RNA编辑酶ADAR1对EV71感染及变异的影响
2017-06-27刘晴晴常章梅白金金龙健儿
刘晴晴 常章梅 白金金 王 艳 龙健儿
(复旦大学基础医学院病原生物学系-卫生部、教育部医学分子病毒学重点实验室 上海 200032)
RNA编辑酶ADAR1对EV71感染及变异的影响
刘晴晴 常章梅 白金金 王 艳 龙健儿△
(复旦大学基础医学院病原生物学系-卫生部、教育部医学分子病毒学重点实验室 上海 200032)
目的 研究RNA编辑酶腺苷酸脱氨酶 (adenosine deaminase acting on RNA,ADAR1)对新型肠道病毒71型(enterovirus 71,EV71)感染及变异的影响。方法 运用RNAi技术筛选ADAR1基因沉默稳定细胞株,通过MTT分析病毒感染后细胞活力变化、噬斑形成分析病毒滴度及细胞对病毒的敏感性,以及Western blot测定病毒蛋白表达水平等,分析ADAR1对EV71感染的影响。由于ADAR1介导的RNA编辑可使基因形成A-G或T-C突变,为确定ADAR1影响EV71感染是否与其编辑EV71基因组导致病毒变异有关,对EV71流行区EV71的突变特征进行分析;并利用EV71感染ADAR1基因沉默细胞,通过对病毒基因组测序分析,研究ADAR1是否直接编辑EV71基因组。结果ADAR1基因沉默后,与对照细胞相比,病毒感染细胞的存活率下降更快,并形成更多、更大的噬斑。病毒感染细胞中的VP1蛋白和细胞培养基上清中的病毒滴度均明显增加。EV71突变特征分析表明,虽然A-G和T-C之间的变异是病毒突变的主要类型,但EV71感染实验初步证明,ADAR1并不直接编辑EV71病毒基因组。结论 ADAR1可能具有抗EV71感染的作用,但ADAR1可能并不直接编辑EV71基因组。
EV71; ADAR1; RNA编辑; 病毒突变
新型肠道病毒71型(enterovirus 71,EV71)是婴幼儿手足口病(hand,food and mouth disease,HFMD)的主要病原体。HFMD临床症状主要表现为手、足、口等部位的疱疹,重症病例可引起神经系统相关并发症,病情进展快,死亡率高[1-2]。虽然EV71预防性灭活疫苗已获批生产,但尚无特异性抗病毒药物[3-5]。因此对EV71的致病机制以及影响EV71感染的宿主因子等进行研究仍十分必要。
EV71为单股正链RNA病毒,在细胞质中复制,复制过程中形成双链RNA(dsRNA)复制中间体。而dsRNA依赖的腺苷酸脱氨酶(adenosine deaminase acting on RNA 1,ADAR1)是一类dsRNA编辑酶,不但能编辑宿主细胞内的dsRNA分子,而且还可编辑部分病毒dsRNA[6]。近来还发现ADAR1是MDA5-MAVS诱生干扰素生成途径的负调控因子[7-8],并且ADAR1本身也是干扰素刺激基因(interferon stimulated gene,ISG)之一[6,9],提示ADAR1在抗病毒感染和免疫应答过程中可能有重要作用。研究表明,ADAR1可影响多种病毒的感染和复制,如水疱性口炎病毒(vesicular stomatitis virus,VSV)[10]、人类免疫缺陷病毒(human immunodeficiency virus,HIV)[11-13]、麻疹病毒(measles virus,MV)[14-15]、丙型肝炎病毒(hepatitis C virus,HCV)[16]和丁型肝炎病毒(hepatitis delta virus,HDV)[17]等。ADAR1对EV71感染是否有影响尚不清楚。
ADAR1的N-端具有双链RNA结合结构域(dsRNA-binding domain,dsRBD),C-端具有RNA编辑酶催化结构域。此外,N-端还具有两个Z-DNA结合域:Zα和Zβ[18-19]。ADAR1可催化dsRNA部分腺苷(A)脱氨基,形成次黄嘌呤(I),次黄嘌呤在转录和翻译时被识别为鸟嘌呤(G),因此可能导致被编辑RNA的基因形成A-G或T-C突变,并影响其表达[20]。因此,本研究拟通过构建ADAR1基因沉默稳定细胞株来研究ADAR1对EV71感染和复制的影响,并探讨ADAR1影响EV71感染是否通过直接编辑EV71基因组而产生影响及是否因此增加EV71的突变率。
材 料 和 方 法
材料和试剂 EV71病毒株(Genbank Access No.HQ891927,064-Shanghai)由本实验室分离并保存。横纹肌肉瘤(rhabdomysarcoma,RD)细胞、子宫颈癌(Henrietta Lacks,HeLa)细胞、人胚肾细胞(human embryonic kidney cell,293FT)购自中科院细胞库。基因沉默载体pLKO.1-TRC克隆载体来源于Addgene(美国)。
ADAR1基因沉默HeLa细胞、RD细胞的构建与筛选 以HeLa细胞为例,RD细胞的构建与此类似。为构建ADAR1基因沉默稳定细胞,设计与人类ADAR1 mRNA(NM_001111) 1398-1418以及4976-4996位点靶向结合的短发夹RNA(short hairpin,shRNA)。将shRNA克隆入慢病毒载体pLKO.1,与病毒包装质粒psPAX2和pMD2.G共转染293FT细胞,包装获得有感染性的慢病毒。然后将慢病毒感染HeLa细胞,通过嘌呤霉素(2 mg/mL)筛选稳定整合慢病毒的细胞株HeLa-pLKO.1-shADAR1。同时构建和筛选整合阴性对照慢病毒载体pLKO.1-SCR的细胞作为对照。
MTT法检测细胞存活力 为确定ADAR1基因沉默后对病毒诱导细胞病变和裂解的影响,以HeLa细胞为例,将HeLa-pLKO.1-shADAR1和对照HeLa-pLKO.1-SCR细胞铺96孔板(1×104/孔),37 ℃培养24 h后,EV71分别以不同的感染复数(multiplicity of infection,MOI)感染上述细胞(从MOI为100 PFU/细胞开始2倍连续稀释)。细胞分别培养72 h和96 h后,加入MTT (10 mL/孔),37 ℃孵育3 h,吸掉培养基,再加入DMSO(100 mL/孔),37 ℃孵育15 min,用酶标仪测定570 nm处的吸光度值。病毒感染后细胞的存活率按以下公式计算:细胞存活率=感染组吸光度值/未感染组吸光度值×100%。
病毒噬斑分析病毒滴度及细胞对病毒的敏感性为测定病毒滴度,铺RD细胞于六孔板(1×106/孔),培养24 h。EV71按梯度稀释感染RD细胞,2 h后弃去病毒上清。然后用0.25%低熔点琼脂胶2 mL覆盖细胞并培养72 h,用0.1%中性红染色3 h后检测噬斑形成数量。为测定细胞对病毒的敏感性,以HeLa细胞为例,铺HeLa-pLKO.1-shADAR1和HeLa-pLKO.1-SCR细胞于六孔板(1.2×106/孔)。培养8 h之后用相同EV71病毒量(4.8×107PFU/mL)按指定倍数稀释分别感染ADAR1基因沉默细胞及对照细胞,2 h后弃去病毒上清,检测EV71在不同细胞上形成的噬斑数量和大小。
Western blot检测ADAR1及病毒VP1蛋白的表达 用含磷酸酶抑制剂、蛋白酶抑制剂、PMSF的细胞裂解液于冰上裂解细胞,用BCA法测定蛋白浓度。经SDS-PAGE电泳分离后电转到PVDF膜,膜封闭2 h后分别与下述单克隆抗体4 ℃摇床过夜,ADAR1抗体(英国Abcam公司,EPR7033,ab126745)稀释5 000倍,EV71 VP1抗体(美国Abnova公司,3D7,MAB1255-M05)稀释2 000倍,β-actin抗体(美国Cell Signaling Technology公司,8H10D10,#3700)稀释3 000倍,洗膜后与HRP标记羊抗兔IgG(1∶5 000稀释)以及HRP标记羊抗鼠IgG(1∶5 000稀释)室温孵育1 h;洗膜后用ECL发光显色,暗室压片,显影定影,扫描后软件定量分析。蛋白表达水平以目的蛋白/β-actin的相对倍数表示。
EV71的突变特征分析 由于ADAR1编辑dsRNA可能导致基因A-G或T-C的突变[20]。为进一步确定ADAR1对EV71感染的影响是否与其编辑EV71基因组并导致病毒变异有关,我们利用NCBI核酸数据库中EV71全基因组序列,对EV71的突变特征进行初步分析:选取曾经EV71感染的5个高度流行区(中国的阜阳、上海和台湾地区,以及马来西亚和越南),每个流行区随机挑选10株,进行序列比对,获得各个流行区EV71病毒基因组保守序列作为参考序列,将各流行区靶基因序列与相应流行区的保守序列进行比较,统计分析其碱基变化情况,获得各流行区EV71的主要碱基突变特征。
RNA编辑分析 EV71感染HeLa-pLKO.1-shADAR1和对照细胞,36 h后收集培养上清,用新增殖病毒再去感染重新铺板的细胞,不断感染和传代,直至第15代(G15)。细胞培养上清中病毒RNA用Trizol试剂抽提,利用随机引物进行逆转录,随后取5 mL cDNA进行PCR,对PCR产物进行测序。以PCR产物测序图谱为分析对象,仔细比对RNA编辑热点的测序峰值,以及测序主峰当中可能含有其他碱基的测序峰,测序峰的积分值比例表明碱基在此位置的百分含量。如果在ADAR1编辑热点出现A主峰中含G峰,或T主峰中含C峰,并随病毒传代该峰出现升高,则认为出现ADAR1对EV71 RNA的A-G或T-C的定向编辑[11]。
统计学分析 不同组间的数据使用Student′st检验进行分析,所有数据均使用SPSS 16.0软件进行处理。P<0.05为差异有统计学意义。
结 果
ADAR1基因沉默细胞的构建与筛选 为确定ADAR1基因沉默后对EV71感染和复制的影响,设计2对分别作用于ADAR1 mRNA的特异性shRNA(图 1A),通过构建慢病毒、筛选稳定下调ADAR1的细胞株。首先构建HeLa细胞ADAR1基因沉默稳定细胞株ShADAR1-1398和ShADAR1-4976。Sh-ADAR1-4976稳定细胞株下调ADAR1蛋白表达水平约90%,而Sh-ADAR1-1398稳定细胞株下调ADAR1蛋白表达水平达96%,均有非常显著的下调作用。故选择Sh-ADAR1-1398稳定细胞株完成后续的实验(图 1B)。同时使用Sh-ADAR1-1398慢病毒感染并筛选RD细胞,并筛选ADAR1基因沉默稳定细胞株RD-pLKO.1-shADAR1(图 1C)。
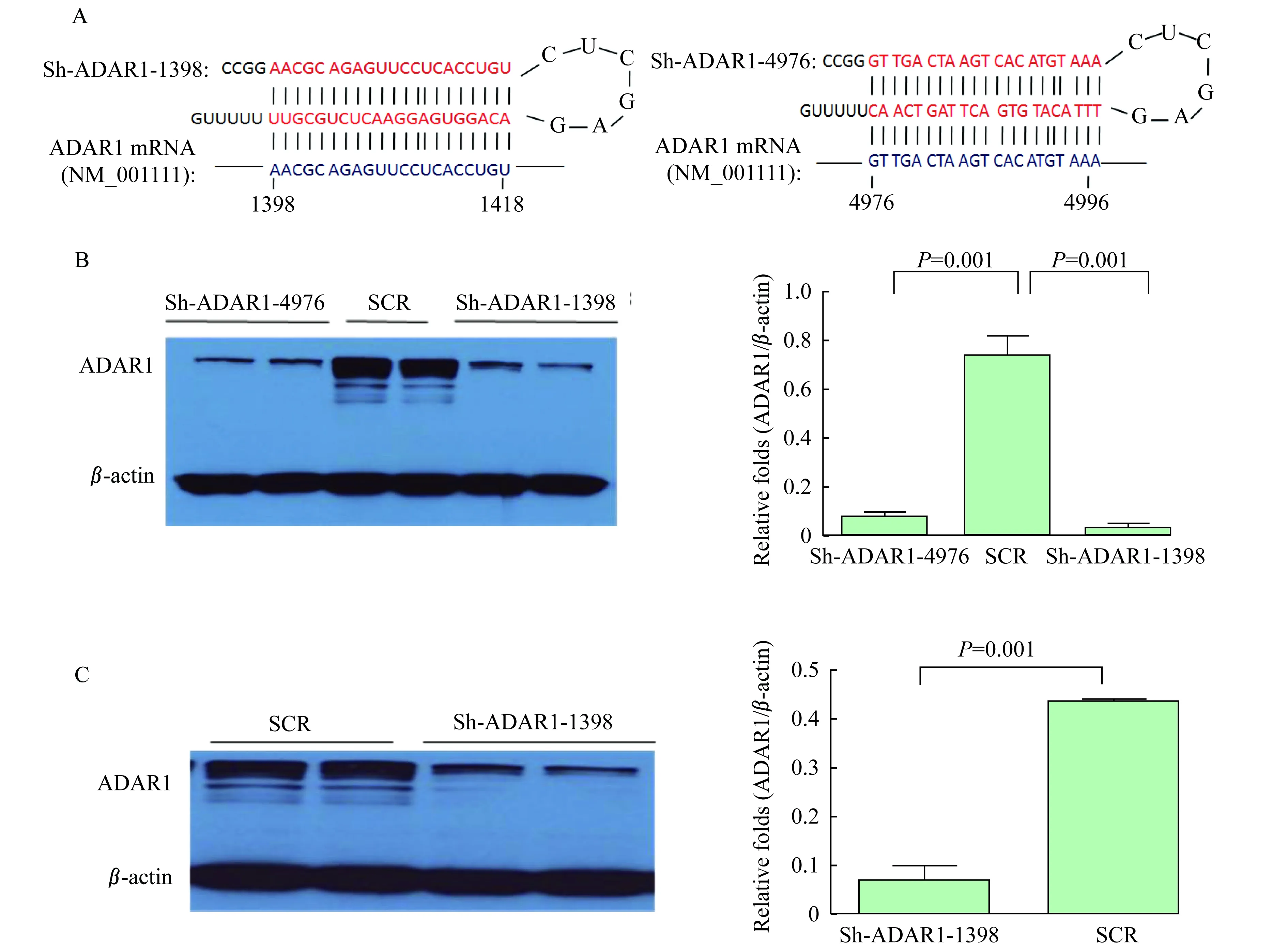
A:Design of shRNA targetingADAR1 mRNA located at 1398-1418 and 4976-4996,respectively;B:ADAR1 expression levels were detected by immunoblotting and knocked down by shRNAs in HeLa cells;C:ADAR1 expression levels were detected in RD cells.The immunoblotting data were quantified and normalized with-actin.
图1ADAR1基因沉默细胞的构建与筛选
Fig 1 Construction and selection of stable cell lines withADAR1 knock-down
ADAR1基因沉默促进EV71诱导细胞病变、裂解 EV71在不同MOI下感染HeLa-pLKO.1-shADAR1细胞和对照HeLa-pLKO.1-SCR细胞,随着感染时间延长,HeLa-pLKO.1-shADAR1细胞和对照细胞的存活率都不断降低,但在相同条件下,HeLa-pLKO.1-shADAR1细胞的存活率均低于对照细胞(图2A)。提示ADAR1基因沉默后促进病毒诱导HeLa细胞病变和裂解。利用ADAR1基因沉默RD细胞也发现类似结果,即ADAR1基因沉默后促进病毒诱导RD细胞的病变和裂解,与对照细胞相比,细胞存活率下降更快(图2B)。
ADAR1基因沉默提高细胞对EV71的敏感性分别用等量EV71病毒感染HeLa-pLKO.1-shADAR1细胞和对照细胞2 h,HeLa-pLKO.1-shADAR1细胞形成的噬斑数量约为对照细胞的3倍,且噬斑明显较大(图3A)。提示ADAR1基因沉默后可提高HeLa细胞对EV71的敏感性。观察EV71感染RD-pLKO.1-shADAR1细胞和对照细胞后形成的噬斑情况,得到一致结果,即细胞ADAR1基因下调后,细胞更容易被病毒感染,形成更多、更大的噬斑(图3B)。
ADAR1基因沉默促进EV71的复制 观察EV71感染HeLa-pLKO.1-shADAR1细胞和对照细胞后病毒的复制情况。在感染后12~48 h,HeLa-pLKO.1-shADAR1细胞中EV71 VP1蛋白表达水平明显高于对照细胞(图 4A);在感染后6~48 h,HeLa-pLKO.1-shADAR1细胞上清中病毒滴度较对照细胞明显升高(P<0.05,图4A)。提示ADAR1基因沉默后可促进EV71在HeLa细胞中的复制。EV71感染RD-pLKO.1-shADAR1细胞和对照细胞后检测病毒VP1含量以及上清中病毒滴度,得到类似的结果(图4B)。
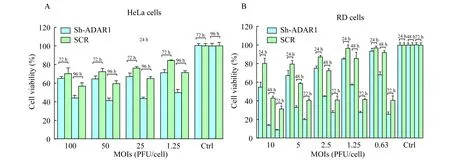
A:Cell viability of HeLa cells withADAR1 knock-down and the control cells were analyzed after EV71 infection;B:Cell viability of RD cells withADAR1 knock-down and the control cells were analyzed.
图2ADAR1基因沉默促进EV71诱导细胞病变和裂解
Fig 2ADAR1 knock-down promoted EV71-induced cell lysis
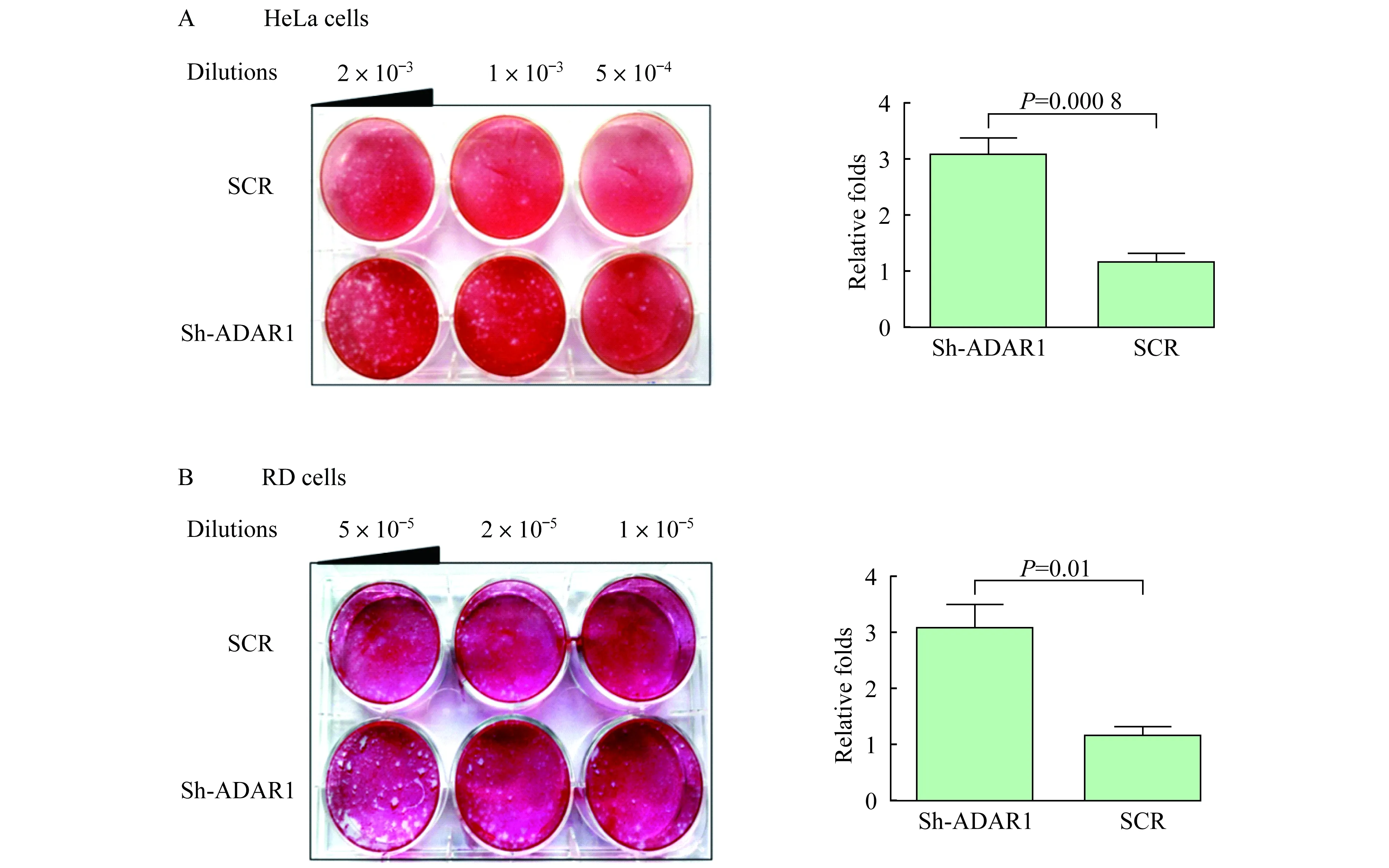
Plaques of EV71 formed on HeLa cells (A) and RD cells (B) withADAR1 knock-down.Graph showed the relative folds of plaque number normalized by the control cells.
图3ADAR1基因沉默提高细胞对EV71的敏感性
Fig 3ADAR1 knock-down increased the cellular susceptibility to EV71
EV71的突变特征及ADAR1对EV71基因组的编辑分析 由于ADAR1具有RNA编辑酶活性,编辑EV71复制中间体dsRNA可导致病毒A-G或T-C的突变[20]。分析5个EV71流行地区病毒的突变特征,结果显示A-G和T-C之间突变确实为EV71的主要突变类型(表1)。为确定ADAR1是否直接编辑EV71基因组并影响病毒感染和突变,根据预测分析的ADAR1编辑热点(AAG、UAG、AAU、UAU和CTT、CTA、ATT、ATA)序列特征,选择EV71基因组中5个编辑热点聚集区进行测序分析。结果显示病毒感染ADAR1基因沉默细胞及其不断传代后(至G15),尚未发现EV71基因组中被ADAR1定向编辑的位点(图5),提示ADAR1可能并不通过直接编辑EV71基因组而影响病毒的感染。
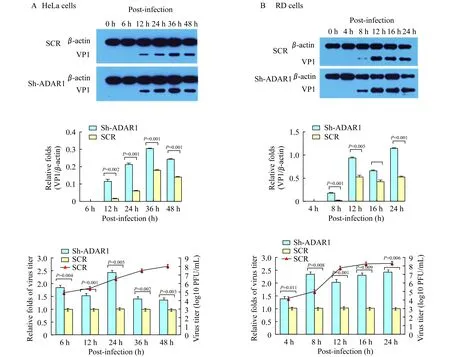
A:EV71 VP1 expressions (upper panel) in HeLa cells withADAR1 knock-down,and virus titer (lower panel) were detected after EV71 infection (MOI=50 PFU/cell).B:EV71 VP1 expressions (upper panel) in RD cells withADAR1 knock-down,and virus titer (lower panel) were detected after EV71 infection (MOI=10 PFU/cell).The data were normalized by the control cells.
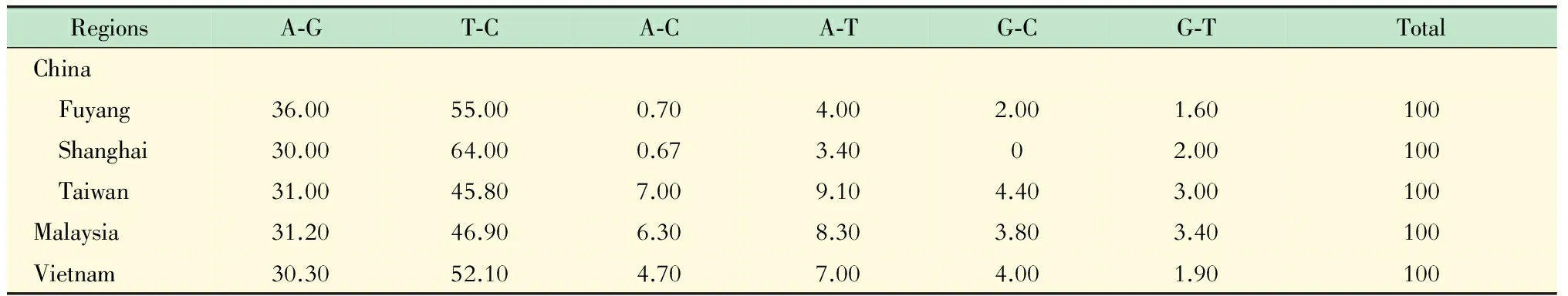
图4 ADAR1基因沉默对EV71复制的影响
EV71 full-length genome sequences from five epidemic regions were collected.The Genbank Acesss No.showed as the following:EU703812,EU703814,HQ188292,JX025561,EU703813,FJ439769,EU812515,GU459070,GU459071 and GU198371 in Fuyang;FJ713137,HQ891925,HQ891926,KC570452,JQ736684,HQ891929,HQ891927,HQ891923,HQ891924 and HQ891928 in Shanghai;GQ231925,GQ231942,GQ231934,GQ231941,GQ231933,KJ186973,KF134486,KF154354,KF154355 and GQ231943 in Taiwan;AB550334,AB550335,DQ341367,DQ341362,DQ341363,DQ341365,DQ341366,AB550340,AB550341 and DQ341358 in Malaysia;KJ686299,KJ686301,KJ686304,KJ686302,KJ686308,KJ686306,KJ686300,KJ686303,KJ686305 and KJ686307 in Vietnam.

Five fragments of EV71 genome (sequence locations indicated with red box) clustering with RNA-editing hot sites by ADAR1 were analyzed by sequencing from G0 to G15.Arrows indicated the potential editing-sites.
图5 EV71基因组RNA编辑分析结果示例
Fig 5 Representative RNA-editing assay for EV71 genome
讨 论
新型肠道病毒EV71感染较其他HFMD病原体更容易致神经性重症[21],但是其致病机制并不清楚。研究表明一些重要宿主因子与EV71感染相关[21]。EV71与其他RNA病毒一样,基因组易产生突变,而且在宿主抗病毒免疫系统的压力下,EV71也容易产生逃逸突变,而高突变率引起的新基因亚型与EV71流行密切相关[3]。
RNA编辑酶ADAR1既可编辑宿主细胞因子,也可编辑病毒dsRNA[6],因而影响多种病毒的感染。ADAR1对EV71感染有何影响,及是否对该病毒进行编辑并不清楚。通过MTT分析及噬斑形成分析发现,ADAR1基因沉默后可促进EV71诱导细胞病变和裂解,增强细胞对EV71的敏感性。Toth等[14]用RNAi技术沉默HeLa细胞ADAR1后,发现MV引起的细胞病变增强;Ward等[15]通过敲除MEF细胞ADAR1 p150异构体,发现细胞对MV感染的敏感性增强。我们通过Western blot检测发现ADAR1基因沉默后可以促进EV71的复制。敲除ADAR1 p150后MV增殖病毒滴度显著增高[15]。Taylor等[16]运用小分子RNA特异性抑制ADAR1,发现HCV的复制能力增加了40倍。我们还通过基因过表达研究了ADAR1对EV71感染的影响,获得了基本一致的结果。但由于细胞中ADAR1的基础表达水平非常高,与看家基因表达水平相近(图1),ADAR1过表达所能提升的蛋白表达水平有限,远未及ADAR1基因沉默改变明显(最多约20倍),因此ADAR1过表达对病毒感染的影响也相应较小(结果未显示)。
本研究初步发现ADAR1具有抗EV71感染的作用,但其分子机制并不清楚。有研究表明ADAR1基因沉默后,MV引起的细胞病变增强与激活PKR和干扰素调节因子3 (IRF3)有关[14]。ADAR1也可以通过RNA编辑影响病毒感染,如ADAR1可能通过对病毒RNA的编辑来达到清除HCV RNA的目的[16]。在HDV感染中,ADAR1过表达使amber/W处被过度编辑,导致大HDV抗原(HDAg-L)的异常表达而使复制过早的终止[17]。尽管通过EV71突变特征分析发现A-G和T-C的突变为EV71的主要突变类型,但本研究并未发现ADAR1可以直接编辑EV71 RNA,所以ADAR1影响EV71感染可能不是ADAR1编辑EV71基因组的结果,而可能与ADAR1诱导或抑制某些重要宿主因子的表达或通过ADAR1编辑某些影响病毒感染的宿主因子有关,具体分子机制尚需进一步研究。由于ADAR1不直接编辑EV71 RNA,因此EV71易突变形成新的基因型与ADAR1的关系可能并不密切。病毒基因突变除与病毒RNA聚合酶有关外[22-24],还有哪些因素影响基因突变的频率和方式,也是目前研究的难点和热点,相关研究进展将对防控病毒的感染和流行具有重要的理论和实际意义。
[1] SOLOMON T,LEWTHWAITE P,PERERA D,etal.Virology,epidemiology,pathogenesis,and control of enterovirus 71[J].LancetInfectDis,2010,10(11):778-790.
[2] YI L,LU J,KUNG HF,etal. The virology and developments toward control of human enterovirus 71[J].CritRevMicrobiol,2011,37(4):313-327.
[3] MAO QY,WANG Y,BIAN L,etal.EV71 vaccine,a new tool to control outbreaks of hand,foot and mouth disease (HFMD).ExpertRevVaccines,2016,15(5):599-606.
[4] TAN CW,LAI JK,SAM IC,etal.Recent developments in antiviral agents against enterovirus 71 infection[J].JBiomedSci,2014,21:14.
[5] LI R,LIU L,MO Z,etal.An inactivated enterovirus 71 vaccine in healthy children[J].NEnglJMed,2014,370(9):829-837.
[6] GEORGE CX,GAN Z,LIU Y,etal. Adenosine deaminases acting on RNA,RNA editing,and interferon action[J].JInterferonCytokineRes,2011,31(1):99-117.
[7] LIDDICOAT BJ,PISKOL R,CHALK AM,etal. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself[J].Science,2015,349(6252):1115-1120.
[8] PESTAL K,FUNK CC,SNYDER JM,etal.Isoforms of RNA-editing enzyme ADAR1 independently control nucleic acid sensor MDA5-driven autoimmunity and multi-organ development[J].Immunity,2015,43(5):933-944.
[9] MCLAUGHLIN PJ,JANTSCH MF,DORIN J,etal. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA [J].CellRep,2014,9(4):1482-1494.
[10] NIE Y,HAMMOND GL,YANG JH.Double-stranded RNA deaminase ADAR1 increases host susceptibility to virus infection[J].JVirol,2007,81(2):917-923.
[11] DORIA M,NERI F,GALLO A,etal.Editing of HIV-1 RNA by the double-stranded RNA deaminase ADAR1 stimulates viral infection[J].NucleicAcidsRes,2009,37(17):5848-5858.
[12] PHUPHUAKRAT A,KRAIWONG R,BOONARKART C,etal. Double-stranded RNA adenosine deaminases enhance expression of human immunodeficiency virus type 1 proteins[J].JVirol,2008,82(21):10864-10872.
[13] CLERZIUS G,GELINAS JF,DAHER A,etal.ADAR1 interacts with PKR during human immunodeficiency virus infection of lymphocytes and contributes to viral replication[J].JVirol,2009,83(19):10119-10128.
[14] TOTH AM,LI Z,CATTANEO R,etal.RNA-specific adenosine deaminase ADAR1 suppresses measles virus-induced apoptosis and activation of protein kinase PKR[J].JBiolChem,2009,284(43):29350-29356.
[15] WARD SV,GEORGE CX,WELCH MJ,etal.RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis[J].ProcNatlAcadSciUSA,2011,108(1):331-336.
[16] TAYLOR DR,PUIG M,DARNELL ME,etal.New antiviral pathway that mediates hepatitis C virus replicon interferon sensitivity through ADAR1[J].JVirol,2005,79(10):6291-6298.
[17] CASEY JL.Control of ADAR1 editing of hepatitis delta virus RNAs[J].CurrTopMicrobiolImmunol,2012,353:123-143.
[18] NISHIKURA K.Functions and regulation of RNA editing by ADAR deaminases[J].AnnuRevBiochem,2010,79:321-349.
[19] HERBERT A,ALFKEN J,KIM YG,etal.A Z-DNA binding domain present in the human editing enzyme,double-stranded RNA adenosine deaminase[J].ProcNatlAcadSciUSA,1997,94(16):8421-8426.
[20] GOODMAN RA,MACBETH MR,BEAL PA.ADAR proteins:structure and catalytic mechanism [J].CurrTopMicrobiolImmunol,2012,353:1-33.
[21] WENG KF,CHEN LL,HUANG PN,etal.Neural pathogenesis of enterovirus 71 infection[J].MicrobesInfect,2010,12(7):505-510.
[22] CROTTY S,MAAG D,ARNOLD JJ,etal.The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen[J].NatMed,2000,6(12):1375-1379.
[24] DRAKE JW,CHARLESWORTH B,CHARLESWORTH D,etal.Rates of spontaneous mutation[J].Genetics,1998,148(4):1667-1686.
The roles of RNA-editing enzyme ADAR1 in EV71 infection and virus mutation
LIU Qing-qing, CHANG Zhang-mei, BAI Jin-jin, WANG Yan, LONG Jian-er△
(KeyLaboratoryofMedicalMolecularVirology,MinistriesofEducationandHealth-DepartmentofMedicalMicrobiologyandParasitology,SchoolofBasicMedicalSciences,FudanUniversity,Shanghai200032,China)
Objective To identify the role of RNA-editing enzyme ADAR1 (adenosine deaminase acting on RNA) in EV71 infection and virus mutation. Methods RNAi technology was applied to establish ADAR1 knock-down stable cell lines.Then the cells were served to evaluate the role of ADAR1 in EV71 infection by MTT assay for detecting virus-induced cell viability,virus plaque assay for quantification of the virus titer and the cellular susceptibility to the virus,and Western blot for virus protein expressions.ADAR1-mediated RNA editing can result in the genetic A-G and T-C mutations.To further determine whether the effects ofADAR1 on EV71 infection were correlated withADAR1-mediated EV71 RNA editing and therefore increased the viral mutations during the infection,the characteristics of EV71 mutation were analyzed based on the different full-length viral genomes from epidemic regions.The viral genome was also sequenced from the infected ADAR1 knock-down cells. Results AfterADAR1 knock-down,the cell viability decreased quickly after the virus infection,and formed much more and larger sizes of plaques than the control cells.The virus capsid protein VP1 expressions and virus titer in the cells culture media were both increased inADAR1 knock-down cells.Statistic analysis showed that A-G and T-C mutations were the major mutations of EV71,which were believed to be the hot sites for RNA-editing.However,the results of viral RNA genomic sequencing data indicated that ADAR1 did not edit EV71 genome directly. Conclusions ADAR1 was a restriction factor for controlling EV71.However,ADAR1 does not directly edit EV71 genome.
EV71; ADAR1; RNA-editing; virus mutation
国家自然科学基金(31570156);传染病防治国家科技重大专项(2012ZX10004503-003)
Q343.1+3
A
10.3969/j.issn.1672-8467.2017.03.001
2016-06-27;编辑:段佳)
△Corresponding author E-mail:longjianer@fudan.edu.cn
*This work was supported by the National Natural Science Foundation of China (31570156) and the National Science and Technology Major Project on Infectious Diseases (2012ZX10004503-003).
