ERα特异性抑制剂MPP降低小鼠2-细胞胚G1/S期过渡相关因子Nanog、cyclin D1和cyclin E水平
2017-06-23逄丽丽贺琳宋婵婵连秀丽刘玥许颂华王世鄂
逄丽丽,贺琳,宋婵婵,连秀丽,刘玥,许颂华,王世鄂,*
(福建医科大学基础医学院1细胞与发育工程中心,2人体解剖学与组织胚胎学系,福州 350122)
论著
ERα特异性抑制剂MPP降低小鼠2-细胞胚G1/S期过渡相关因子Nanog、cyclin D1和cyclin E水平
逄丽丽1,贺琳2,宋婵婵1,连秀丽2,刘玥1,许颂华2,王世鄂1,2*
(福建医科大学基础医学院1细胞与发育工程中心,2人体解剖学与组织胚胎学系,福州 350122)
目的观察雌激素受体(ERα)特异性抑制剂甲基哌啶吡唑(methyl-piperidino-pyrazole,MPP)对小鼠2-细胞胚G1/S期过渡相关因子Nanog、cyclin D1和cyclin E表达水平的影响,阐明ERα对小鼠2-细胞胚G1/S期过渡的作用,为进一步探讨ERα在小鼠植入前胚合子基因组激活中的作用机制提供理论依据。方法收集昆明雌性小鼠与雄性小鼠交配所得的受精卵,分别置于无MPP的培养液和含20μmol/L MPP的培养液中培养,获取体外发育的2-细胞胚,采用免疫荧光染色技术检测细胞内Nanog、cyclin D1和cyclin E水平。结果免疫荧光染色结果显示,含20μmol/L MPP培养液培养的受精卵其体外发育2-细胞胚内Nanog、cyclin D1和cyclin E免疫反应性明显降低。结论ERα可通过调控小鼠2-细胞胚内Nanog、cyclinD1和cyclinE水平,影响细胞从G1期到S期的过渡。
雌激素受体α;植入前胚;Nanog;细胞周期蛋白;小鼠
雌激素受体α(estrogen receptor alpha, ERα)是类固醇激素受体超家族成员之一,可通过经典及非经典途径调控基因表达,对细胞与组织的增殖、分化和发育起着重要作用[1]。前期研究发现,ERα对小鼠植入前胚体外发育有一定的作用,经ERα特异性抑制剂甲基哌啶吡唑(methyl-piperidino-pyrazole,MPP)处理后,小鼠植入前胚发育停滞在2-细胞胚时期,且合子基因组激活(zygotic gene activation, ZGA)相关基因MuERV-L mRNA表达水平下调[2]。ERα的时空动态表达模式也提示,ERα可能调控小鼠植入前胚第一次卵裂的细胞周期进程,参与ZGA[3]。进一步研究发现,经MPP作用后,小鼠2-细胞胚核呈较小的圆形核,属于G0/G1期细胞核象,提示MPP处理后小鼠2-细胞胚(hCG后42h)发育阻滞于DNA复制前期。BrdU标记实验显示,ERα在小鼠2-细胞胚S期中的作用与S期启动有关[4]。但ERα是否通过调节2-细胞胚G1/S期监测点相关蛋白的表达来影响后续发育进程尚不明了。
研究表明,参与G1/S期过渡的关键蛋白主要有cyclinD和cyclinE等。脊椎动物G1期的细胞周期素主要有cyclin D1、cyclin D2和cyclin D3,但它们的含量变化并不依赖于细胞周期进程,而主要依赖于细胞生长或生长促进信号[5]。无论是在正常细胞中,还是在肿瘤细胞中,若cyclin D1蛋白表达受到抑制,则细胞周期停滞在G1/S期,无法顺利过渡到后续发育阶段[6-8]。G1/S期的另一重要调控因子Cyclin E的角色则更加复杂,cyclin D1-细胞周期依赖性蛋白激酶(CDK)4/6只催化部分pRB的磷酸化和E2F的激活,而cyclin E-CDK2可促使E2F完全激活,并通过pRB蛋白的磷酸化,对E2F依赖的基因表达起主要的协调作用[9]。大多哺乳动物细胞中DNA的复制由S期CDKs启动,cyclin E-CDk2复合物作为复制起始的直接调控者引起S期CDKs抑制蛋白的酶解[10]。此外,胚胎干细胞转录因子Nanog对细胞周期的推动也有重要作用。Nanog能推动高分化的小鼠胚胎干细胞G1/S期过渡[11],也可通过调控下游的CDK6及CDC25A,推动细胞由G1期进入S期[12]。可见,Nanog可作用于细胞周期关键调控因子,参与G1/S期的过渡。本研究通过免疫荧光染色技术检测MPP处理后小鼠2-细胞胚中Nanog及细胞周期蛋白cyclin D1和cyclin E的表达情况,进一步阐明ERα在小鼠植入前胚G1/S期过渡中的作用机制。
材料和方法
1 实验动物
昆明(kunming,KM)小鼠购自上海斯莱克公司,雌鼠4~6周,雄鼠8周以上,继续饲养3~5d调节生理周期,使其适应环境供实验处理。
2 主要试剂和仪器
孕马血清促性腺激素(PMSG)(宁波激素制药二厂),人绒毛膜促性腺激素(hCG)(Prospec公司),ERα特异性抑制剂(MPP)(Tocris Bioscience),Nanog兔多克隆抗体(Cell Signaling Technology),cyclin D1鼠单克隆抗体(武汉博士德),cyclin E兔多克隆抗体(Santa Cruz),Alexa Fluor® 488标记驴抗鼠二抗、Alexa Fluor® 488标记驴抗兔二抗(Life Technology),DAPI(碧云天),倒置显微镜(Nikon),水套式二氧化碳培养箱(Thermo)。
3 胚胎的收集和培养
KM雌性小鼠腹腔注射10IU PMSG,46~48h后注射10IU hCG,随后与KM雄性小鼠1∶1合笼(♀KM×♂KM),次日清晨检查阴栓判断是否受精。在注射hCG后27h,从有阴栓雌鼠输卵管中收集受精卵,经M2洗涤,37℃、5% CO2平衡的potassium-supplemented simplex optimized medium (KSOM)漂洗3次,将形态完好的受精卵移至预孵育好的培养液滴(其中分别含0μmol/L、20μmol/L的MPP)中,置于37℃、5% CO2饱和湿度的培养箱中连续培养,每天观察和记录植入前胚发育情况,重复3次以上,确认20μmol/L MPP使1-细胞胚体外发育阻滞于2-细胞期。
4 免疫荧光染色
收集hCG注射后27h的1-细胞胚,分别置于KSOM和含MPP(20μmol/L)KSOM培养液中培养18h获取2-细胞胚,经固定、通透、封闭、一抗(Nanog兔多抗1∶800;cyclin D1鼠单抗 1∶400;cyclin E兔多抗1∶3200)4℃孵育过夜;二抗(Alexa Fluor 488标记驴抗兔1∶1000;Alexa Fluor® 488标记驴抗鼠1∶1000)孵育、DAPI复染,倒置荧光显微镜观察、拍照,所得图片用SmartScape软件进行荧光强度分析。
5 统计学处理
利用SmartScape软件计算免疫荧光图片中各组2-细胞胚内荧光灰度值,采用SPSS17.0统计软件对实验数据进行统计分析,检验方差齐性后运用独立样本t检验进行组间比较,均以P<0.05为差异具有统计学意义。
结 果
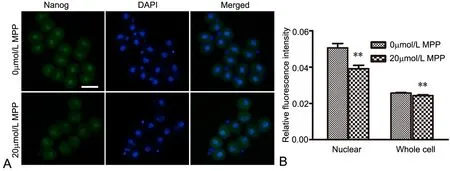
图1 MPP对2-细胞胚内Nanog影响的免疫荧光检测。A,Nanog免疫反应性荧光显微镜成像;B,Nanog免疫反应性统计学分析;比例尺,50μm;**,与对照组比较,P<0.01。Fig. 1 Immunofuorescent detection of the efect of MPP on Nanog expression in 2-cell embryos. A, fuorescence microscope images of Nanog immunoreactivity; B, statistical analysis of Nanog immunoreactivity; scale bar, 50μm; **, P<0.01, compared with control

图2 MPP对2-细胞胚内cyclin D1影响的免疫荧光检测。A,cyclin D1免疫反应性荧光显微镜成像;B,Nanog免疫反应性统计学分析;比例尺,50μm;**,与对照组比较,P<0.01。Fig. 2 Immunofuorescent detection of the efect of MPP on cyclin D1 expression in 2-cell embryos. A, fuorescence microscope images of cyclin D1 immunoreactivity; B, statistical analysis of cyclin D1 immunoreactivity; scale bar, 50μm; **, P<0.01, compared with control
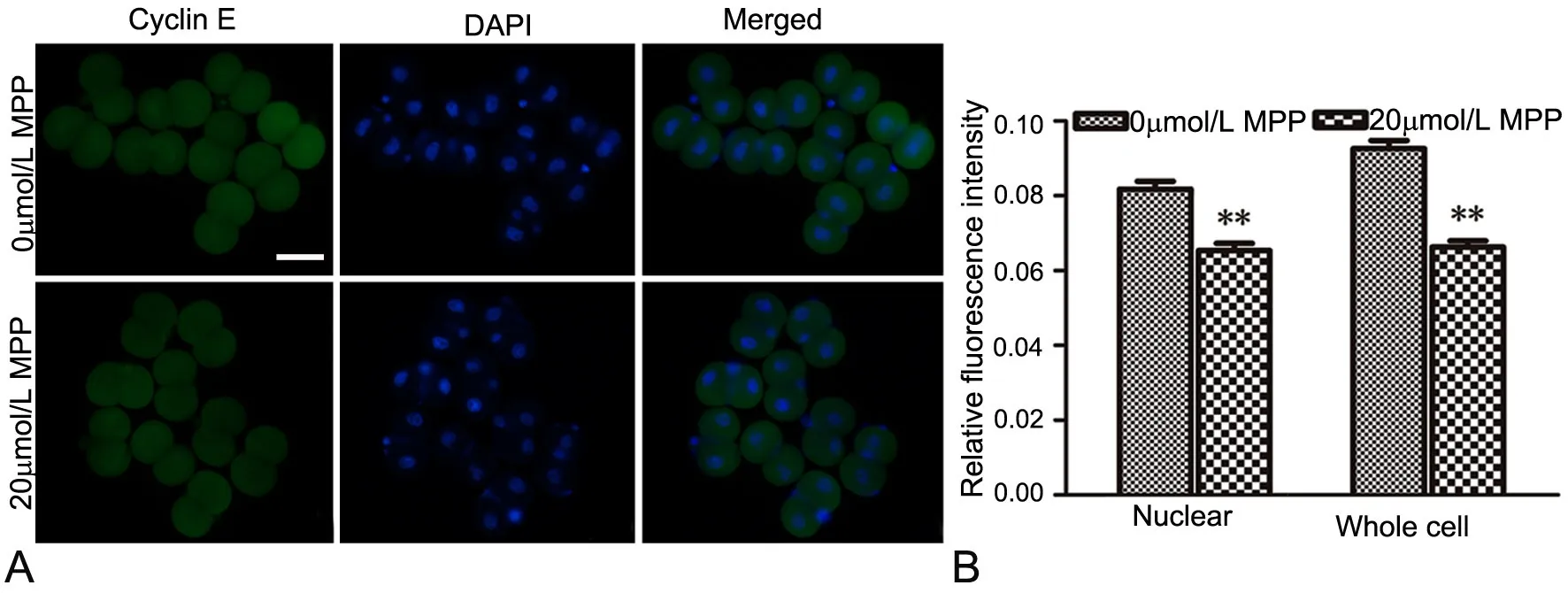
图3 MPP对2-细胞胚内cyclin E影响的免疫荧光检测。A,cyclin E免疫反应性荧光显微镜成像;B,cyclin E免疫反应性统计学分析;比例尺,50μm;**,与对照组比较,P<0.01。Fig. 3 Immunofuorescent detection of the efect of MPP on cyclin E expression in 2-cell embryos. A, fuorescence microscope images of cyclin E immunoreactivity; B, statistical analysis of cyclin E immunoreactivity; scale bar, 50μm; **, P<0.01, compared with control
免疫荧光染色观察显示,Nanog、cyclin D1和Cyclin E免疫反应性在2-细胞胚的胞质和胞核均有分布(图1A,图2A,图3A)。三者在无MPPKSOM培养的2-细胞胚内核定位更为明显(图1A上行,图2A上行,图3A上行);经MPP处理后,三者在2-细胞胚核内和细胞整体的水平均显著降低(图1A下行,图2A下行,图3A下行,图1B,图2B,图3B)。
讨 论
前期研究表明,ERα特异性抑制剂MPP可抑制小鼠合子型标志基因的表达[2],ERα可能通过调节2-细胞胚“G1/S期检测点”相关蛋白的功能,影响小鼠2-细胞胚G1/S期过渡,进而调控ZGA的发生,以及后续的早胚发育进程[4]。Nanog最早被发现作为转录因子,对胚胎干细胞全能性的维持有重要作用,后期另有大量研究发现其对细胞周期调控,尤其是G1/S期过渡亦有重要作用。Nanog表达水平的改变可影响细胞周期关键调节因子的表达,从而影响细胞从G1期到S期的过渡。有研究发现,Nanog的C端可结合于CDK6和CDC25A的调控区,从而推动人胚胎干细胞顺利由G1期进入S期[12]。Nanog可与Oct4、Sox2协同作用,通过影响小分子非编码RNA的表达,调控细胞周期:三者共同结合于miR-302的启动子区域,减少G1期细胞数,增加S期细胞数,促进细胞进入S期[13]。Tomonori等人提出CIBZ可通过PI3K信号调控Nanog表达水平,从而促进人胚胎干细胞G1/S期的进程[11]。本研究发现,经ERα特异性抑制剂MPP(20μmol/L)处理后,小鼠植入前胚发育阻滞在2-细胞阶段, Nanog水平降低,提示ERα可能影响小鼠2-细胞胚中Nanog表达。
此外,cyclin D1作为G1/S期特异性周期蛋白,推动细胞周期进程,促进细胞的增殖。真核生物细胞周期调节存在“开关”样的调控模式,细胞周期依赖性蛋白激酶(CDKs)作为细胞周期的“按钮”持续存在于细胞中,其活性受到相应细胞周期蛋白(cyclins)的严格调控,只有当细胞周期蛋白表达量到达一定阈值时方可激活CDKs,从而推动细胞周期进程[14]。Cyclin D1通过结合并激活G1期CDK4/6,使G1期的抑制蛋白Rb磷酸化失活,与转录因子E2F解离,E2F转录因子启动转录相关基因,从而推动细胞周期由G1期进入S期[15-17]。本研究显示,hCG后45h体外正常发育小鼠2-细胞胚核增大,大部分呈蚕豆形核象,此时处于小鼠植入前胚第二轮细胞周期的G1/S期的关键调控时期,核内cyclin D1水平正常,从而顺利完成CDK4/6激活,使2-细胞胚顺利过渡到S期、G2期,启动ZGA,克服“2-细胞阻滞”,完成后续发育。而MPP(20μmol/L)处理后,小鼠2-细胞胚核则呈较小类圆形G0/G1期细胞核象,cyclin D1明显减少,2-细胞胚发育停滞在第二轮细胞周期G0/G1期,而无法完成后续的植入前胚发育进程。
G1/S期的Start检验点,是真核生物检验细胞是否做好遗传物质复制的一个重要检验点,一旦通过Start点则标志着细胞周期不可逆地开启[18]。Start检验点的中心事件是G1/S期周期蛋白cyclin E-CDk2复合物的激活。动物细胞在Start检验点进入细胞周期为G1-、G1/S-、S-Cdks复合物的激活所触发,同时还伴随E2F依赖的基因表达激活[19,20]。研究发现,cyclin E主要在G1末期表达,在S期表达量达高峰,以保证G1/S期的顺利过渡以及S期的维持。Cyclin E-CDk2复合物参与维持Rb、Cdc6、NPAT以及核仁磷酸蛋白的高磷酸化状态,并且在G1/S期磷酸化P27,诱导其降解,促进G1/S的过渡、S期DNA复制以及细胞增殖[21,22]。以上研究表明,cyclin E蛋白表达量的高低对CDK2的激活,G1-、G1/S-、S-Cdks复合物的形成,G1期到S期的顺利过渡,以及S期遗传物质复制的维持等有重要作用。本研究发现,MPP处理后,小鼠2-细胞胚cyclin E水平也明显下降。这些结果表明,除了Nanog和cyclin D1外,cyclin E也参与ERα在小鼠2-细胞胚中的调控作用。
由此可见,ERα可通过调控小鼠2-细胞胚内Nanog、cyclin D1和cyclin E蛋白的表达水平,而影响细胞从G1期到S期的过渡。但在肝癌细胞中,敲低Nanog可导致Cyclin D1水平下调,阻滞在G0/G1期的细胞比率增高,而S期与G2期细胞明显减少[23]。那么ERα是否通过影响Nanog的蛋白表达,从而调控Cyclin D1、Cyclin E的表达水平,有待进一步研究探讨。
[1] O’Brien JE, Peterson TJ, Tong MH, et al. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem, 2006, 281(36)∶ 26683-26692.
[2] Zhang Y, Jiang Y, Lian X, et al. Efects of ERalpha-specifc antagonist on mouse preimplantation embryo development and zygotic genome activation. J Steroid Biochem Mol Biol, 2015, 145∶ 13-20.
[3] Xu S, Lian X, Cheng X, et al. Dynamic subcellular localization of estrogen receptor alpha during the frst two cleavages of mouse preimplantation embryos. Acta Histochem, 2016, 118(3)∶ 317-321.
[4] 许颂华,程霄翔,宋婵婵,等. 雌激素受体α抑制剂MPP对小鼠合子型基因组激活的影响及与S期启动的关系. 中国组织化学与细胞化学杂志, 2015, 24(6):507-511.
[5] Zhang Q, Sakamoto K, Wagner KU. D-type Cyclins are important downstream efectors of cytokine signaling that regulate the proliferation of normal and neoplastic mammary epithelial cells. Mol Cell Endocrinol, 2014, 382(1)∶ 583-592.
[6] Yang M, Zhong J, Zhao M, et al. Overexpression of nuclear apoptosis-inducing factor 1 altered the proteomic profle of human gastric cancer cell MKN45 and induced cell cycle arrest at G1/S phase. PLoS One. 2014, 9(6)∶ e100216.
[7] Liang J, Pan Y, Zhang D, et al. Cellular prion protein promotes proliferation and G1/S transition of human gastric cancer cells SGC7901 and AGS. FASEB J, 2007, 21(9)∶2247-2256.
[8] Pal D, Sur S, Mandal S, et al. Prevention of liver carcinogenesis by amarogentin through modulation of G1/S cell cycle check point and induction of apoptosis. Carcinogenesis, 2012, 33(12)∶ 2424-2431.
[9] Sherr CJ, Roberts JM. CDK inhibitors∶ positive and negative regulators of G1-phase progression. Gene Dev, 1999, 13(12)∶ 1501-1512.
[10] Zhao H, Chen X, Gurian-West M, et al. Loss of cyclin-dependent kinase 2 (CDK2) inhibitory phosphorylation in a CDK2AF knock-in mouse causes misregulation of DNA replication and centrosome duplication. Mol Cell Biol, 2012, 32(8)∶ 1421-1432.
[11] Nishii T, Oikawa Y, Ishida Y, et al. CtBP-interacting BTB zinc fnger protein (CIBZ) promotes proliferation and G1/S transition in embryonic stem cells via Nanog. J Biol Chem, 2012, 287(15)∶ 12417-12424.
[12] Zhang X, Neganova I, Przyborski S, et al. A role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A. J Cell Biol, 2009, 184(1)∶ 67-82.
[13] Card DA, Hebbar PB, Li L, et al. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol, 2008, 28(20)∶ 6426-6438.
[14] Blain SW. Switching cyclin D-Cdk4 kinase activity on and of. Cell Cycle, 2008, 7(7)∶ 892-898.
[15] Narasimha AM, Kaulich M, Shapiro GS, et al. Cyclin D activates the Rb tumor suppressor by mono-phosphorylation. Elife, 2014, 3.
[16] Alinari L, Prince CJ, Edwards RB, et al. Dual targeting of the cyclin/Rb/E2F and mitochondrial pathways in mantle cell lymphoma with the translation inhibitor silvestrol. Clin Cancer Res, 2012, 18(17)∶ 4600-4611.
[17] Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219∶ in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs, 2014, 32(5)∶825-837.
[18] Toone WM, Aerne BL, Morgan BA, et al. Getting started∶regulating the initiation of DNA replication in yeast. Annu Rev Microbiol, 1997, 51∶ 125-149.
[19] Westendorp B, Mokry M, Groot Koerkamp MJ, et al. E2F7 represses a network of oscillating cell cycle genes to control S-phase progression. Nucleic Acids Res, 2012, 40(8)∶ 3511-3523.
[20] Kyo M, Nagano A, Yamaji N, et al. Timing of the G1/S transition in tobacco pollen vegetative cells as a primary step towards androgenesis in vitro. Plant Cell Rep, 2014, 33(9)∶ 1595-1606.
[21] Mazumder S, DuPree EL, Almasan A. A dual role of cyclin E in cell proliferation and apoptosis may provide a target for cancer therapy. Curr Cancer Drug Targets, 2004, 4(1)∶65-75.
[22] Prall OW, Sarcevic B, Musgrove EA, et al. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem, 1997, 272(16)∶ 10882-10894.
[23] Zhou JJ, Chen RF, Deng XG, et al. Hepatitis C virus core protein regulates NANOG expression via the stat3 pathway. FEBS Lett, 2014, 588(4)∶ 566-573.
Estrogen receptor α specific antagonist MPP inhibits G1/S transitionrelated factors Nanog, cyclin D1 and cyclin E in mouse 2-cell embryos
Pang Lili1, He Lin2, Song Chanchan1, Lian Xiuli2, Liu Yue1, Xu Songhua2, Wang Shie1,2*(1Cellular and Developmental Engineering Center,2Department of Human Anatomy, Histology and Embryology, School of Basic Medical Sciences, Fujian Medical University, Fuzhou 350122, China)
ObjectiveTo investigate the efect of estrogen receptor α (ERα) specifc antagonist methyl-piperidino-pyrazole (MPP) on the expression of G1/S transition-related factors Nanog, cyclin D1 and cyclin E in mouse 2-cell embryos, so as to demonstrate the role of ERα in G1/S transition in mouse 2-cell embryos and provide a theoretical basis for further research on the mechanism of the activation of pre-implantation mouse zygotic genome by ERα.MethodsZygotes were collected from Kunming female mice that had been mated with male mice, and cultured in media without MPP and media supplemented with 20mol/L MPP, respectively. The 2-cell embryos were obtained, and the levels of Nanog, cyclin D1 and cyclin E were detected by immunofuorescent staining.ResultsImmunofuorescent staining results showed that the immunoreactivity of Nanog, cyclin D1 and cyclin E in the 2-cell embryos cultured in 20mol/L MPP was obviously lower than in those cultured without MPP treatment.ConclusionERα might afect G1/S transition via regulating the expression of Nanog, cyclin D1 and cyclin E in mouse 2-cell embryos.
Estrogen receptor alpha; pre-implantation embryo; Nanog; cyclin; mouse
R321
A
10.16705/ j. cnki. 1004-1850.2017.02.002
2017-01-17
2017-03-30
国家自然科学基金(81170624、81671526)
逄丽丽,女(1980年),汉族,实验师
*通讯作者(To whom correspondence should be addressed):shineww@163.com
