[67] Guo Y, Zhang Y, Huang W, et al. Dose-response effect of berberine on bile acid profile and gut microbiota in mice[J]. BMC Complement Altern Med,2016,16(1):394.
[68] Cao Y, Pan Q, Cai W, et al. Modulation of gut microbiota by berberine improves steatohepatitis in high-fat diet-fed BALB/C mice[J]. Arch Iran Med,2016,19(3):197.
[69] Aronsson L, Huang Y, Parini P, et al. Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4)[J]. PLoS ONE, 2010, doi: 10.1371.
[70] Xie W, Gu D, Li J, et al. Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice[J]. PLoS ONE,2011,6(9):e24520.
[71] Cani P D, Amar J, Iglesias M A, et al. Metabolic endotoxemia initiates obesity and insulin resistance[J]. Diabetes,2007,56(7):1761.
[72] Cani P D, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice[J]. Diabetes,2008,57(6):1470.
[73] de La Serre C B, Ellis C L, Lee J, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation[J]. Am J Physiol Gastrointest Liver Physiol,2010,299(2):G440.
[74] Gu L, Li N, Gong J, et al. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia[J]. J Infect Dis,2011,203(11):1602.
[75] Shan C Y, Yang J H, Kong Y, et al. Alteration of the intestinal barrier and GLP2 secretion in berberine-treated type 2 diabetic rats[J]. J Endocrinol,2013,218(3):255.
[76] Zhang Q, Piao X L, Piao X S, et al. Preventive effect of Coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides[J]. Food Chem Toxicol,2011,49(1):61.
[77] den Besten G, van Eunen K, Groen A K, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism[J]. J Lipid Res,2013,54(9):2325.
[78] Tilg H, Moschen A R. Microbiota and diabetes: an evolving relationship[J]. Gut,2014,63(9):1513.
[79] Everard A, Cani P D. Gut microbiota and GLP-1[J]. Rev Endocr Metab Disord,2014,15(3):189.
[80] Menzel T, Luhrs H, Zirlik S, et al. Butyrate inhibits leukocyte adhesion to endothelial cells via modulation of VCAM-1[J]. Inflamm Bowel Dis,2004,10(2):122.
[81] Duncan S H, Belenguer A, Holtrop G, et al. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces[J]. Appl Environ Microbiol,2007,73(4):1073.
[82] Liu J, Pang Z P. Glucagon-like peptide-1 drives energy metabolism on the synaptic highway[J]. FEBS J,2016,283(24):4413.
[83] Delzenne N M, Cani P D. Nutritional modulation of gut microbiota in the context of obesity and insulin resistance: potential interest of prebiotics[J]. Int Dairy J,2010,20(4):277.
[84] Yu Y, Liu L, Wang X, et al. Modulation of glucagon-like peptide-1 release by berberine: in vivo and in vitro studies[J]. Biochem Pharmacol,2010,79(7):1000.
[85] Zhang Q, Xiao X, Li M, et al. Berberine moderates glucose metabolism through the GnRH-GLP-1 and MAPK pathways in the intestine[J]. BMC Complement Altern Med,2014,14(1):188.
[86] Sun H, Wang N, Cang Z, et al. Modulation of microbiota-gut-brain axis by berberine resulting in improved metabolic status in high-fat diet-fed rats[J]. Obes Facts,2016,9(6):365.
[責任编辑 曹阳阳]
