肌细胞特异性microRNAs对收缩舒张生物学效应调控机制的研究进展
2017-06-27段婷婷综述韩晓杰徐玉东杨永清尹磊淼审校
段婷婷(综述) 韩晓杰 庞 雨 徐玉东 王 宇 杨永清 尹磊淼(审校)
(上海中医药大学上海市针灸经络研究所 上海 201203)
肌细胞特异性microRNAs对收缩舒张生物学效应调控机制的研究进展
段婷婷(综述) 韩晓杰 庞 雨 徐玉东 王 宇 杨永清 尹磊淼△(审校)
(上海中医药大学上海市针灸经络研究所 上海 201203)
肌细胞特异性microRNAs (muscle-specific microRNAs,myomiRs)是一类特异性表达在肌组织中的内源性非编码小分子RNA,通过转录后水平负调控相关基因的表达,广泛参与到一系列生物学过程中,影响疾病的发生发展。肌细胞相关疾病(如慢性阻塞性肺炎、肥厚型心肌病等)的发生、发展可引起myomiRs及其下游靶基因表达改变,从而进一步影响疾病的发展、预后及转归。本文将综述miR-1、miR-133、miR-206、miR-208和miR-499等常见myomiRs在横纹肌和非横纹肌收缩舒张机制中的作用,重点关注myomiRs对肌细胞收缩舒张生物学效应的影响,以期为肌细胞相关疾病治疗提供新思路。
肌细胞特异性microRNAs; 平滑肌; 骨骼肌; 心肌; 肌肉收缩舒张机制
microRNAs (miRNAs)是含20~22个核苷酸的非编码小分子RNA,通过转录后水平负调控相关基因的表达。MiRNAs分布具有组织特异性,其中肌细胞特异性miRNAs (muscle-specific microRNAs,myomiRs)[1]仅表达在肌组织包括平滑肌、心肌、骨骼肌中[2],只针对肌细胞相关基因进行转录后表达调控,并显著影响肌细胞收缩舒张、增殖和分化[3]。目前常见的myomiRs包括miR-1、miR-133、miR-206、miR-208及miR-499等[4]。myomiRs可影响肌细胞收缩舒张、增殖及分化等重要生物学功能,在转录后水平负性调控相应靶基因表达,并和相关转录因子、信号蛋白、激酶等相互作用形成复杂的生物调控网络[3,5]。myomiRs在肌细胞收缩舒张生物学效应中扮演重要角色,如myomiRs参与调节兰尼碱受体(ryanodine,RyR)、1,4,5-三磷酸肌醇受体(inositol 1,4,5-triphosphate
receptor,IP3R)控制钙离子释放,从而影响肌细胞收缩[6-7]。
慢性阻塞性肺疾病(chronic obstructive pulmonary disease,COPD)、肥厚型心肌病(hypertrophic cardiomyopathy,HCM)等肌细胞相关疾病可引起myomiRs表达改变,影响下游靶基因表达,诱发肌细胞的过度收缩、增殖,最终影响到疾病的发展[8-11](表1)。阐明myomiRs参与肌细胞收缩舒张过程的生物学机制,将为平滑肌收缩舒张功能障碍相关疾病提供新的治疗策略和思路。
鉴于肌球蛋白轻链(myosin light chain,MLC)在肌细胞收缩舒张过程中所起关键调控作用,学界将肌肉收缩舒张分为MLC磷酸化依赖性途径和非MLC磷酸化依赖性途径[12-13]。本文将从myomiRs对非横纹肌(平滑肌)和横纹肌(心肌与骨骼肌)的收缩舒张效应进行综述。
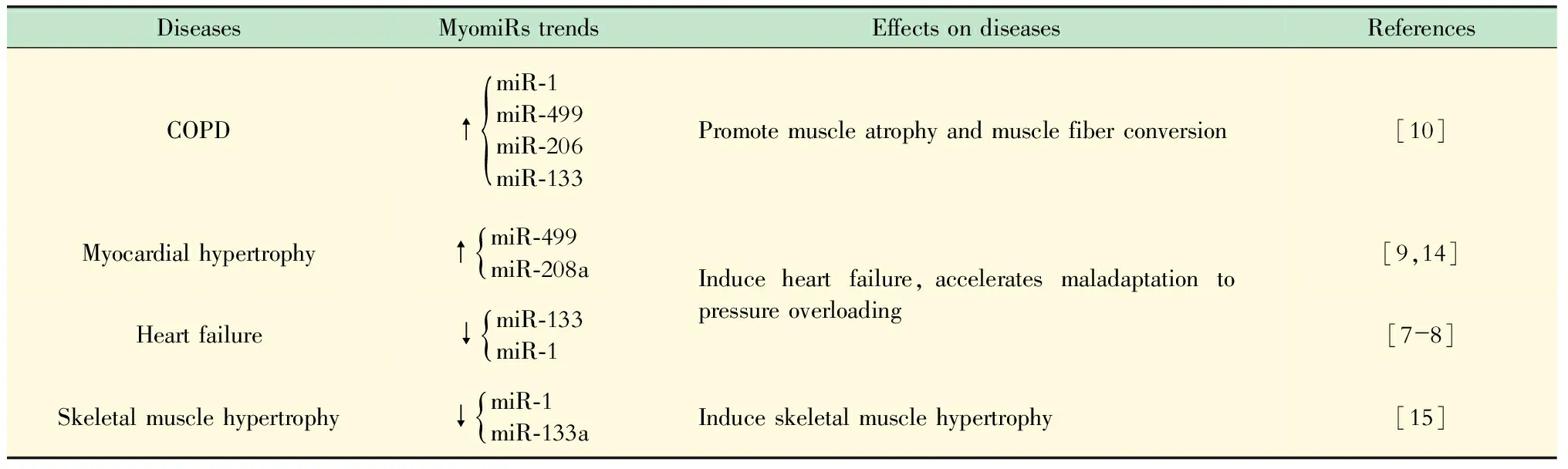
表1 myomiRs表达与肌细胞相关疾病
COPD:Chronic obstructive pulmonary disease.
平滑肌收缩舒张机制及myomiRs对其影响 MLC在平滑肌细胞收缩舒张过程中起重要作用,据此可将收缩舒张机制分为MLC磷酸化依赖性途径(包括Ca2+-CaM-MLCK机制和Rho-ROK-MLCP、PKC-CPI-17-MLCP机制)及非MLC磷酸化依赖性途径(细肌丝相关蛋白的调节机制)[12-13,16]。
MyomiRs在MLC磷酸化依赖性收缩的作用MLC磷酸化水平是决定平滑肌收缩程度的一个重要因素,其磷酸化水平受到Ca2+/钙调蛋白(calmodulin,CaM)依赖的肌球蛋白轻链激酶(myosin light chain kinase,MLCK)和非Ca2+依赖的肌球蛋白磷酸酶(myosin light chain phosphatase,MLCP)的双重调节[17]。MLCK磷酸化MLC促进肌球蛋白单体形成肌丝,收缩平滑肌细胞;MLCP脱磷酸化肌球蛋白舒张平滑肌细胞。平滑肌细胞舒缩状态取决于MLC的磷酸化程度,由MLCK/MLCP比值大小直接决定,并受胞质内钙离子浓度、Rho激酶和钙调蛋白等因素调控[17-18]。
MyomiRs参与 Ca2+-CaM-MLCK机制 该机制也可被称作为钙依赖机制[19]。当平滑肌细胞受到外界刺激时,膜成分磷脂酰肌醇4,5二磷酸(phosphatidylinositol 4,5-bisphosphate,PIP2)在磷脂酶C (phospholipase C,PLC)的作用下分解为三磷酸肌醇(inositol 1,4,5-trisphosphate,IP3)和二酰甘油(diacylglycerol,DAG),前者激活肌浆网使内钙释放,后者通过L型钙通道开放,引起钙离子内流,进一步激活肌浆网上的兰尼碱受体,促进内钙释放。这种“外钙内流和内钙释放”方式使胞质内的Ca2+浓度升高[16],Ca2+/CaM复合体形成进而激活MLCK,磷酸化MLC第19位丝氨酸,收缩平滑肌细胞[20]。当Ca2+浓度下降至10-7mmol/L时[21],CaM与MLCK分离,MLCK活性消失,此时MLCP作用占主导地位,MLCP降低MLC磷酸化程度,从而舒张平滑肌细胞(图1)。
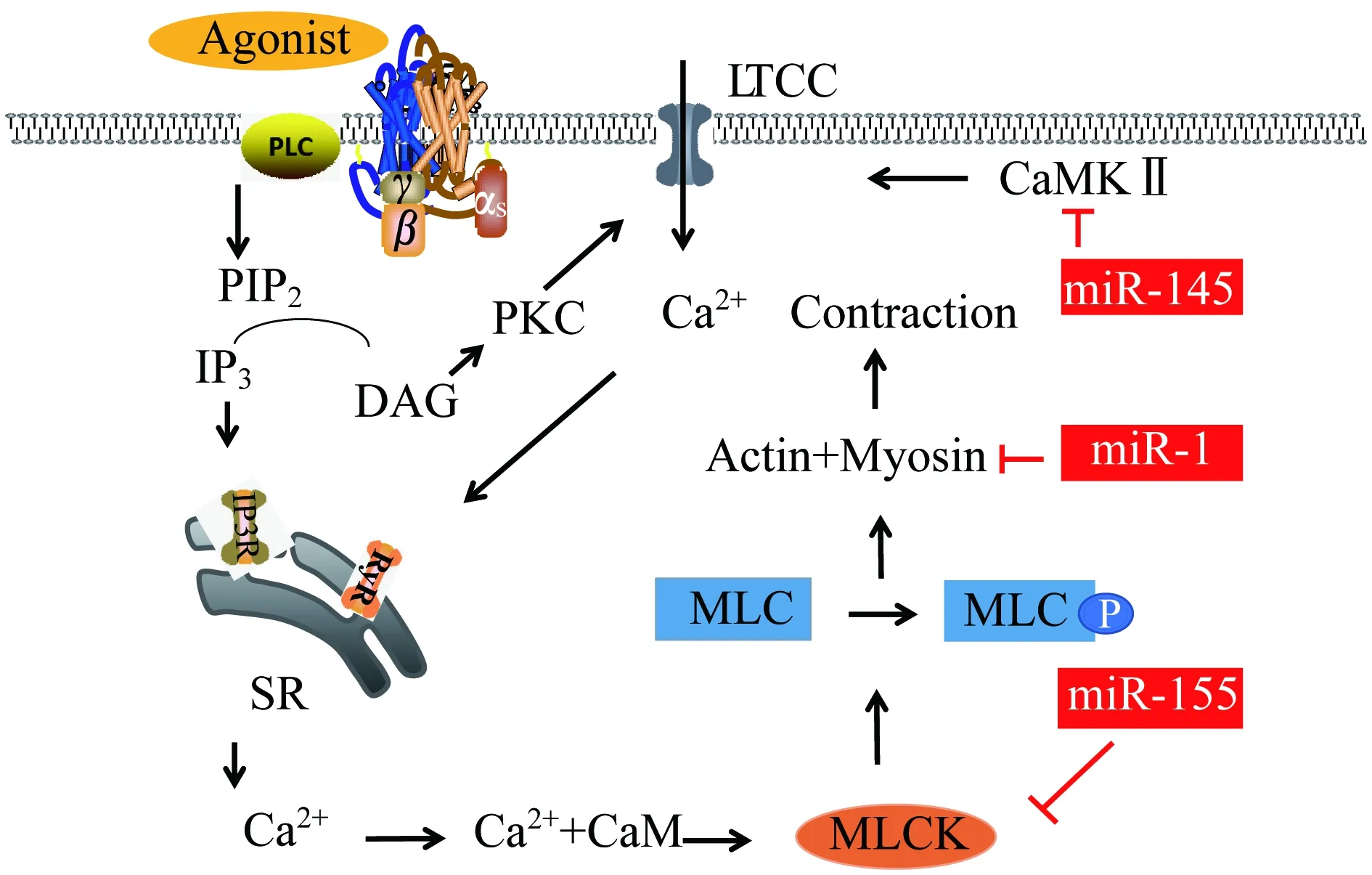
GPCR:G protein coupled receptor;PLC:Phospholipase C;PIP2:Phosphatidylinositol 4,5-bisphosphate;IP3:Inositol 1,4,5-trisphosphate;DAG:Diacylglycerol;IP3R:Inositol 1,4,5-trisphosphate receptor;RyR:Ryanodine receptors;SR:Sarcoplasmic reticulum;CaM:Calmodulin;MLCK:Myosin light chain kinase;MLC:Myosin light chain;PKC:Protein kinase C;LTCC:L-type calcium channel;CaMKⅡ:Calmodulin kinase type Ⅱ.
图1 钙依赖性收缩机制通路图
Fig 1 Pathway of calcium dependent muscle contraction mechanism
研究发现miR-1通过抑制α-平滑肌肌动蛋白(α-smooth muscle actin)和平滑肌22蛋白(smooth muscle-22,SM22)表达,减少肌动蛋白细胞骨架形成,从而抑制心肌素诱导的血管平滑肌收缩[22]。miR-145下调钙调蛋白激酶Ⅱ (calmodulin kinase type Ⅱ,CaMKⅡ)增加L型钙通道(L-type calcium channel,LTCC)表达,胞外Ca2+内流增多,诱发SR释放出更多Ca2+,胞质内Ca2+总浓度上升,间接促进血管平滑肌的收缩[23]。目前虽未找到相关myomiRs能够直接作用MLCK的文献,但有研究表明在内皮细胞中,miR-155能够直接作用RhoA和MLCK,抑制其表达,使p-MLC表达减少,抑制细胞的收缩及应力纤维的形成[24]。
MyomiRs参与Rho-ROK-MLCP与PKC-CPI-17-MLCP机制 该机制又被称为钙敏化机制,主要是指对MLCP的抑制作用,增强了MLC磷酸化促使平滑肌进一步收缩[25]。钙敏化机制主要通过Rho-ROK通路和PKC-CPI-17通路抑制MLCP活性。Rho-ROK通路使肌球蛋白靶亚基(myosin phosphatase target subunit,MYPT1)上T853和T696位点磷酸化,直接导致MLCP失活[26-27]。而PKC磷酸化蛋白激酶C抑制蛋白(C kinase-potentiated phosphatase inhibitor of 17 kD,CPI-17)与MLCP催化亚基紧密结合,间接使MLCP失活[12,28](图2)。
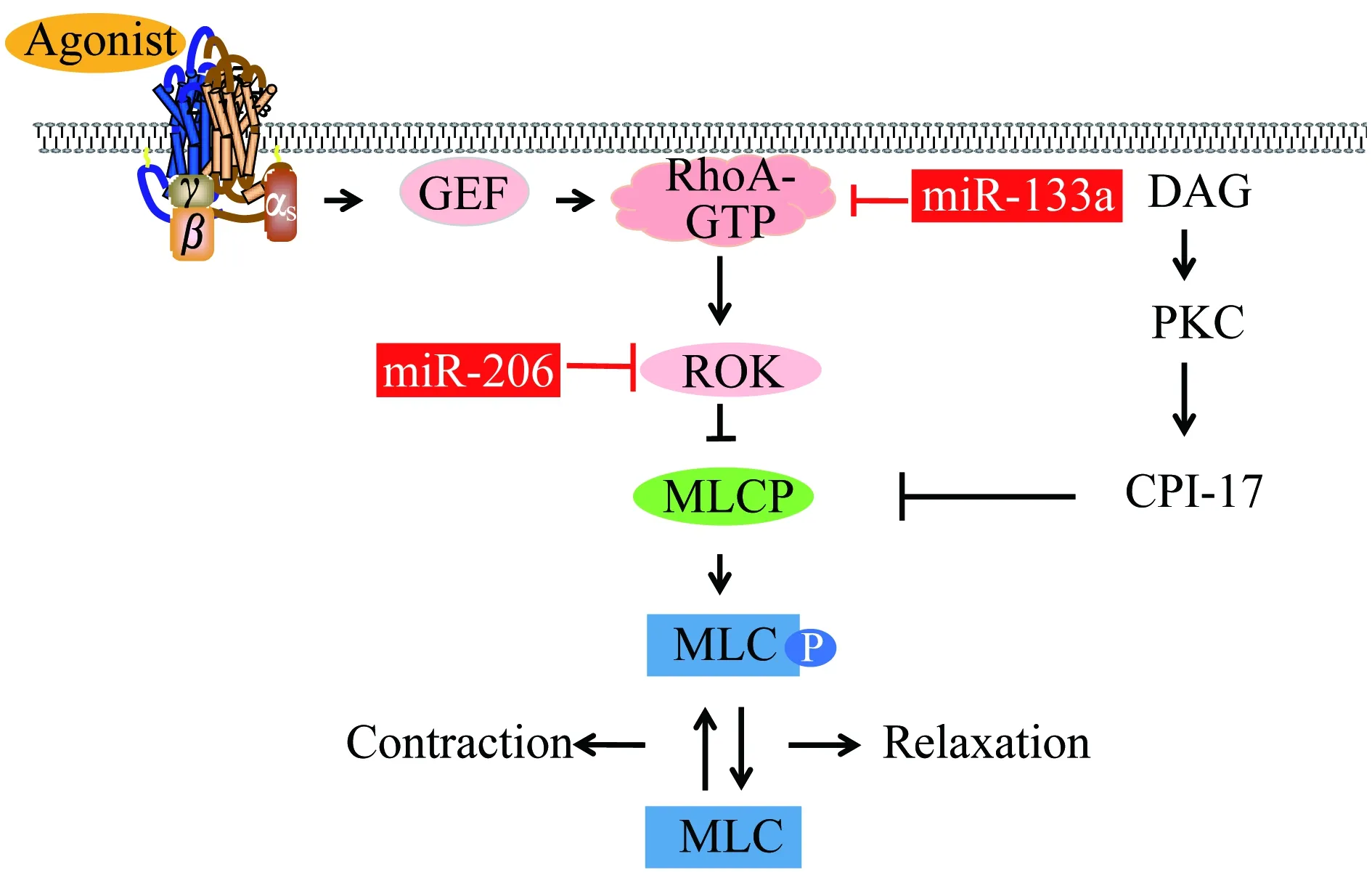
GPCR:G protein coupled receptor;GEF:Guanine nucleotide exchange factors;ROK:Rho-associated kinase;MLCP:Myosin light chain phosphatase;MLC:Myosin light chain;DAG:Diacylglycerol;PKC:Protein kinase C;CPI-17:Protein kinase-potentiated phosphatase inhibitor of 17 kD.
图2 钙敏化收缩机制通路图
Fig 2 Pathway of calcium sensitization contraction mechanism
研究发现气道平滑肌中RhoA表达受到miR-133a负调控[29]。给予气管平滑肌细胞IL-13,发现在干预后第3、6小时miR-133a的表达显著降低,RhoA表达增多,气管平滑肌收缩[30]。糖尿病和高血脂介导的炎性反应能够通过下调miR-206和其他一些非myomiRs表达,如miR-10a、miR-139b,增加ROK和连接蛋白的表达,从而诱导血管平滑肌细胞收缩和血管高反应性[31]。
MyomiRs在非MLC磷酸化依赖性收缩的作用
MyomiRs参与细肌丝相关蛋白机制 在平滑肌的收缩舒张过程中除了粗肌丝的调节外,细肌丝也可调节其收缩舒张[32]。钙调结合蛋白(caldesmon,CaD)和原肌球蛋白(tropomyosin,TM)是参与细肌丝相关调节的重要蛋白,静息状态下,二者能够分别抑制actin-myosin的结合和myosin ATP酶的活性,此时肌肉处于舒张状态[33]。在收缩信号的刺激下,CaD、TM和HSP27被磷酸化,磷酸化CaD构象改变与actin-TM解离,磷酸化HSP27与磷酸化TM结合在actin上滑动,暴露出myosin结合位点。actin与myosin结合,引发收缩[34]。
研究发现在血管平滑肌细胞中,miR-143和miR-145高表达能够通过下调枯否样因子(krupple-like factor,KLF)4和KLF5,增加钙调理蛋白(calponin,CaP)、平滑肌细胞肌球蛋白重链(smooth muscle myosin heavy chain,SM-MHC)等收缩蛋白的表达[35]。干扰素β和γ能够同时提高miR-143、145和气道平滑肌中α肌动蛋白表达量,这可能和气管平滑肌收缩相关[36]。
临床疾病 MyomiRs通过调控下游靶基因,参与哮喘、动脉粥样硬化、肺动脉高压等疾病。miR-133是气管平滑肌高反应性的重要调节者。小鼠过敏性哮喘模型气道高反应性显著增高并伴有IL-13、RhoA蛋白表达增强和miR-133a降低[30]。进一步研究发现,IL-13通过降低miR-133a表达,增高RhoA,从而诱发气道平滑肌收缩增强并出现气道高反应性[29]。
miR-145在动脉粥样硬化和肺动脉高压的形成中也发挥着关键作用。临床研究发现在动脉粥样硬化血管壁上miR-145表达较正常组显著降低[37]。而血管平滑肌细胞表型转换作为动脉粥样硬化的起始过程,受到miR-145负调节,即miR-145降低会促进血管平滑肌细胞分化,从而加快动脉粥样硬化病变的进程[38]。在肺动脉高压的形成中,miR-145等myomiRs也可通过调控血管细胞的收缩增殖、凋亡可诱发肺动脉管壁压力升高[39]。
横纹肌收缩舒张机制及myomiRs对其调控 根据MLC在横纹肌中收缩和舒张中所起的作用,将横纹肌的收缩舒张机制也可按照MLC磷酸化依赖性途径(Ca2+-CaM-MLCK机制)和非MLC磷酸化依赖性途径,包括Ca2+-TN-TM机制和肌球蛋白重链(myosin heavy chain,MHC)调节机制。
MyomiRs在MLC磷酸化依赖性收缩的作用
MyomiRs参与调控Ca2+-CaM-MLCK机制 类似于平滑肌,横纹肌胞内Ca2+增高能够激活CaM,Ca2+-CaM复合体继而激活MLCK,从而引起MLC磷酸化[40-41]。MLC磷酸化能够倾斜、旋转横桥,促进actin与myosin结合,收缩平滑肌[42]。胞内钙离子的升高激活CaM,Ca2+-CaM复合体激活MLCK,MLCK磷酸化MLC,促进横桥与actin结合,从而促进收缩。
研究发现高表达的miR-1能够通过调节亚基B56α,抑制蛋白磷酸酶2A (protein phosphatase 2A,PP2A)生物学功能,激活RyR2,促使肌质网上自发的钙离子释放,提高胞内钙离子浓度引起细胞收缩。在压力负荷和神经激素刺激下,miR-133a下调使IP3RⅡ表达增多,增加促肥厚钙信号(IP3-induced calcium release,IICR)形成和心肌病理性重构,使得代偿性心肌总量增加、心肌收缩力加强[43]。miR-1能通过对钙依赖信号分子CaM、肌细胞强化因子2a (myocyte enhancer factor-2a,Mef2a)、转录因子GATA4负调控,使得代偿性心肌总量减弱、心肌收缩力减弱,减弱小鼠心肌肥厚发生[44]。
MyomiRs在非MLC磷酸化依赖性收缩的作用
Ca2+-TN-TM机制 横纹肌不同于平滑肌,诱发肌肉收缩的是Ca2+与肌钙蛋白(troponin,TN)结合[42]。当肌细胞兴奋而使胞质内Ca2+增加时,Ca2+便与细丝上的TN结合,构象发生变化、牵拉原肌球蛋白滑动移位,暴露结合位点,actin与myosin结合,横桥周期生成,牵拉细肌丝向粗肌丝内滑行,肌节缩短,出现肌肉收缩。
MyomiRs参与MHC调节机制 MHC是心肌细胞控制收缩性能的主要决定因素[45]。α-MHC和β-MHC的表达比可影响心肌肌小节的收缩[46]。用丙基硫氧嘧啶处理雄性鼠使之心肌MHC产生α-MHC并向β-MHC转换,发现α-MHC表达减少伴随着横桥周期速率减少,与横桥周期动力学之间存在线性关系[47](图3)。
研究发现当机体处于应激或者甲状腺功能减退时,能诱导成人心肌α-MHC向β-MHC转换[48],横桥再生速率减缓,从而出现收缩效率减退[49]。miR-208a通过抑制甲状腺受体相关蛋白(thyroid receptor-associated protein 1,THRAP1)抑制β-MHC表达。依赖miR-208α,miR-208b和miR-499能控制肌球蛋白表达量,肌纤维种类和性能[50],从而调控收缩。

GPCR:G protein coupled receptor;PLC:Phospholipase C;PIP2:Phosphatidylinositol 4,5-bisphosphate;IP3:Inositol 1,4,5-trisphosphate;DAG:Diacylglycerol;SR:Sarcoplasmic reticulum;CaM:Calmodulin;MLCK:Myosin light chain kinase;MLC:Myosin light chain;PP2A:Protein phosphatase 2A;MHC:Myosin heavy chain.
图3 横纹肌收缩调节机制通路图
Fig 3 Pathways of strained muscle contraction regulation
临床疾病 大量研究表明myomiRs与心衰及HCM紧密相关。对心衰患者使用醛固酮受体拮抗剂(依普利酮)能够有效抑制miR-208a表达,提高THRAP1,从而抑制心肌病理性肥厚[51]。机制研究表明miR-208a通过抑制THRAP1降低α-MHC、上调β-MHC表达发挥诱导心衰作用[14]。而MHC组成的微小改变对心肌收缩具有一定作用,如用β-MHC替换掉12%的α-MHC,心肌纤维ATP酶活性下降23%,收缩力下降15%[52]。HCM发病过程中,miR-1和miR-133可负调控Ca2+及CaM、Mef2a等信号蛋白,增加肌总量和肌收缩力[53]。
MyomiRs在心肌梗死中起到预测和监控作用。研究发现急性心梗患者血清miR-1、miR-208a和miR-499表达量显著升高,分别为正常组300、2000和250倍[54]。进一步分析显示上述三者均可作为预测心肌梗死生物学标记,而miR-208a和miR-499预测效果优于miR-1(特异性和敏感性约高10%)[55]。在对ST段抬高心梗患者预测中,miR-208b敏感性和特异性均高达100%[56]。
结语 MyomiRs生物学功能多样,可通过作用相应下游靶基因发挥不同作用。myomiRs表达失调与肌细胞疾病发生发展密切相关。在肌细胞相关疾病中,myomiRs改变可调节肌细胞收缩舒张机制生物网络中的转录因子、信号蛋白、激酶等表达,改变肌细胞舒缩功能,最终影响疾病预后与转归。
临床应用上,目前myomiRs主要用于诊断肌细胞相关性疾病,如miR-499等可作为新一代心肌梗死生物标志物。治疗方面,myomiRs表现出强劲的潜力:哮喘患者可通过特异性增高气管平滑肌细胞miR-133a表达,舒张气道平滑肌、缓解哮喘气道高反应性;提高miR-206表达,可以舒张血管平滑肌、降低高血脂诱发的血管高反应性,达到保护心血管的作用[34]。在心衰患者治疗中,可通过慢病毒给药、使用醛固酮受体抑制剂等方式降低miR-208a表达,有效抑制心肌病理性修复,提高心衰患者存活率。提高心衰患者血清miR-133和miR-1表达,抑制肌纤维量和肌收缩力,减缓心肌肥厚,从而保护心脏。因此,进一步探索和研究myomiRs对肌肉收缩舒张机制的影响,发展myomiRs相关替代疗法,将为肌细胞相关疾病提供新的治疗方案。
[1] SIMIONESCU-BANKSTON A,KUMAR A.Noncoding RNAs in the regulation of skeletal muscle biology in health and disease [J].JMolMed,2016,94(8):853-866.
[2] CHISTIAKOV DA,OREKHOV AN,BOBRYSHEV YV.Cardiac-specific miRNA in cardiogenesis,heart function,and cardiac pathology (with focus on myocardial infarction) [J].JMolCellCardiol,2016,94:107-121.
[3] 韩晓杰,杨莎莎,段婷婷,等.肌细胞特异性microRNAs生物学效应研究进展[J]中国细胞生物学学报,2016,38(6):729-735.
[4] HORAK M,NOVAK J,BIENERTOVA-VASKU J.Muscle-specific microRNAs in skeletal muscle development [J].DevBiol,2016,410(1):1-13.
[5] PHILIPPEN LE,DIRKX E,DA COSTA-MARTINS PA,etal.Non-coding RNA in control of gene regulatory programs in cardiac development and disease [J].JMolCellCardiol,2015,89(PtA):51-58.
[6] SCHLOSSMANN J,AMMENDOLA A,ASHMAN K,etal.Regulation of intracellular calcium by a signaling complex of IRAG,IP3 receptor and cGMP kinase Ibeta [J].Nature,2000,404(6774):197-201.
[7] HARADA M,LUO X,MUROHARA T,etal.MicroRNA regulation and cardiac calcium signaling:role in cardiac disease and therapeutic potential [J].CircRes,2014,114(4):689-705.
[8] DORN GW,MATKOVICH SJ,ESCHENBACHER WH,etal.A human 3′ miR-499 mutation alters cardiac mRNA targeting and function [J].CircRes,2012,110(7):958-967.
[9] MATKOVICH SJ,HU Y,ESCHENBACHER WH,etal.Direct and indirect involvement of microRNA-499 in clinical and experimental cardiomyopathy [J].CircRes,2012,111(5):521-531.
[10] DONALDSON A,NATANEK SA,LEWIS A,etal.Increased skeletal muscle-specific microRNA in the blood of patients with COPD [J].Thorax,2013,68(12):1140-1149.
[11] MCCARTHY JJ,ESSER KA.MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy [J].JApplPhysiol,2006,102(1):306-313.
[12] GAO N,HUANG J,HE W,etal.Signaling through myosin light chain kinase in smooth muscles [J].JBiolChem,2013,288(11):7596-7605.
[13] ZHANG Y,MORELAND S,MORELAND RS.Regulation of vascular smooth muscle contraction:myosin light chain phosphorylation dependent and independent pathways [J].CanJPhysiolPharmacol,1994,72(11):1386-1391.
[14] VAN ROOIJ E,SUTHERLAND LB,QI X,etal.Control of stress-dependent cardiac growth and gene expression by a microRNA [J].Science,2007,316(5824):575-579.
[15] MCCARTHY JJ,ESSER KA,ANDRADE FH.MicroRNA-206 is overexpressed in the diaphragm but not the hindlimb muscle of mdx mouse [J].AmJPhysiolCellPhysiol,2007,293(1):C451-C457.
[16] WEBB RC.Smooth muscle contraction and relaxation [J].AdvPhysiolEduc,2003,27(1-4):201-206.
[17] MIZUNO Y,ISOTANI E,HUANG J,etal.Myosin light chain kinase activation and calcium sensitization in smooth muscleinvivo[J].AmJPhysiolCellPhysiol,2008,295(2):C358-C364.
[18] SOMLYO AP,SOMLYO AV.Ca2+Sensitivity of smooth muscle and nonmuscle myosin Ⅱ:modulated by G proteins,kinases,and myosin phosphatase [J].PhysiolRev,2003,83(4):1325-1358.
[19] TANG Z,CHEN H,YANG J,etal.The comparison of Ca2+/CaM-independent and Ca2+/CaM-dependent phosphorylation of myosin light chains by MLCK [J].PhysiolRes,2005,54(6):671-678.
[20] PIWKOWSKA A,ROGACKA D,AUDZEYENKA I,etal.Intracellular calcium signaling regulates glomerular filtration barrier permeability:the role of the PKGIα-dependent pathway [J].FEBSLett,2016,590(12):1739-1748.
[21] 陈哲宇.胃肠平滑肌运动的细胞信号转导机制 [J].国外医学消化系疾病分册,2003,23(3):138-141.
[22] JIANG Y,YIN H,ZHENG XL.MicroRNA-1 inhibits myocardin-induced contractility of human vascular smooth muscle cells [J].JCellPhysiol,2010,225(2):506-511.
[24] WEBER M,KIM S,PATTERSON N,etal.MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells [J].AmJPhysiolHeartCircPhysiol,2014,306(8):H1192-H1203.
[25] AN C,BHETWAL BP,SANDERS KM,etal.Role of telokin in regulating murine gastric fundus smooth muscle tension [J].PLoSOne,2015,10(8):e0134876.
[26] LIU B,LEE YC,ALWAAL A,etal. Carbachol-induced signaling through Thr696-phosphorylation of myosin phosphatase-targeting subunit 1 (MYPT1) in rat bladder smooth muscle cells [J].IntUrolNephrol,2016,48(8):1237-1242.
[27] HIRANO K.Current topics in the regulatory mechanism underlying the Ca2+sensitization of the contractile apparatus in vascular smooth muscle [J].JPharmacolSci,2007,104(2):109-115.
[28] SWARD K,MITA M,WILSON DP,etal.The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction [J].CurrHypertensRep,2003,5(1):66-72.
[29] CHIBA Y,MISAWA M.MicroRNAs and their therapeutic potential for human diseases:MiR-133a and bronchial smooth muscle hyperresponsiveness in asthma [J].JPharmacolSci,2010,114(3):264-268.
[30] CHIBA Y,TANABE M,GOTO K,etal.Down-regulation of miR-133a contributes to up-regulation of Rhoa in bronchial smooth muscle cells [J].AmJRespirCritCareMed,2009,180(8):713-719.
[31] LI T,YANG G M,ZHU Y,etal.Diabetes and hyperlipidemia induce dysfunction of VSMCs:contribution of the metabolic inflammation/miRNA pathway [J].AmJPhysiolEndocrinolMetab,2015,308(4):E257-E269.
[32] PUETZ S,LUBOMIROV LT,PFITZER G.Regulation of smooth muscle contraction by small GTPases [J].Physiol,2009,24(6):342-356.
[33] MORGAN KG,GANGOPADHYAY SS.Signal transduction in smooth muscle.Invited review:Cross-bridge regulation by thin filament-associated proteins [J].JApplPhysiol,2001,91(2):953-962.
[34] SOMARA S,GILMONT R,BITAR KN.Role of thin-filament regulatory proteins in relaxation of colonic smooth muscle contraction [J].AmJPhysiolGastrointestLiverPhysiol,2009,297(5):G958-G966.
[35] XIN M,SMALL EM,SUTHERLAND LB,etal.MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury [J].GenesDev,2009,23(18):2166-2178.
[36] GONCHAROVA EA,LIM PN,CHISOLM A,etal.Interferons modulate mitogen-induced protein synthesis in airway smooth muscle.[J].AmJPhysiolLungCellMolPhysiol,2010,299(1):25-35.
[37] CORDES KR,SHEEHY NT,WHITE MP,etal.miR-145 and miR-143 regulate smooth muscle cell fate and plasticity [J].Nature,2009,460(7256):705-710.
[38] ZHANG C.MicroRNA and vascular smooth muscle cell phenotype:new therapy for atherosclerosis? [J].GenomeMed,2009,1(9):1-3.
[39] ALBINSSON S,SWARD K.Targeting smooth muscle microRNAs for therapeutic benefit in vascular disease [J].PharmacolRes,2013,75(5):28-36.
[40] KAMPOURAKIS T,IRVING M.Phosphorylation of myosin regulatory light chain controls myosin head conformation in cardiac muscle [J].JMolCellCardiol,2015,85:199-206.
[41] STULL JT,KAMM KE,VANDENBOOM R.Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle [J].ArchBiochemBiophys,2011,510(2):120-128.
[42] SZCZESNA D.Regulatory light chains of striated muscle myosin.Structure,function and malfunction [J].CurrDrugTargets,2003,3(2):187-197.
[43] DRAWNEL FM,WACHTEN D,MOLKENTIN JD,etal.Mutual antagonism between IP(3)RII and miRNA-133a regulates calcium signals and cardiac hypertrophy [J].JCellBiol,2012,199(5):783-798.
[44] IKEDA S,HE A,KONG SW,etal.MicroRNA-1 negatively regulates expression of the hypertrophy-associated calmodulin and Mef2a genes [J].MolCellBiol,2009,29(8):2193-2204.
[45] ROOIJ EV,QUIAT D,JOHNSON BA,etal.A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance [J].DevCell,2009,17(5):662-673.
[46] MORKIN E.Control of cardiac myosin heavy chain gene expression [J].MicrosResTech,2000,50(50):522-531.
[47] RUNDELL VL,MANAVES V,MARTIN AF,etal. Impact of beta-myosin heavy chain isoform expression on cross-bridge cycling kinetics [J].AmJPhysiolHeartCircPhysiol,2005,288(2):H896-H903.
[48] VANDERHEYDEN M,MULLENS W,DELRUE L,etal.Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders [J].JAmCollCardiol,2008,51(2):129-136.
[49] STELZER JE,BRICKSON S L,LOCHER MR,etal.Role of myosin heavy chain composition in the stretch activation response of rat myocardium [J].JPhysiol,2007,579(1):161-173.
[50] MCCARTHY JJ.The MyomiR network in skeletal muscle plasticity [J].ExercSportSciRev,2011,39(3):150-154.
[51] GONG H,PENG F,ZHANG H,etal. Eplerenone regulates hypertrophy in heart failure by microRNA-208a inhibiting on THRAP1.[J].IntJClinExpPathol,2016,9(2):2424-2434.
[52] TARDIFF JC,HEWETT TE,FACTOR SM,etal.Expression of the beta (slow)-isoform of MHC in the adult mouse heart causes dominant-negative functional effects [J].AmJPhysiolHeartCircPhysiol,2000,278(2):582-591.
[53] KRINGS A,NATEGH S,WALLMARK O,etal. From life to death:microRNA s in the fine tuning of heart [J].CardiovascRes,2013,1(1):3-22.
[54] XU J,ZHAO J,EVAN G,etal.Circulating microRNAs:novel biomarkers for cardiovascular diseases [J].EurHeartJ,2011,90(8):865-875.
[55] LIU X,FAN Z,ZHAO T,etal.Plasma miR-1,miR-208,miR-499 as potential predictive biomarkers for acute myocardial infarction:An independent study of Han population [J].ExpGerontol,2015,72:230-238.
[56] GIDLÖF O,ANDERSSÖN P,VAN DER PALS J,etal.Cardiospecific microRNA plasma levels correlate with troponin and cardiac function in patients with ST elevation myocardial infarction,are selectively dependent on renal elimination,and can be detected in urine samples [J].Cardiol,2011,118(4):217-226.
Progress in the biological effects of muscle-specific microRNAs on muscle contraction and relaxation
DUAN Ting-ting, HAN Xiao-jie, PANG Yu, XU Yu-dong, WANG Yu, YANG Yong-qing, YIN Lei-miao△
(ShanghaiResearchInstituteofAcupunctureandMeridian,ShanghaiUniversityofTraditionalChineseMedicine,Shanghai201203,China)
Muscle-specific microRNAs (myomiRs) are a class of small endogenous non-coding RNAs that expressed specifically in the muscle tissue.By negatively regulating related gene expression at post-translational level,they participate in a variety of biological processes and affects the occurrence and development of diseases.The occurrence and development of muscle-related diseases,such as chronic obstructive pneumonia disease,hypertrophic cardiomyopathy and so on,induce the expression changes of myomiRs and downstream target genes.The effects of myomiRs on the muscle contraction will affect the development of the disease.This paper will review the biological effects of common myomiRs,such as miR-1,miR-133,miR-206,miR-208 and miR-499 in muscle contraction and relaxation,including striated and non-striated muscle.Better understanding of the effects of myomiRs on the biological effects of muscle contraction and relaxation will provide a new idea for the treatment of muscle-related diseases.
muscle-specific microRNAs; smooth muscle; skeletal muscle; cardiac muscle;mechanism of muscle contraction and relaxation
国家自然科学基金( 81473760,81574058);上海市人才发展基金(201610);上海市卫生系统优秀青年人才培养计划( XYQ2013081);上海市中医药事业发展三年行动计划重大研究项目(ZY3-CCCX-3-3005)
R34
B
10.3969/j.issn.1672-8467.2017.03.023
2016-09-01;编辑:张秀峰)
△Corresponding author E-mail:collegeylm@shutcm.edu.cn
*This work was supported by the National Natural Science Foundation of China (81473760,81574058),Shanghai Talent Development Fund (201610),Training Plan for the Excellent Youth Scholars of Shanghai Health System (XYQ2013081) and the Three-year Action Plan for Development of Chinese Traditional Medicine in Shanghai (ZY3-CCCX-3-3005).
